Abstract
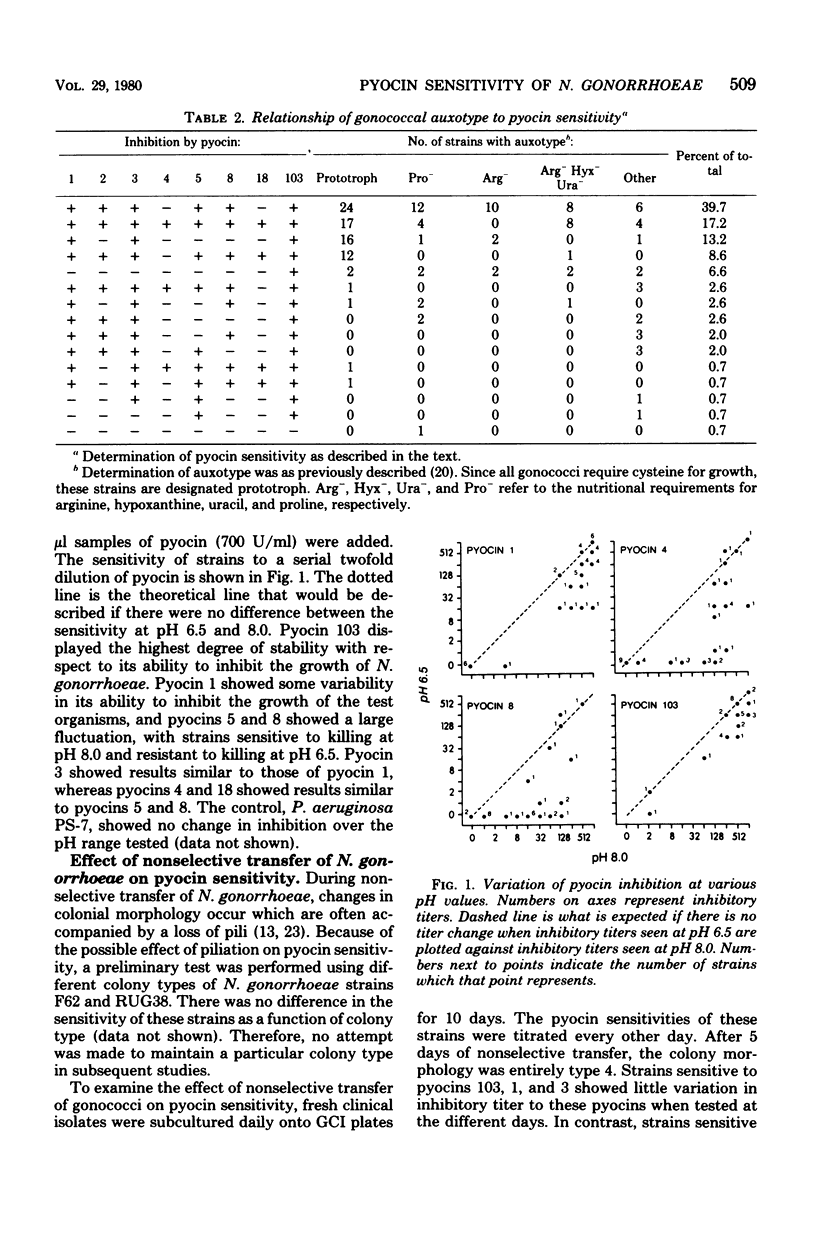
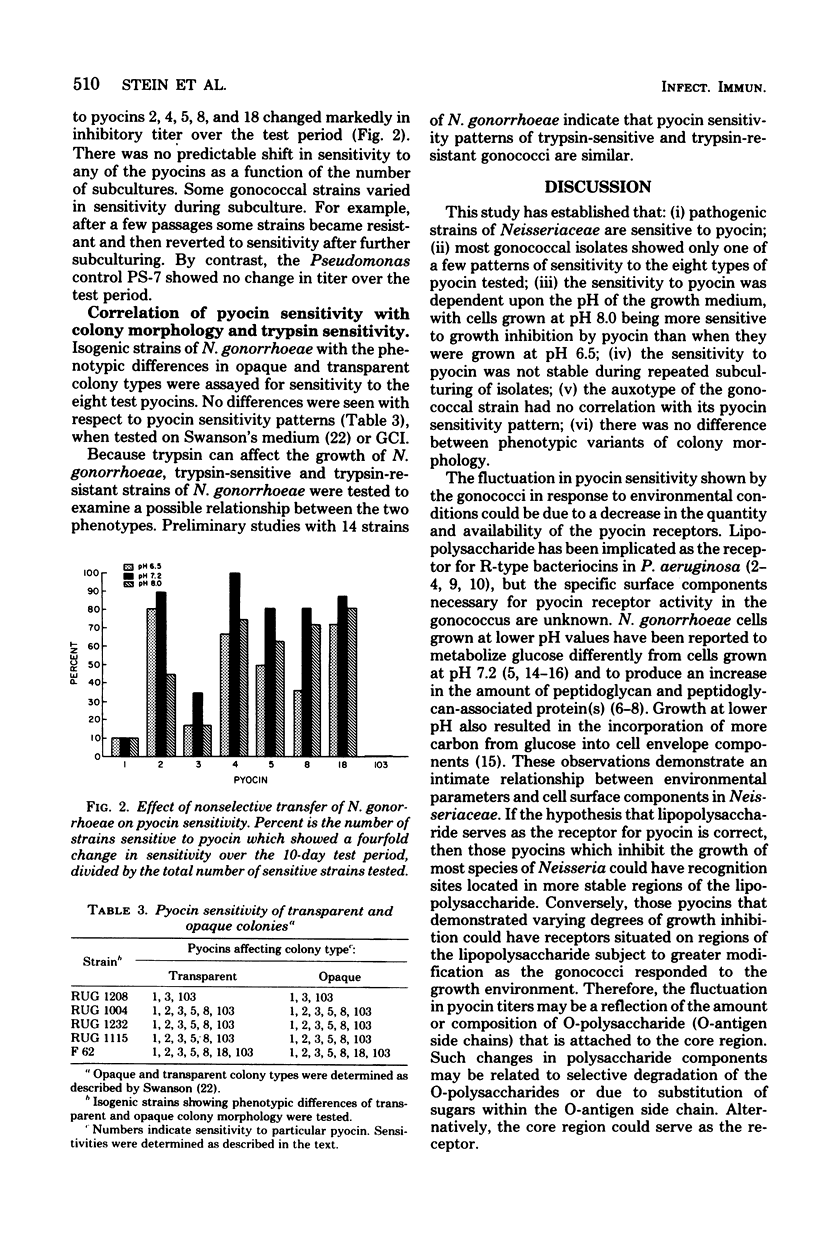
The effect of environmental variation on the susceptibility of Neisseria gonorrhoeae to pyocin produced by Pseudomonas aeruginosa was examined. Susceptibility to at least one pyocin was demonstrated in strains of N. gonorrhoeae (99%), N. meningitidis (35%), and N. lactamica (47%). The degree of sensitivity to pyocin displayed by N. gonorrhoeae was affected by varying the pH of the growth environment. Gonococcal strains were more sensitive to growth inhibition by pyocins at an alkaline pH and less sensitive to growth inhibition at an acid pH. Inhibitory titers fluctuated during nonselective subculture of fresh clinical isolates. There was no apparent correlation between auxotype and sensitivity to pyocin. Also, no relationship between colony morphology and pyocin sensitivity was seen.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brookes R., Hedén C. G. Dense cultures of Neisseria gonorrhoeae in liquid medium. Appl Microbiol. 1967 Mar;15(2):219–223. doi: 10.1128/am.15.2.219-223.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyke J., Berk R. S. Growth inhibition and pyocin receptor properties of endotoxin from Pseudomonas aeruginosa. Proc Soc Exp Biol Med. 1974 Apr;145(4):1405–1408. doi: 10.3181/00379727-145-38023. [DOI] [PubMed] [Google Scholar]
- Govan J. R. Studies on the pyocins of Pseudomonas aeruginosa: morphology and mode of action of contractile pyocins. J Gen Microbiol. 1974 Jan;80(1):1–15. doi: 10.1099/00221287-80-1-1. [DOI] [PubMed] [Google Scholar]
- Govan J. R. Studies on the pyocins of Pseudomonas aeruginosa: production of contractile and flexuous pyocins in Pseudomonas aeruginosa. J Gen Microbiol. 1974 Jan;80(1):17–30. doi: 10.1099/00221287-80-1-17. [DOI] [PubMed] [Google Scholar]
- Hebeler B. H., Morse S. A. Physiology and metabolism of pathogenic neisseria: tricarboxylic acid cycle activity in Neisseria gonorrhoeae. J Bacteriol. 1976 Oct;128(1):192–201. doi: 10.1128/jb.128.1.192-201.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebeler B. H., Morse S. A., Wong W., Young F. E. Evidence for peptidoglycan-associated protein(s) in Neisseria gonorrhoeae. Biochem Biophys Res Commun. 1978 Apr 14;81(3):1011–1017. doi: 10.1016/0006-291x(78)91451-1. [DOI] [PubMed] [Google Scholar]
- Hebeler B. H., Wong W., Morse S. A., Young F. E. Cell envelope of Neisseria gonorrhoeae CS7: peptidoglycan protein complex. Infect Immun. 1979 Feb;23(2):353–359. doi: 10.1128/iai.23.2.353-359.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K., Egami F. Receptor substance for pyocin R. I. Partial purification and chemical properties. J Biochem. 1969 Apr;65(4):603–609. doi: 10.1093/oxfordjournals.jbchem.a129053. [DOI] [PubMed] [Google Scholar]
- JACOB F. Biosynthèse induite et mode d'action d'une pyocine, antibiotique de Pseudomonas pyocyanea. Ann Inst Pasteur (Paris) 1954 Feb;86(2):149–160. [PubMed] [Google Scholar]
- KAGEYAMA M., EGAMI F. On the purification and some properties of a pyocin, a bacteriocin produced by Pseudomonas aeruginosa. Life Sci. 1962 Sep;1:471–476. doi: 10.1016/0024-3205(62)90055-3. [DOI] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Vaughan P., Johnson D., Iglewski B. H. Inhibition of Neisseria gonorrhoeae by a bacteriocin from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1976 Aug;10(2):354–362. doi: 10.1128/aac.10.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny P., Short J. A., Walker P. D. An electron-microscope study of naturally occurring and cultured cells of Neisseria Gonorrhoeae. J Med Microbiol. 1975 Aug;8(3):413–427. doi: 10.1099/00222615-8-3-413. [DOI] [PubMed] [Google Scholar]
- Penn C. W., Veale D. R., Smith H. Selection from gonococci grown in vitro of a colony type with some virulence properties of organisms adapted in vivo. J Gen Microbiol. 1977 May;100(1):147–158. doi: 10.1099/00221287-100-1-147. [DOI] [PubMed] [Google Scholar]
- Short H. B., Ploscowe V. B., Weiss J. A., Young F. E. Rapid method for auxotyping multiple strains of Neisseria gonorrhoeae. J Clin Microbiol. 1977 Sep;6(3):244–248. doi: 10.1128/jcm.6.3.244-248.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidberry H. D., Sadoff J. C. Pyocin sensitivity of Neisseria gonorrhoeae and its feasibility as an epidemiological tool. Infect Immun. 1977 Feb;15(2):628–637. doi: 10.1128/iai.15.2.628-637.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Kraus S. J., Gotschlich E. C. Studies on gonococcus infection. I. Pili and zones of adhesion: their relation to gonococcal growth patterns. J Exp Med. 1971 Oct 1;134(4):886–906. doi: 10.1084/jem.134.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]


