Abstract
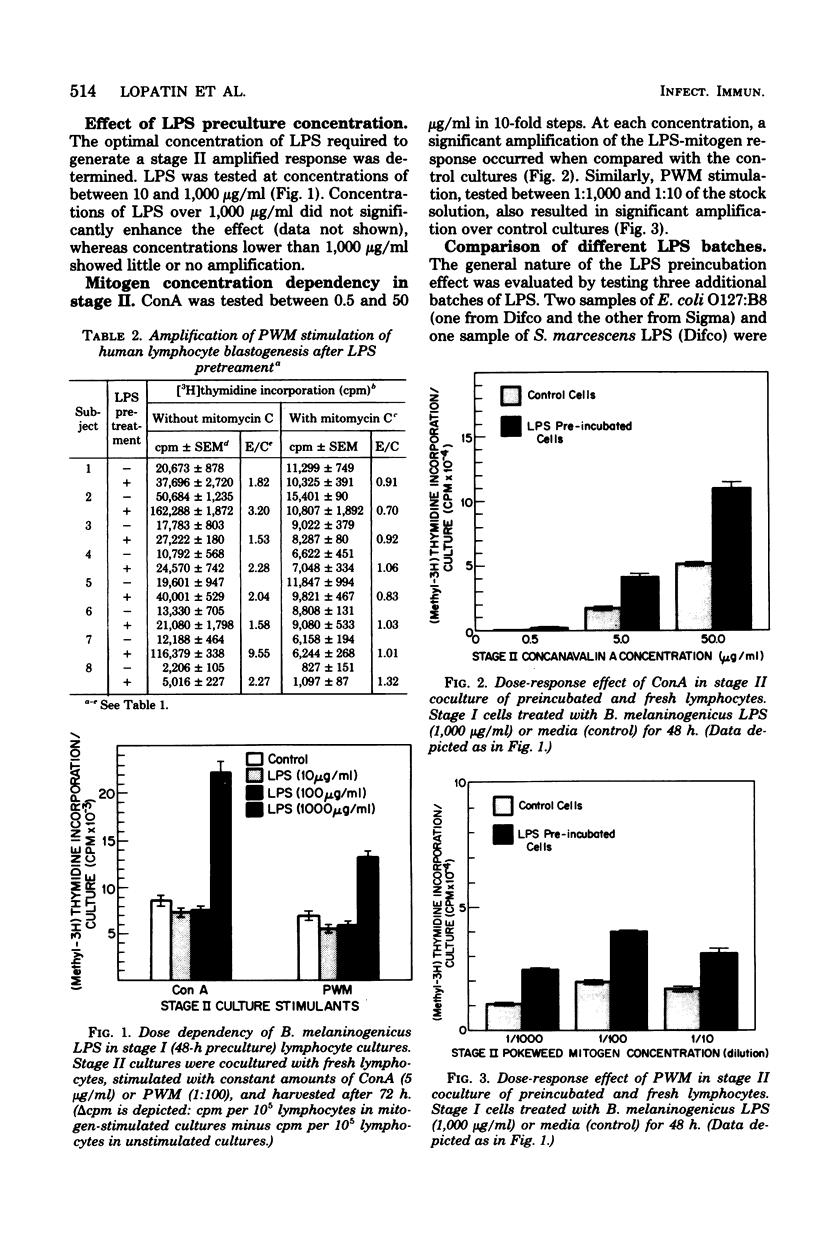
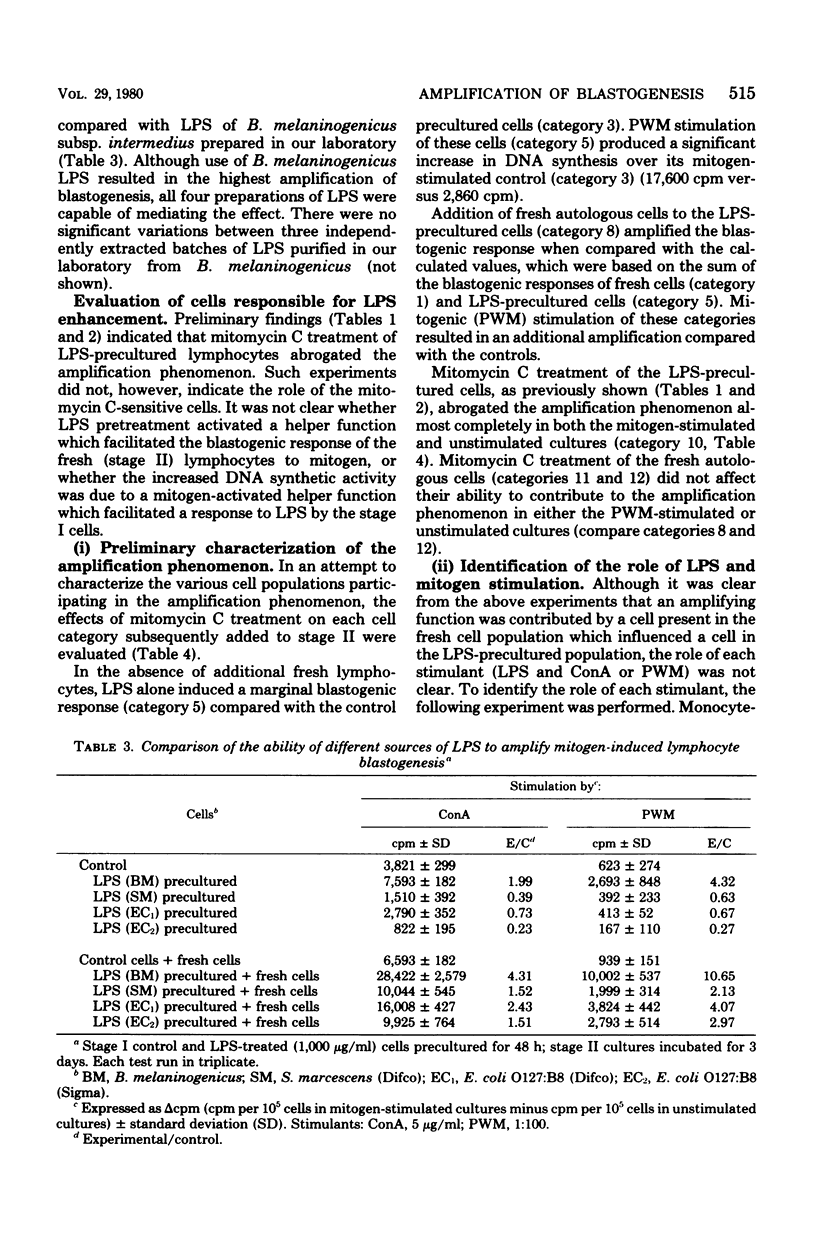
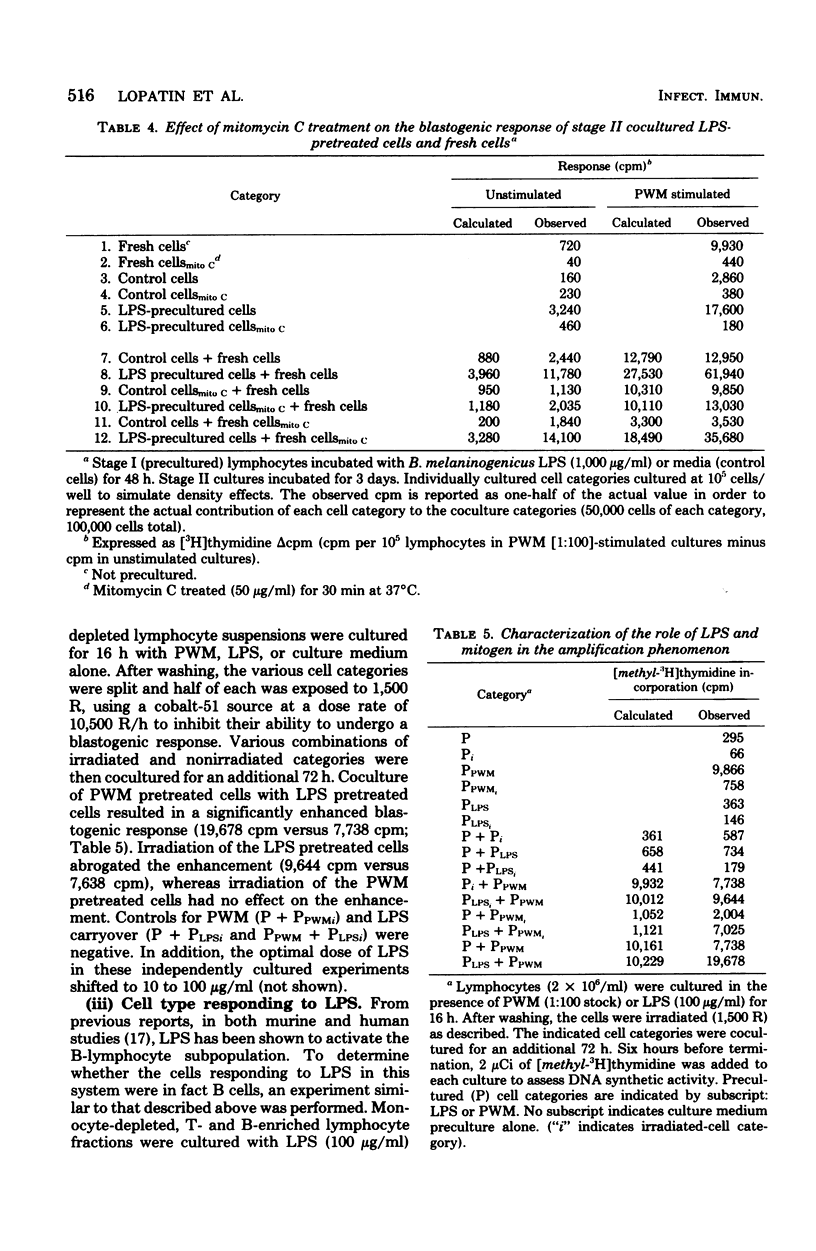
Human peripheral blood lymphocytes were cultured in vitro with lipopolysaccharide (LPS) for 48 h. After washing, stimulation with a concanavalin A (ConA) or pokeweed mitogen (PWM) resulted in a synergistic blastogenic response that was greater than the sum of the independently stimulated control cultures. Addition of fresh autologous lymphocytes after LPS preculture produced an additional increment of synergy. The nature of the responding and helping effects was determined by coculturing irradiated or nonirradiated lymphocyte suspensions that had been precultured with either LPS or ConA/PWM. Such studies indicated that amplification was the result of a mitogen-activated helper activity, which facilitated the blastogenic response to LPS. Experiments with lymphocytes resolved into T- and B-cell-enriched fractions indicated that the LPS-responsive cells were of the B type and that the help was provided by a mitogen-activated T-cell population. These studies indicated that LPS can induce human B-cell blastogenesis; however, a helper function must be provided by a T-cell subpopulation. This helper activity is inducible by pretreatment of the T cells with plant lectins.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso C. M., Bernabé R. R., Moreno E., Diaz de Espada F. Depletion of monocytes from human peripheral blood leucocytes by passage through sephadex G-10 columns. J Immunol Methods. 1978;22(3-4):361–368. doi: 10.1016/0022-1759(78)90043-1. [DOI] [PubMed] [Google Scholar]
- Andersson J., Möller G., Sjöberg O. Selective induction of DNA synthesis in T and B lymphocytes. Cell Immunol. 1972 Aug;4(4):381–393. doi: 10.1016/0008-8749(72)90040-8. [DOI] [PubMed] [Google Scholar]
- Bresnihan B., Jasin H. E. Suppressor function of peripheral blood mononuclear cells in normal individuals and in patients with systemic lupus erythematosus. J Clin Invest. 1977 Jan;59(1):106–116. doi: 10.1172/JCI108607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton R. W. Inhibitory and stimulatory effects of concanavalin A on the response of mouse spleen cell suspensions to antigen. I. Characterization of the inhibitory cell activity. J Exp Med. 1972 Dec 1;136(6):1445–1460. doi: 10.1084/jem.136.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon R. K. T cell control of antibody production. Contemp Top Immunobiol. 1974;3:1–40. doi: 10.1007/978-1-4684-3045-5_1. [DOI] [PubMed] [Google Scholar]
- Gery I., Krüger J., Spiesel S. Z. Stimulation of B-lymphocytes by endotoxin. Reactions of thymus-deprived mice and karyotypic analysis of dividing cells in mice bearing T 6 T 6 thymus grafts. J Immunol. 1972 Apr;108(4):1088–1091. [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A. Functional analysis of B cell heterogeneity. Transplant Rev. 1975;24:3–40. doi: 10.1111/j.1600-065x.1975.tb00164.x. [DOI] [PubMed] [Google Scholar]
- Hallgren H. M., Yunis E. J. Suppressor lymphocytes in young and aged humans. J Immunol. 1977 Jun;118(6):2004–2008. [PubMed] [Google Scholar]
- Hubert C., Delespesse G., Govaerts A. Concanavalin A-activated suppressor cells in normal human peripheral blood lymphocytes. Clin Exp Immunol. 1976 Oct;26(1):95–98. [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Dean J. H., Richardson G. L., Herberman R. B. Depletion of monocytes from human peripheral blood mononuclear leukocytes: comparison of the sephadex G-10 column method with other commonly used techniques. J Immunol Methods. 1980;32(1):11–29. doi: 10.1016/0022-1759(80)90113-1. [DOI] [PubMed] [Google Scholar]
- Kaplan M. E., Clark C. An improved rosetting assay for detection of human T lymphocytes. J Immunol Methods. 1974 Jul;5(2):131–135. doi: 10.1016/0022-1759(74)90003-9. [DOI] [PubMed] [Google Scholar]
- Lopatin D. E., Peebles F. L., Horner I. S. The influence of mitogen costimulation on the human lymphocyte blastogenic response to Actinomyces viscosus ultrasonicates. Clin Immunol Immunopathol. 1980 May;16(1):75–83. doi: 10.1016/0090-1229(80)90168-3. [DOI] [PubMed] [Google Scholar]
- MacDermott R. P., Nash G. S., Bertovich M. J., Merkel N. S., Weinrieb I. J. Human B-cell mitogenic responsiveness to lectins: the requirement for T cells. Cell Immunol. 1978 Jun;38(1):198–202. doi: 10.1016/0008-8749(78)90047-3. [DOI] [PubMed] [Google Scholar]
- Miller R. A., Gartner S., Kaplan H. S. Stimulation of mitogenic responses in human peripheral blood lymphocytes by lipopolysaccharide: serum and T helper cell requirements. J Immunol. 1978 Dec;121(6):2160–2164. [PubMed] [Google Scholar]
- Nowotny A., Cundy K. R., Neale N. L., Nowotny A. M., Radvany R., Thomas S. P., Tripodi D. J. Relation of structure to function in bacterial O-antigens. IV. Fractionation of the components. Ann N Y Acad Sci. 1966 Jun 30;133(2):586–603. doi: 10.1111/j.1749-6632.1966.tb52391.x. [DOI] [PubMed] [Google Scholar]
- Oppenheim J. J., Leventhal B. G., Hersh E. M. The transformation of column-purified lymphocytes with nonspecific and specific antigenic stimuli. J Immunol. 1968 Aug;101(2):262–267. [PubMed] [Google Scholar]
- Peavy D. L., Adler W. H., Smith R. T. The mitogenic effects of endotoxin and staphylococcal enterotoxin B on mouse spleen cells and human peripheral lymphocytes. J Immunol. 1970 Dec;105(6):1453–1458. [PubMed] [Google Scholar]
- Sakane T., Green I. Human suppressor T cells induced by concanavalin A: suppressor T cells belong to distinctive T cell subclasses. J Immunol. 1977 Sep;119(3):1169–1178. [PubMed] [Google Scholar]
- Schmidtke J. R., Najarian J. S. Synergistic effects on DNA synthesis of phytohemagglutinin or concanavalin A and lipopolysaccharide in human peripheral blood lymphocytes. J Immunol. 1975 Feb;114(2 Pt 2):742–746. [PubMed] [Google Scholar]
- Schwartz S. A., Shou L., Good R. A., Choi Y. S. Suppression of immunoglobulin synthesis and secretion by peripheral blood lymphocytes from normal donors. Proc Natl Acad Sci U S A. 1977 May;74(5):2099–2103. doi: 10.1073/pnas.74.5.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou L., Schwartz S. A., Good R. A. Suppressor cell activity after concanavalin A treatment of lymphocytes from normal donors. J Exp Med. 1976 May 1;143(5):1100–1110. doi: 10.1084/jem.143.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Bois M. J., Bierhorst-Eijlander A., Meinesz A., Schellekens P. T. Cellular requirements for lymphocyte restimulation after a first challenge in vitro. Cell Immunol. 1978 Dec;41(2):338–346. doi: 10.1016/0008-8749(78)90231-9. [DOI] [PubMed] [Google Scholar]


