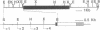Abstract
Milk protein regulation involves synergistic action of lactogenic hormones and extracellular matrix (ECM). It is well established that substratum has a dramatic effect on morphology and function of mammary cells. The molecular mechanisms that regulate the ECM- and hormone-dependent gene expression, however, have not been resolved. To address this question, a subpopulation (designated CID 9) of the mouse mammary epithelial cell strain COMMA-1D has been developed in which more than 35% of the cells express beta-casein, form alveoli-like structures when plated onto a reconstituted basement membrane, and secrete beta-casein unidirectionally into a lumen. These cells were stably transfected with a series of chloramphenicol acetyltransferase (CAT) fusion genes to study transcriptional regulation of the bovine beta-casein gene. The expression of CAT in these lines demonstrated a striking matrix and hormone dependency (greater than 150-fold induction in some cases). This regulation occurred primarily at the transcriptional level and was dependent on the length of the 5' flanking region of the beta-casein promotor. Both matrix and hormonal control of transcription occurred within at least the first 1790 base pairs upstream and/or 42 base pairs downstream of the transcriptional initiation site. The ECM effect was independent of glucocorticoid stimulation. However, prolactin was essential and hydrocortisone further increased CAT expression. Endogenous beta-casein expression in these lines was similar to that of the parent CID 9 cells. Our data indicate the existence of matrix-dependent elements that regulate transcription.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball R. K., Friis R. R., Schoenenberger C. A., Doppler W., Groner B. Prolactin regulation of beta-casein gene expression and of a cytosolic 120-kd protein in a cloned mouse mammary epithelial cell line. EMBO J. 1988 Jul;7(7):2089–2095. doi: 10.1002/j.1460-2075.1988.tb03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-Hoff M. H., Aggeler J., Ram T. G., Bissell M. J. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989 Feb;105(2):223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron J. M. Induction of albumin gene transcription in hepatocytes by extracellular matrix proteins. Mol Cell Biol. 1990 Mar;10(3):1239–1243. doi: 10.1128/mcb.10.3.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. H., Bissell M. J. A novel regulatory mechanism for whey acidic protein gene expression. Cell Regul. 1989 Nov;1(1):45–54. doi: 10.1091/mbc.1.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Danielson K. G., Oborn C. J., Durban E. M., Butel J. S., Medina D. Epithelial mouse mammary cell line exhibiting normal morphogenesis in vivo and functional differentiation in vitro. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3756–3760. doi: 10.1073/pnas.81.12.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doppler W., Groner B., Ball R. K. Prolactin and glucocorticoid hormones synergistically induce expression of transfected rat beta-casein gene promoter constructs in a mammary epithelial cell line. Proc Natl Acad Sci U S A. 1989 Jan;86(1):104–108. doi: 10.1073/pnas.86.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doppler W., Höck W., Hofer P., Groner B., Ball R. K. Prolactin and glucocorticoid hormones control transcription of the beta-casein gene by kinetically distinct mechanisms. Mol Endocrinol. 1990 Jun;4(6):912–919. doi: 10.1210/mend-4-6-912. [DOI] [PubMed] [Google Scholar]
- Eisenstein R. S., Rosen J. M. Both cell substratum regulation and hormonal regulation of milk protein gene expression are exerted primarily at the posttranscriptional level. Mol Cell Biol. 1988 Aug;8(8):3183–3190. doi: 10.1128/mcb.8.8.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman J. T., Pitelka D. R. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977 May;13(5):316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Gorodetsky S. I., Tkach T. M., Kapelinskaya T. V. Isolation and characterization of the Bos taurus beta-casein gene. Gene. 1988 Jun 15;66(1):87–96. doi: 10.1016/0378-1119(88)90227-2. [DOI] [PubMed] [Google Scholar]
- Jeang K. T., Rawlins D. R., Rosenfeld P. J., Shero J. H., Kelly T. J., Hayward G. S. Multiple tandemly repeated binding sites for cellular nuclear factor 1 that surround the major immediate-early promoters of simian and human cytomegalovirus. J Virol. 1987 May;61(5):1559–1570. doi: 10.1128/jvi.61.5.1559-1570.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Hassell J. R., Star V. L., Cannon F. B., Laurie G. W., Martin G. R. Basement membrane complexes with biological activity. Biochemistry. 1986 Jan 28;25(2):312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- Kliman H. J., Feinberg R. F. Human trophoblast-extracellular matrix (ECM) interactions in vitro: ECM thickness modulates morphology and proteolytic activity. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3057–3061. doi: 10.1073/pnas.87.8.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. Y., Lee W. H., Kaetzel C. S., Parry G., Bissell M. J. Interaction of mouse mammary epithelial cells with collagen substrata: regulation of casein gene expression and secretion. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1419–1423. doi: 10.1073/pnas.82.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. Y., Parry G., Bissell M. J. Modulation of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. J Cell Biol. 1984 Jan;98(1):146–155. doi: 10.1083/jcb.98.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. F., Atiee S. H., Rosen J. M. Differential regulation of rat beta-casein-chloramphenicol acetyltransferase fusion gene expression in transgenic mice. Mol Cell Biol. 1989 Feb;9(2):560–565. doi: 10.1128/mcb.9.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. L., Aggeler J., Farson D. A., Hatier C., Hassell J., Bissell M. J. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc Natl Acad Sci U S A. 1987 Jan;84(1):136–140. doi: 10.1073/pnas.84.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina D., Li M. L., Oborn C. J., Bissell M. J. Casein gene expression in mouse mammary epithelial cell lines: dependence upon extracellular matrix and cell type. Exp Cell Res. 1987 Sep;172(1):192–203. doi: 10.1016/0014-4827(87)90105-4. [DOI] [PubMed] [Google Scholar]
- Schneider W., Shyamala G. Glucocorticoid receptors in primary cultures of mouse mammary epithelial cells: characterization and modulation by prolactin and cortisol. Endocrinology. 1985 Jun;116(6):2656–2662. doi: 10.1210/endo-116-6-2656. [DOI] [PubMed] [Google Scholar]
- Schuetz E. G., Li D., Omiecinski C. J., Muller-Eberhard U., Kleinman H. K., Elswick B., Guzelian P. S. Regulation of gene expression in adult rat hepatocytes cultured on a basement membrane matrix. J Cell Physiol. 1988 Mar;134(3):309–323. doi: 10.1002/jcp.1041340302. [DOI] [PubMed] [Google Scholar]
- Streuli C. H., Bissell M. J. Expression of extracellular matrix components is regulated by substratum. J Cell Biol. 1990 Apr;110(4):1405–1415. doi: 10.1083/jcb.110.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suard Y. M., Haeuptle M. T., Farinon E., Kraehenbuhl J. P. Cell proliferation and milk protein gene expression in rabbit mammary cell cultures. J Cell Biol. 1983 May;96(5):1435–1442. doi: 10.1083/jcb.96.5.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucker D. S., Yamamoto K. R. Early events in the stimulation of mammary tumor virus RNA synthesis by glucocorticoids. Novel assays of transcription rates. J Biol Chem. 1984 Jun 25;259(12):7416–7420. [PubMed] [Google Scholar]
- Watt F. M., Jordan P. W., O'Neill C. H. Cell shape controls terminal differentiation of human epidermal keratinocytes. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5576–5580. doi: 10.1073/pnas.85.15.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicha M. S., Lowrie G., Kohn E., Bagavandoss P., Mahn T. Extracellular matrix promotes mammary epithelial growth and differentiation in vitro. Proc Natl Acad Sci U S A. 1982 May;79(10):3213–3217. doi: 10.1073/pnas.79.10.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M., Oka T. Transfection of beta-casein chimeric gene and hormonal induction of its expression in primary murine mammary epithelial cells. Proc Natl Acad Sci U S A. 1990 May;87(10):3670–3674. doi: 10.1073/pnas.87.10.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu-Lee L. Y., Rosen J. M. A transfected alpha-casein minigene bypasses posttranscriptional control by hormones, but retains cell-substratum regulation in mammary epithelial cells. Mol Endocrinol. 1988 May;2(5):431–443. doi: 10.1210/mend-2-5-431. [DOI] [PubMed] [Google Scholar]
- Zurfluh L. L., Bolten S. L., Byatt J. C., McGrath M. F., Tou J. S., Zupec M. E., Krivi G. G. Isolation of genomic sequence encoding a biologically active bovine TGF-alpha protein. Growth Factors. 1990;3(4):257–266. doi: 10.3109/08977199009003668. [DOI] [PubMed] [Google Scholar]