Abstract
The adherence and aggregation properties of 46 human oral Streptococcus salivarius isolates were examined. A total of 41% of the isolates aggregated with whole human saliva, 50% aggregated with human erythrocytes, and 85% adhered to human buccal epithelial cells. Strains that aggregated with saliva and erythrocytes usually reacted with Streptococcus group K typing serum whereas the non-hemagglutinating strains did not. K+ strains also adhered more strongly to human buccal epithelial cells than K- strains. All isolates coaggregated with Fusobacterium nucleatum LF and Bacteroides asaccharolyticus 2D, 91% coaggregated with Veillonella alcalescens V1, and 50% coaggregated with Veillonella parvula V4. S. salivarius HB aggregated with saliva from 15 different human donors and aggregated with human erythrocytes irrespective of the blood group. This strain only weakly aggregated with rat saliva or rat erythrocytes. We isolated mutants which concomitantly lost the ability to agglutinate erythrocytes, aggregate with saliva, and bind to buccal epithelial cells, but retained their interbacterial aggregation properties. A second class of mutants lost the ability to coaggregate with Veillonella, but these mutants retained all of the other aggregation properties. Treatment of S. salivarius HB cells with pronase or subtilisin destroyed their ability to aggregate with saliva and erythrocytes and to bind to buccal epithelial cells. The unique characteristics of the aggregation and adherence reactions were suggested by differences in the rate of loss of activity during protease treatment and in the response to chemical modification. The presence of saliva did not affect hemagglutination and adherence to buccal epithelial cells. Binding of the salivary aggregating factor to the bacteria could be distinguished from aggregation on the basis that the latter required divalent cations. The factor involved in coaggregation with F. nucleatum LF was physicochemically different from the other factors, since it was resistant to heat and to extraction with trichloroacetic acid, aqueous phenol, sodium dodecyl sulfate, and formamide, but was sensitive to proteases and was present in both classes of mutants. Coaggregation with V. alcalescens was not sensitive to proteases. A variety of mono- and disaccharides had no influence on any of the reactions tested.
Full text
PDF





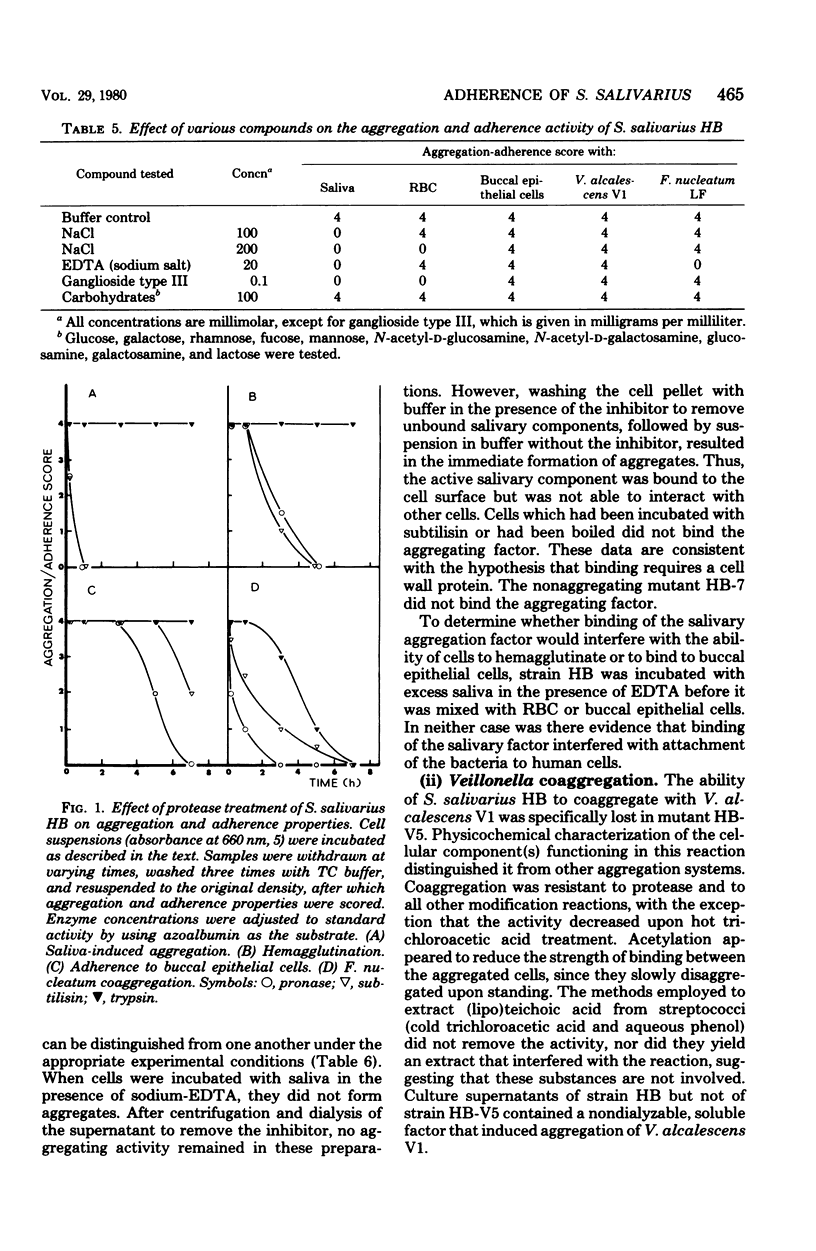



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avery J. K., Cox C. F., Corpron R. E. The effects of combined nerve resection and cavity preparation and restoration on response dentine formation in rabbit incisors. Arch Oral Biol. 1974 Jul;19(7):539–548. doi: 10.1016/0003-9969(74)90069-7. [DOI] [PubMed] [Google Scholar]
- Beachey E. H., Ofek I. Epithelial cell binding of group A streptococci by lipoteichoic acid on fimbriae denuded of M protein. J Exp Med. 1976 Apr 1;143(4):759–771. doi: 10.1084/jem.143.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Grahnén H., Jonsson G., Wikner S. Early establishment of Streptococcus salivarius in the mouth of infants. J Dent Res. 1970 Mar-Apr;49(2):415–418. doi: 10.1177/00220345700490023601. [DOI] [PubMed] [Google Scholar]
- Ciardi J. E., Rölla G., Bowen W. H., Reilly J. A. Adsorption of Streptococcus mutans lipoteichoic acid to hydroxyapatite. Scand J Dent Res. 1977 Sep;85(6):387–391. doi: 10.1111/j.1600-0722.1977.tb00570.x. [DOI] [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Gibbons R. J. Parameters affecting the adherence and tissue tropisms of Streptococcus pyogenes. Infect Immun. 1974 Jan;9(1):85–91. doi: 10.1128/iai.9.1.85-91.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam R. R. Physiological differentiation of viridans streptococci. J Clin Microbiol. 1977 Feb;5(2):184–201. doi: 10.1128/jcm.5.2.184-201.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkler W. A., Jr, Hawley C. E. Hemagglutinating activity of Fusobacterium nucleatum. Infect Immun. 1977 Jan;15(1):230–238. doi: 10.1128/iai.15.1.230-238.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Chludzinski A. M., Schachtele C. F. Streptococcus mutans dextransucrase: requirement for primer dextran. J Bacteriol. 1974 Oct;120(1):287–294. doi: 10.1128/jb.120.1.287-294.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Interbacterial aggregation of plaque bacteria. Arch Oral Biol. 1970 Dec;15(12):1397–1400. doi: 10.1016/0003-9969(70)90031-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Qureshi J. V. Selective binding of blood group-reactive salivary mucins by Streptococcus mutans and other oral organisms. Infect Immun. 1978 Dec;22(3):665–671. doi: 10.1128/iai.22.3.665-671.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Spinell D. M., Skobe Z. Selective adherence as a determinant of the host tropisms of certain indigenous and pathogenic bacteria. Infect Immun. 1976 Jan;13(1):238–246. doi: 10.1128/iai.13.1.238-246.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Van Houte J., Liljemark W. F. Parameters that effect the adherence of Streptococcus salivarius to oral epithelial surfaces. J Dent Res. 1972 Mar-Apr;51(2):424–435. doi: 10.1177/00220345720510023101. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Selective bacterial adherence to oral epithelial surfaces and its role as an ecological determinant. Infect Immun. 1971 Apr;3(4):567–573. doi: 10.1128/iai.3.4.567-573.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket S., Donaldson C. G. Saliva-induced aggregation of oral streptococci. J Bacteriol. 1972 Dec;112(3):1127–1133. doi: 10.1128/jb.112.3.1127-1133.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo W., Sato M., Ozawa H. Haemagglutinating activity of Leptotrichia buccalis cells and their adherence to saliva-coated enamel powder. Arch Oral Biol. 1976;21(6):363–369. doi: 10.1016/s0003-9969(76)80004-0. [DOI] [PubMed] [Google Scholar]
- Levine M. J., Herzberg M. C., Levine M. S., Ellison S. A., Stinson M. W., Li H. C., van Dyke T. Specificity of salivary-bacterial interactions: role of terminal sialic acid residues in the interaction of salivary glycoproteins with Streptococcus sanguis and Streptococcus mutans. Infect Immun. 1978 Jan;19(1):107–115. doi: 10.1128/iai.19.1.107-115.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride B. C., Gisslow M. T. Role of sialic acid in saliva-induced aggregation of Streptococcus sanguis. Infect Immun. 1977 Oct;18(1):35–40. doi: 10.1128/iai.18.1.35-40.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Vatter A. E., Baros J., Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978 Sep;21(3):978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikx F. H., Van der Hoeven J. S. Symbiosis of Streptococcus mutans and Veillonella alcalescens in mixed continuous cultures. Arch Oral Biol. 1975 Jul;20(7):407–410. doi: 10.1016/0003-9969(75)90224-1. [DOI] [PubMed] [Google Scholar]
- Montague E. A., Knox K. W. Antigenic components of the cell wall of Streptococcus salivarius. J Gen Microbiol. 1968 Dec;54(2):237–246. doi: 10.1099/00221287-54-2-237. [DOI] [PubMed] [Google Scholar]
- Olsson J., Glantz P. O. Effect of pH and counter ions on the zeta-potential of oral streptococci. Arch Oral Biol. 1977;22(8-9):461–466. doi: 10.1016/0003-9969(77)90038-3. [DOI] [PubMed] [Google Scholar]
- Olsson J., Glantz P. O., Krasse B. Electrophoretic mobility of oral streptococci. Arch Oral Biol. 1976;21(10):605–609. doi: 10.1016/0003-9969(76)90030-3. [DOI] [PubMed] [Google Scholar]
- Orstavik D., Kraus F. W., Henshaw L. C. In vitro attachment of streptococci to the tooth surface. Infect Immun. 1974 May;9(5):794–800. doi: 10.1128/iai.9.5.794-800.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson P. J., Shapiro I. M. Effect of diphosphonates on root resorption. J Dent Res. 1976 Jan-Feb;55(1):166–166. doi: 10.1177/00220345760550011201. [DOI] [PubMed] [Google Scholar]
- Rölla G. Formation of dental integuments--some basic chemical considerations. Swed Dent J. 1977;1(6):241–251. [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Zur chemischen Zusammensetzung der Zellwand der Streptokokken. I. Die Aminosäuresequenz des Mureins von Str. thermophilus und Str. faecalis. Arch Mikrobiol. 1967 Jul 6;57(4):335–364. [PubMed] [Google Scholar]
- Sherman J. M., Niven C. F., Smiley K. L. Streptococcus salivarius and Other Non-hemolytic Streptococci of the Human Throat. J Bacteriol. 1943 Mar;45(3):249–263. doi: 10.1128/jb.45.3.249-263.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Gibbons R. J. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect Immun. 1978 Jan;19(1):254–264. doi: 10.1128/iai.19.1.254-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M., Wagner B. An electron microscopic study of the location of peptidoglycan in group A and C streptococcal cell walls. J Gen Microbiol. 1978 Oct;108(2):283–294. doi: 10.1099/00221287-108-2-283. [DOI] [PubMed] [Google Scholar]
- Weerkamp A., Bongaerts-Larik L., Vogels G. D. Bacteriocins as factors in the in vitro interaction between oral streptococci in plaque. Infect Immun. 1977 Jun;16(3):773–780. doi: 10.1128/iai.16.3.773-780.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Gibbons R. J. Inhibition of streptococcal attachment to receptors on human buccal epithelial cells by antigenically similar salivary glycoproteins. Infect Immun. 1975 Apr;11(4):711–718. doi: 10.1128/iai.11.4.711-718.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberger C. L., Beaman A. J., Lee L. N. Tween 80 effect on glucosyltransferase synthesis by Streptococcus salivarius. J Bacteriol. 1978 Jan;133(1):231–239. doi: 10.1128/jb.133.1.231-239.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


