Abstract
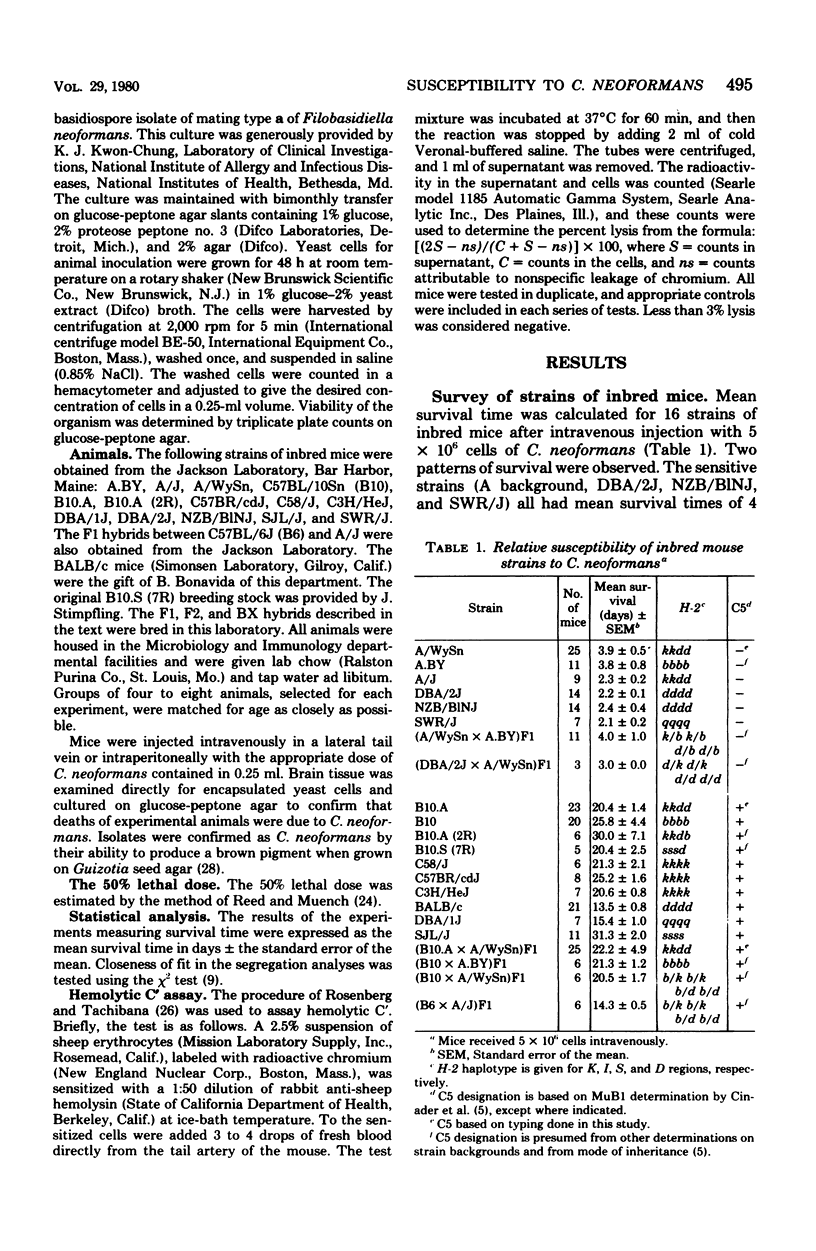
Inbred mice injected intravenously with 5 × 106 cells of Cryptococcus neoformans showed two patterns of survival: sensitive (A/WySn, A.BY, A/J, DBA/2J, NZB/B1NJ, and SWR/J) and resistant [C57BL/10Sn, B10.A, B10.A (2R), B10.S (7R),C57BR/cdJ, C58/J, C3H/HeJ, BALB/c, DBA/1J, and SJL/J]. Relative susceptibility based on survival time was shown to correspond to differences obtained for 50% lethal dose values. Either decreasing the dose of organisms or changing to the intraperitoneal route of inoculation resulted in prolonged survival times, but neither change affected the observed patterns of survival. F1 hybrids between different sensitive strains were also sensitive, whereas F1 hybrids between sensitive and resistant strains were resistant, indicating a dominant mode of inheritance. Sensitivity and resistance were shown to be under single gene control by segregation analysis in F2 progeny produced by inbreeding (B10.A × A/WySn)F1 hybrids and in (F1 × A/WySn) backcross progeny. Blood obtained from parental strains, F1, F2, and backcross hybrids was tested for the presence or absence of hemolytic complement. Mice lacking hemolytic complement activity in their sera are homozygous for the Hc0 allele at the Hc locus on chromosome 2 and are deficient in the complement component C5. A 1:1 correspondence was found between C5 deficiency and sensitivity to C. neoformans. Resistance was shown to cosegregate with the presence of hemolytic complement in the F2 and the backcross progenies.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley D. J., Taylor B. A., Blackwell J., Evans E. P., Freeman J. Regulation of Leishmania populations within the host. III. Mapping of the locus controlling susceptibility to visceral leishmaniasis in the mouse. Clin Exp Immunol. 1979 Jul;37(1):7–14. [PMC free article] [PubMed] [Google Scholar]
- CINADER B., DUBISKI S., WARDLAW A. C. DISTRIBUTION, INHERITANCE, AND PROPERTIES OF AN ANTIGEN, MUB1, AND ITS RELATION TO HEMOLYTIC COMPLEMENT. J Exp Med. 1964 Nov 1;120:897–924. doi: 10.1084/jem.120.5.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caren L. D., Rosenberg L. T. The role of complement in resistance to endogenous and exogenous infection with a common mouse pathogen, Corynebacterium kutscheri. J Exp Med. 1966 Oct 1;124(4):689–699. doi: 10.1084/jem.124.4.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chick E. W., Roberts G. D. The varying susceptibility of different genetic strains of laboratory mice to Histoplasma capsulatum. Mycopathol Mycol Appl. 1974 Apr 30;52(3):251–253. doi: 10.1007/BF02198750. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., May J. E., Kane M. A., Frank M. M., Bennett J. E. The role of the classical and alternate complement pathways in host defenses against Cryptococcus neoformans infection. J Immunol. 1974 Jun;112(6):2260–2270. [PubMed] [Google Scholar]
- Diamond R. D., May J. E., Kane M., Frank M. M., Bennett J. E. The role of late complement components and the alternate complement pathway in experimental cryptococcosis. Proc Soc Exp Biol Med. 1973 Oct 1;144(1):312–315. doi: 10.3181/00379727-144-37580. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Root R. K., Bennett J. E. Factors influencing killing of Cryptococcus neoformans by human leukocytes in vitro. J Infect Dis. 1972 Apr;125(4):367–376. doi: 10.1093/infdis/125.4.367. [DOI] [PubMed] [Google Scholar]
- HERZENBERG L. A., TACHIBANA D. K., HERZENBERG L. A., ROSENBERG L. T. A gene locus concerned with hemolytic complement in Mus musculus. Genetics. 1963 May;48:711–715. doi: 10.1093/genetics/48.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hormaeche C. E. Genetics of natural resistance to salmonellae in mice. Immunology. 1979 Jun;37(2):319–327. [PMC free article] [PubMed] [Google Scholar]
- Hormaeche C. E. Natural resistance to Salmonella typhimurium in different inbred mouse strains. Immunology. 1979 Jun;37(2):311–318. [PMC free article] [PubMed] [Google Scholar]
- Miller M. E., Nilsson U. R. A familial deficiency of the phagocytosis-enhancing activity of serum related to a dysfunction of the fifth component of complement (C5). N Engl J Med. 1970 Feb 12;282(7):354–358. doi: 10.1056/NEJM197002122820702. [DOI] [PubMed] [Google Scholar]
- Morelli R., Rosenberg L. T. Role of complement during experimental Candida infection in mice. Infect Immun. 1971 Apr;3(4):521–523. doi: 10.1128/iai.3.4.521-523.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli R., Rosenberg L. T. The role of complement in the phagocytosis of Candida albicans by mouse peripheral blood leukocytes. J Immunol. 1971 Aug;107(2):476–480. [PubMed] [Google Scholar]
- Muchmore H. G., Felton F. G., Salvin S. B., Rhoades E. R. Delayed hypersensitivity to cryptococcin in man. Sabouraudia. 1968 Oct;6(4):285–288. doi: 10.1080/00362176885190561. [DOI] [PubMed] [Google Scholar]
- Nilsson U. R., Müller-Eberhard H. J. Deficiency of the fifth component of complement in mice with an inherited complement defect. J Exp Med. 1967 Jan 1;125(1):1–16. doi: 10.1084/jem.125.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi Y. M., Colten H. R. Genetic defect in secretion of complement C5 in mice. Nature. 1979 Nov 8;282(5735):207–208. doi: 10.1038/282207a0. [DOI] [PubMed] [Google Scholar]
- Patton R. M., Riggs A. R., Compton S. B., Chick E. W. Histoplasmosis in purebred mice: influence of genetic susceptibility and immune depression on treatment. Mycopathologia. 1976 Dec 10;60(1):39–43. doi: 10.1007/BF00442546. [DOI] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Genetics of resistance to infection with Salmonella typhimurium in mice. J Infect Dis. 1976 Jan;133(1):72–78. doi: 10.1093/infdis/133.1.72. [DOI] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Locating salmonella resistance gene on mouse chromosome 1. Clin Exp Immunol. 1979 Jul;37(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Natural resistance to Salmonella infection, delayed hypersensitivity and Ir genes in different strains of mice. Nature. 1974 Mar 22;248(446):345–347. doi: 10.1038/248345a0. [DOI] [PubMed] [Google Scholar]
- Pérez H., Arredondo B., González M. Comparative study of American cutaneous leishmaniasis and diffuse cutaneous leishmaniasis in two strains of inbred mice. Infect Immun. 1978 Nov;22(2):301–307. doi: 10.1128/iai.22.2.301-307.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSENBERG L. T., TACHIBANA D. K. Activity of mouse complement. J Immunol. 1962 Dec;89:861–867. [PubMed] [Google Scholar]
- Robson H. G., Vas S. I. Resistance of inbred mice to Salmonella typhimurium. J Infect Dis. 1972 Oct;126(4):378–386. doi: 10.1093/infdis/126.4.378. [DOI] [PubMed] [Google Scholar]
- Ruddy S., Gigli I., Austen K. F. The complement system of man. I. N Engl J Med. 1972 Sep 7;287(10):489–495. doi: 10.1056/NEJM197209072871005. [DOI] [PubMed] [Google Scholar]
- Shields A. B., Ajello L. Medium for selective isolation of Cryptococcus neoformans. Science. 1966 Jan 14;151(3707):208–209. doi: 10.1126/science.151.3707.208. [DOI] [PubMed] [Google Scholar]
- Shin H. S., Smith M. R., Wood W. B., Jr Heat labile opsonins to pneumococcus. II. Involvement of C3 and C5. J Exp Med. 1969 Dec 1;130(6):1229–1241. doi: 10.1084/jem.130.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Durack D. T., McCarty G. A., Ward F. E., Meadows L. Deficiency of the fifth component of complement in human subjects. Clinical, genetic and immunologic studies in a large kindred. Am J Med. 1979 Oct;67(4):638–645. doi: 10.1016/0002-9343(79)90247-x. [DOI] [PubMed] [Google Scholar]
- TACHIBANA D. K., ULRICH M., ROSENBERG L. T. THE INHERITANCE OF HEMOLYTIC COMPLEMENT ACTIVITY IN CF-1 MICE. J Immunol. 1963 Aug;91:230–232. [PubMed] [Google Scholar]
- Walter J. E., Atchison R. W. Epidemiological and immunological studies of Cryptococcus neoformans. J Bacteriol. 1966 Jul;92(1):82–87. doi: 10.1128/jb.92.1.82-87.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]



