Abstract
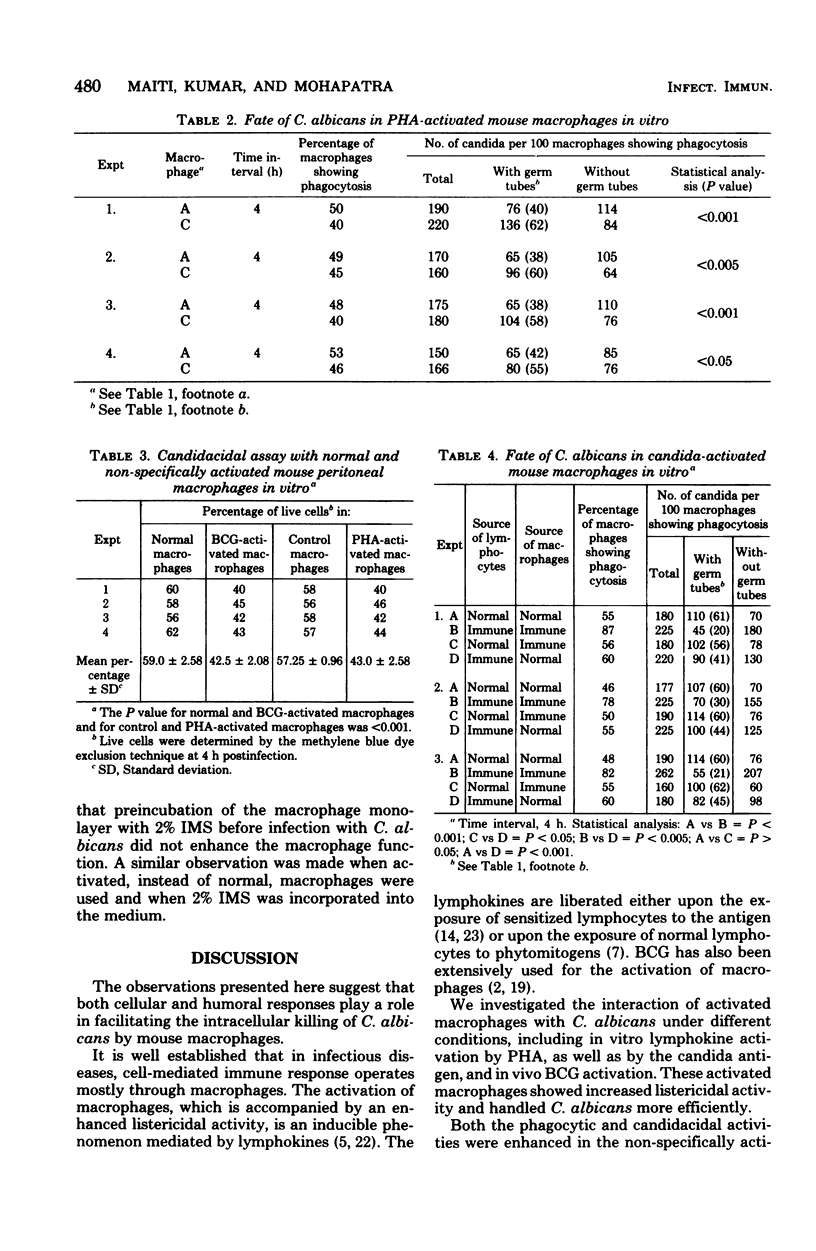
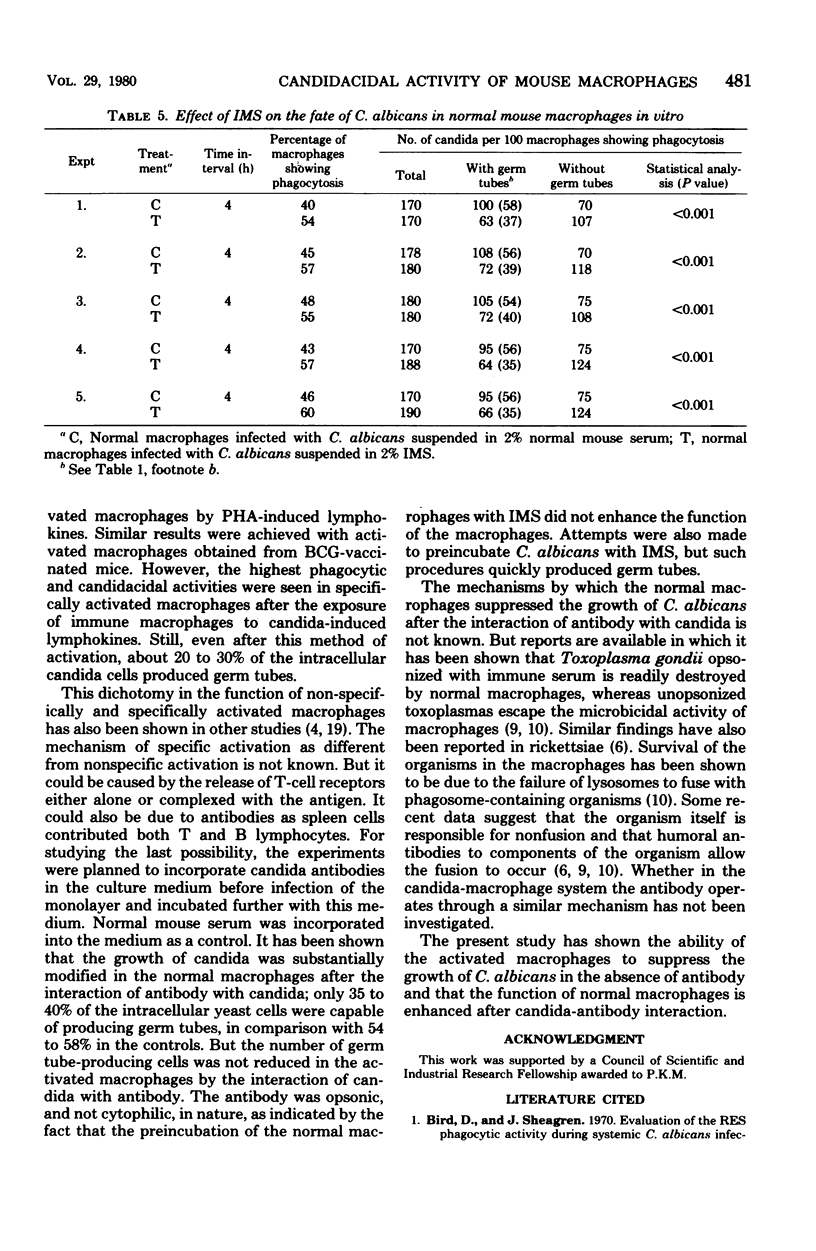
Mouse peritoneal macrophages were infected in vitro with Candida albicans, and the phagocytic and candidacidal activities were estimated by microscopic examination of Giemsa-stained cells. Activated macrophages obtained from either BCG-vaccinated animals or by in vitro exposure of normal macrophages to phytohemagglutinin-induced lymphokines exhibited higher phagocytic and candidacidal activities than did normal macrophages. However, activated macrophages obtained by in vitro exposure of macrophages to candida-induced lymphokines exhibited the highest phagocytic and candidacidal activities. The incorporation of immune mouse serum into the culture medium also enhanced the phagocytic and candidacidal activities of the normal macrophages but failed to improve the function of the activated macrophages. These results suggest that both activated macrophages and antibodies may be required for controlling candida infections in mice.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanden R. V., Lefford M. J., Mackaness G. B. The host response to Calmette-Guérin bacillus infection in mice. J Exp Med. 1969 May 1;129(5):1079–1107. doi: 10.1084/jem.129.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilgren R. A., Meuwissen H. J., Quie P. G., Good R. A., Hong R. The cellular immune defect in chronic mucocutaneous candidiasis. Lancet. 1969 Jun 28;1(7609):1286–1288. doi: 10.1016/s0140-6736(69)92223-5. [DOI] [PubMed] [Google Scholar]
- Cole P. Activation of mouse peritoneal cells to kill Listeria monocytogenes by T-lymphocyte products. Infect Immun. 1975 Jul;12(1):36–41. doi: 10.1128/iai.12.1.36-41.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowles R. E., Fajardo I. M., Leibowitch J. L., David J. R. The enhancement of macrophage bacteriostasis by products of activated lymphocytes. J Exp Med. 1973 Oct 1;138(4):952–964. doi: 10.1084/jem.138.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambrill M. R., Wisseman C. L., Jr Mechanisms of immunity in typhus infections. 3. Influence of human immune serum and complement on the fate of Rickettsia mooseri within the human macrophages. Infect Immun. 1973 Oct;8(4):631–640. doi: 10.1128/iai.8.4.631-640.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M. F., Bauminger S. Activation of T and B lymphocytes by insoluble phytomitogens. Nat New Biol. 1972 Jan 19;235(55):67–70. doi: 10.1038/newbio235067a0. [DOI] [PubMed] [Google Scholar]
- Jones T. C., Hirsch J. G. The interaction between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J Exp Med. 1972 Nov 1;136(5):1173–1194. doi: 10.1084/jem.136.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. C., Len L., Hirsch J. G. Assessment in vitro of immunity against Toxoplasma gondii. J Exp Med. 1975 Feb 1;141(2):466–482. doi: 10.1084/jem.141.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. C. Macrophages and intracellular parasitism. J Reticuloendothel Soc. 1974 May;15(5):439–450. [PubMed] [Google Scholar]
- Kirkpatrick C. H., Ottenson E. A., Smith T. K., Wells S. A., Burdick J. F. Reconstitution of defective cellular immunity with foetal thymus and dialysable transfer factor. Long-term studies in a patient with chronic mucocutaneous candidiasis. Clin Exp Immunol. 1976 Mar;23(3):414–428. [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Interaction of Candida albicans with human leukocytes and serum. J Bacteriol. 1969 Jun;98(3):996–1004. doi: 10.1128/jb.98.3.996-1004.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourad S., Friedman L. Passive immunization of mice against Candida albicans. Sabouraudia. 1968 Feb;6(2):103–105. [PubMed] [Google Scholar]
- Ozato K., Uesaka I. The role of macrophages in Candida albicans infection in vitro. Jpn J Microbiol. 1974 Jan;18(1):29–35. doi: 10.1111/j.1348-0421.1974.tb00740.x. [DOI] [PubMed] [Google Scholar]
- Pearsall N. N., Adams B. L., Bunni R. Immunologic responses to Candida albicans. III. Effects of passive transfer of lymphoid cells or serum on murine candidiasis. J Immunol. 1978 Apr;120(4):1176–1180. [PubMed] [Google Scholar]
- Peterson E. M., Calderone R. A. Growth inhibition of Candida albicans by rabbit alveolar macrophages. Infect Immun. 1977 Mar;15(3):910–915. doi: 10.1128/iai.15.3.910-915.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzan K. R., Musher D. M., Keusch G. T., Weinstein L. Correlation of increased metabolic activity, resistance to infection, enhanced phagocytosis, and inhibition of bacterial growth by macrophages from Listeria- and BCG-infected mice. Infect Immun. 1972 Apr;5(4):499–504. doi: 10.1128/iai.5.4.499-504.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. J., Balish E., Manning D. D. The role of thymus-dependent cell-mediated immunity in resistance to experimental disseminated candidiasis. J Reticuloendothel Soc. 1976 Oct;20(4):291–298. [PubMed] [Google Scholar]
- SALVIN S. B., PETERSON R. D., GOOD R. A. THE ROLE OF THE THYMUS IN RESISTANCE TO INFECTION AND ENDOTOXIN TOXICITY. J Lab Clin Med. 1965 Jun;65:1004–1022. [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Cellular immunity in vitro. I. Immunologically mediated enhancement of macrophage bactericidal capacity. J Exp Med. 1971 Jun 1;133(6):1377–1389. doi: 10.1084/jem.133.6.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Enhancement of macrophage bactericidal capacity by antigenically stimulated immune lymphocytes. Cell Immunol. 1972 Jun;4(2):163–174. doi: 10.1016/0008-8749(72)90015-9. [DOI] [PubMed] [Google Scholar]
- Sweet C. E., Kaufman L. Application of agglutinins for the rapid and accurate identification of medically important Candida species. Appl Microbiol. 1970 May;19(5):830–836. doi: 10.1128/am.19.5.830-836.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. M., Sawyer S., Remington J. S. Role of activated macrophages in resistance of mice to infection with Trypanosoma cruzi. J Infect Dis. 1976 Dec;134(6):610–623. doi: 10.1093/infdis/134.6.610. [DOI] [PubMed] [Google Scholar]


