Abstract
Background
It is well known that transcranial direct current stimulation (tDCS) is capable of modulating corticomotor excitability. However, a source of growing concern has been the observed inter- and intra-individual variability of tDCS-responses. Recent studies have assessed whether individuals respond in a predictable manner across repeated sessions of anodal tDCS (atDCS). The findings of these investigations have been inconsistent, and their methods have some limitations (i.e. lack of sham condition or testing only one tDCS intensity).
Objective
To study inter- and intra-individual variability of atDCS effects at two different intensities on primary motor cortex (M1) excitability.
Methods
Twelve subjects participated in a crossover study testing 7-min atDCS over M1 in three separate conditions (2mA, 1mA, sham) each repeated three times separated by 48hrs. Motor evoked potentials were recorded before and after stimulation (up to 30min). Time of testing was maintained consistent within participants. To estimate the reliability of tDCS effects across sessions, we calculated the Intra-class Correlation Coefficient (ICC).
Results
AtDCS at 2mA, but not 1mA, significantly increased cortical excitability at the group level in all sessions. The overall ICC revealed fair to high reliability of tDCS effects for multiple sessions. Given that the distribution of responses showed important variability in the sham condition, we established a Sham Variability-Based Threshold to classify responses and to track individual changes across sessions. Using this threshold an intra-individual consistent response pattern was then observed only for the 2mA condition.
Conclusion
2mA anodal tDCS results in consistent intra- and inter-individual increases of M1 excitability.
Keywords: Cortical plasticity, Transcranial direct current stimulation, Inter-individual variability, Intra-individual variability
Introduction
Transcranial Direct Current Stimulation (tDCS) has been widely used as a non-invasive brain stimulation method capable of modulating cortical excitability as determined by the change in amplitude of motor evoked potentials (MEP) induced by transcranial magnetic stimulation (TMS) [1, 2]. Commonly assumed, anodal tDCS (atDCS) leads to an increase of neuronal excitability of the cerebral cortex, whereas cathodal tDCS induces a decrease [2–4]. This initial promising finding, the easy application and the ability to induce long-lasting after-effects [2, 5–7] have resulted in an explosion of investigations in motor [8–15], cognitive [16–18] and perceptual domains using tDCS [19–21].
Although different studies have successfully reproduced tDCS neuromodulatory properties, recent investigations have indicated that its response is quite variable (see for review [22, 23]). Besides reported inter-group variability (i.e., inconsistent responses across different groups of individuals to the application of an identical tDCS protocol [24]), other studies described variable responses within the same experimental group resulting in noticeable inter-individual variability [7, 25]. Indeed, many studies have shown that 20 – 60% of a group of individuals experience the classical excitability increase induced by a single atDCS session, whereas the rest have no change or even the opposite effect compared to baseline values [26–32]. With this in mind, another key question is whether individuals respond in a consistent and predictable manner to repeated tDCS sessions. To date, several studies have measured the reliability by intra-individual assessment of tDCS-responses showing inconsistent results across studies [27, 29, 33–35]. Besides other methodological differences, these inconsistencies could be due to the lack of control conditions, which cannot rule out the variability introduced by the measuring tool (TMS), or testing different tDCS intensities.
To better understand tDCS-response variability in a crossover design we assessed intra- and inter-individual atDCS responses using two different tDCS intensities while controlling for extrinsic factors and TMS variability. We predicted that the repeated presentation of atDCS to the same group of individuals would result in consistent responses at the group and individual level across sessions.
Materials and methods
Subjects
Twelve healthy subjects (4 females, 19–34 years old, mean age ± SD: 24.9 ± 5.1), 2 left-handed, non-smokers, with a negative history of neurological or psychiatric conditions participated in this study. Alcohol, recreational drugs and caffeine consumption were not allowed in the 24hrs prior to the study. Participants gave written consent, which was approved by the Johns Hopkins Institutional Review Board and in accordance to the Declaration of Helsinki.
Experimental procedure
We used a single-blinded, crossover and counterbalanced design. Subjects participated in three sessions for each experimental condition of tDCS (2mA, 1mA, sham). Each participant attended all sessions, which started at the same time of the day, and were at least 48 hours apart (mean interval between sessions ± SD: 3.6 ± 4.1 days) to avoid cumulative increases in cortical excitability [36, 37]. Subjects sat in a comfortable chair with both arms resting on a pillow. The experimental procedure was identical in all sessions (except tDCS intensity; Fig. 1). At the beginning of each session we identified the M1 ‘hotspot’. The stimulus intensity required to evoke a MEP of ~1 mV peak-to-peak amplitude was determined, and using this intensity 10 MEPs (minimum number recommended to ensure highest reliability of TMS measures [38]) induced every 7 ± 1 sec were recorded prior to tDCS application. To assess after-effects on MEP amplitude, TMS measurements (10 pulses) were repeated immediately (Post0), 15 min (Post15) and 30 min (Post30) after tDCS. EMG activity was monitored during each session. All sessions were performed by the same experimenter.
Figure 1.
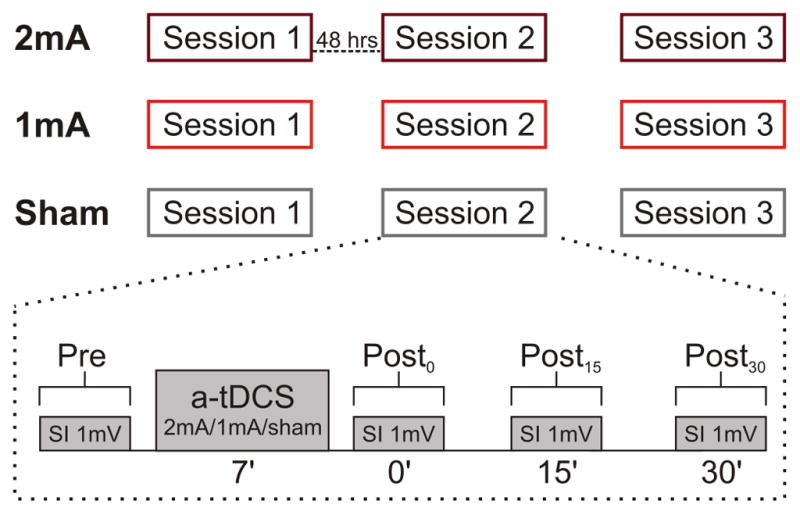
Three identical sessions were performed for each tDCS condition at least 48 hours apart. Each session consisted of TMS pre-measures; defining stimulus intensity required to evoke mean MEP peak-to-peak amplitude of 1mV (SI1mV, Pre). Then, atDCS 1 or 2 mA was applied for 7 min (in sham condition current passed only for 30s) with the active electrode on the FDI ‘hotspot’. Post-measurements were performed using the SI1mV intensity immediately (Post0), 15 (Post15), and 30 (Post30) min after tDCS.
Electromyographic recordings
We recorded electromyographic (EMG) activity from the right first dorsal interosseous (FDI) muscle using disposable surface electrodes. Signals were amplified (1000x; AMT-8 EMG, Bortec Biomedical Ltd., Canada), sampled (2000 Hz) and recorded with CED 1401 and Signal 4 (Cambridge Electronic Design, UK), and finally analyzed off-line using MATLAB (MathWorks).
Transcranial magnetic stimulation
We performed single-pulse TMS using a flat 70-mm figure-eight-shaped magnetic coil connected to a Magstim 2002 stimulator (Magstim Co. Ltd, UK). The coil was held tangential to the scalp with the handle oriented backwards and 45° from the midline. We used a frameless neuronavigation system (BrainSight; Rogue Research, Canada) to guide the coil position with the help of a magnetic resonance imaging template. For each session we determine the optimal area (‘hot spot’) of M1 for eliciting MEPs in the resting FDI. For corticospinal excitability measurement, we determined the TMS output intensity necessary to evoke a peak-to-peak MEP amplitude of ~ 1mV (Stimulus intensity 1 mV, SI1mV).
Transcranial direct current stimulation
We delivered tDCS with a direct-current stimulator (Chattanooga Ionto™, UK) through two 25cm2 sponge electrodes soaked in saline. We implemented a bipolar electrode montage with the active electrode centered over the defined M1 ‘hot spot’ and the reference electrode placed on the right supra-orbital area [2]. We delivered atDCS over the corticomotor hand representation of the FDI. Subjects received in a randomized and counterbalanced order anodal (2mA, 1mA) or sham tDCS. The current densities for 2mA and 1mA were 0.08 mA/cm2 and 0.04 mA/cm2, respectively. For active tDCS conditions, the current was ramped up for 30 s, held constant at the determined intensity for 7 min and then ramped down for 30 s. For sham condition, the current was ramped up and immediately ramped down for 30 s. We chose the stimulation duration of 7 minutes based on previous results showing significant increases in excitability that lasted up to 30mins [39].
Data analysis
We calculated mean MEP amplitudes by averaging MEP values across subjects for each time point (reported as mean value ± standard error of the measurement, SEM). We also calculated the ratio of post-tDCS and pre-tDCS MEP values (post/pre). We used the Kolmogorov-Smirnov Test to check for normal distribution. We applied the Greenhouse-Geisser correction to correct for non-sphericity. We performed repeated measures ANOVA (ANOVARM) to explore differences between CONDITION (2mA, 1mA, sham), SESSION (S1, S2, S3) and TIME (Pre, Post0, Post15, Post30). For all analyses, when a statistical significance was found, we performed post-hoc pairwispe comparisons using Bonferroni correction for multiple comparisons.
To represent individual responses across sessions and with the purpose to compare our data with previous studies, we also calculated the Grand Mean for each subject being the mean of the mean MEP values across the 3 post-tDCS time-points. In addition, for all conditions we classified subject’s responses to different groups (‘increase’, ‘no change’, ‘decrease’) based on the mean MEP ratios and using the standard deviation (SD) of the sham condition as a threshold for response classification.
To estimate individual reliability of tDCS after-effects across sessions, we calculated the Intra-class Correlation Coefficient (ICC), defined as the ratio of the between-subject variance and all sources of variance. Values range from 0 to 1 (though negative values are possible due to the manner in which the statistic is computed), with lower values indicating a lack of reliability. Guidelines propose that ICC ≥ 0.75 indicates high, whereas ICC < 0.4 poor agreement [40]. All statistical analyses were executed using SPSS-software (SPSS Statistics for Windows, IBM Corp., NY).
Results
All participants reported sensations of itching/tingling consistent with those generally described during tDCS [41]. We found no differences for gender, age, and quality of sleep (p > 0.05).
AtDCS elicited consistent group responses
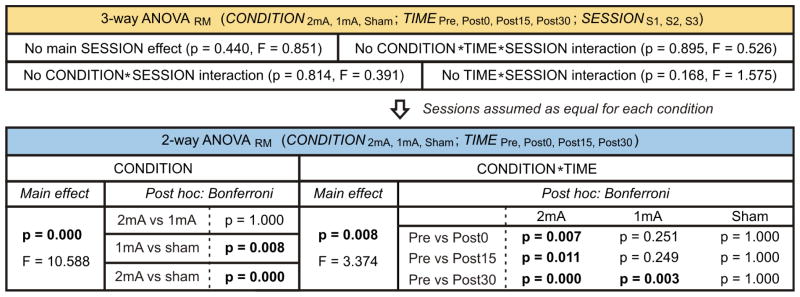
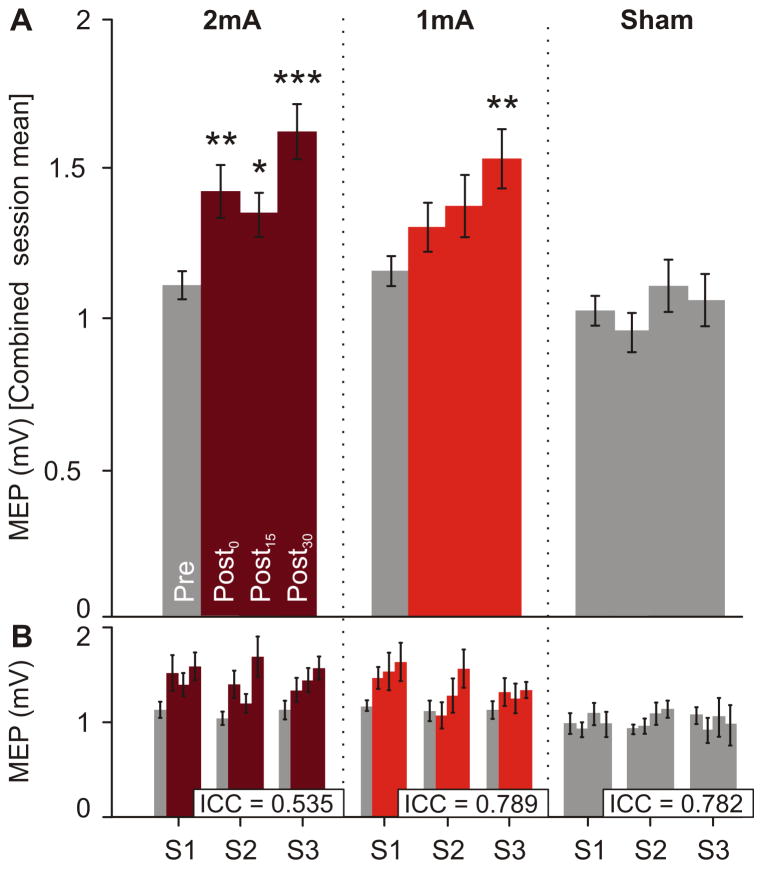
The statistical results are summarized in Table 1. The 3-way ANOVARM comparing the tDCS effects across CONDITION (2mA, 1mA, sham), SESSION (S1, S2, S3) and TIME (Pre, Post0, Post15, Post30) revealed no statistical significance between pre-tDCS values (p > 0.05). Since we did not detect significant main SESSION effect or significant interactions between SESSION and the other variables, sessions were assumed to be equal for each condition. Thus, we applied a 2-way ANOVARM to the combined session mean using CONDITION and TIME as factors (Fig. 2A). This revealed a main CONDITION effect (p ≤ 0.001), where post-hoc analysis showed significant differences for CONDITION 2mA vs. sham and 1mA vs. sham, and no significant differences for 2mA vs. 1mA. We also found a significant CONDITION*TIME interaction (p ≤ 0.01). After multiple comparisons correction, 2mA-tDCS mean MEP amplitudes from all post-time points showed significant differences compared to the corresponding pre-tDCS amplitude, while 1mA-tDCS only induced statistical significance between Post30 and pre-values. We found no significance between pre-and post-stimulation for sham tDCS (Table 1).
Table 1.
Summary of ANOVARM results. Since 3-way ANOVARM revealed no significant effects related to SESSION, we used a 2-way ANOVARM. Post hoc analysis performed using Bonferroni correction. Statistically significant cases are shown in bold
Figure 2.
Overall atDCS effect and reliability across consecutive sessions. A) Combined session average of MEP amplitude (mV) for each time point (Pre, Post0, Post15, Post30) and condition (2mA, 1mA, sham). Two-way ANOVARM was performed with CONDTION and TIME, comparing pre with post-values. Post-hoc pairwise comparisons were performed using Bonferroni correction (*, p≤0.05; **, p≤0.01; ***, p≤0.001). B) Mean MEP amplitudes for each session and condition across time. ICC, calculated with all post-time points from the 3 sessions for each condition (n = 9/condition), showed fair to high reliability. Errors bars represent SEM.
Analysis based on Grand Mean show low reliability
To determine intra-individual variability of tDCS-induced effects for multiple sessions we calculated the ICC including all nine post-tDCS time points from the three sessions for each condition. This analysis revealed fair to high ICC reliability (ICC range = 0.535 – 0.789, Fig. 2B).
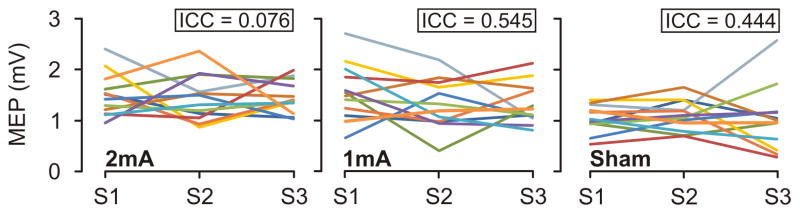
To better compare our findings with previous studies, we also calculated the ICCs for Grand Mean post-tDCS amplitudes (the mean of all post-tDCS measures, as done in [27, 29, 33, 34]. This analysis showed poor to fair reliability of tDCS post-effects (ICC range = 0.076 – 0.545; Fig. 3).
Figure 3.
Grand Mean values of MEP amplitude for each individual across sessions and conditions (2mA, 1mA, sham). Each colored line represents a single subject across all nine sessions. ICC calculated for each condition reveals poor to fair reliability of tDCS post-effects.
Sham standard deviation as a threshold helps classify the type of tDCS response
To understand whether atDCS results in the expected increase of excitability or not we developed a threshold based on the SD of the sham condition. First we calculated the mean MEP ratios (post/pre) for each session of each condition to analyze their frequency distribution. As expected, the normal distributions corresponding to the conditions 2mA and 1mA showed a higher mean compared to sham (Fig. 4). Interestingly, we found a considerable overlap of sham values with the active atDCS conditions (Fig. 4, grey area). In order to classify sessions by the actual tDCS-induced effect in three different categories (‘increase’, ‘no change’, ‘decrease’), we defined the SD of the sham ratios (1σ = 0.33) as the Sham Variability-Based Threshold (VBTsham). Sessions with a mean MEP ratio > 1.3 were classified as ‘increase’, while sessions with values between 0.7 < ratio < 1.3 were considered as the ‘no change’ group, and sessions with < 0.7 were classified as ‘decrease’.
Figure 4.
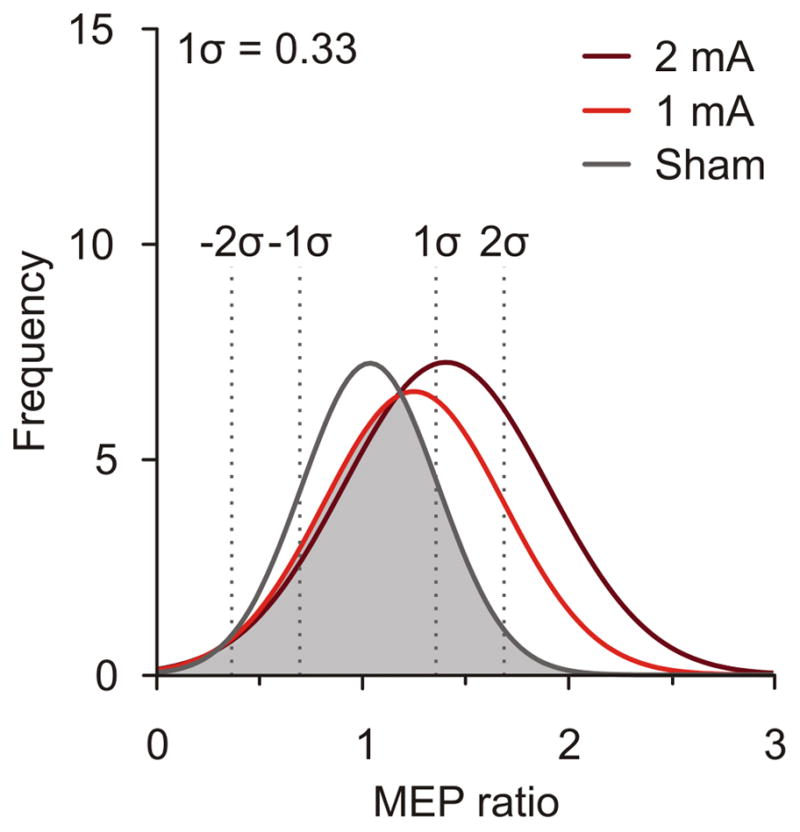
Normal frequency distribution plots of mean MEP ratios (post/pre) for all sessions within each condition (n= 36/condition). The grey area represents overlapping ratios found in the sham, 2mA and 1mA conditions. Dotted lines indicate SDs from mean sham values.
2mA elicits a more consistent expected increase in MEP amplitude than 1mA
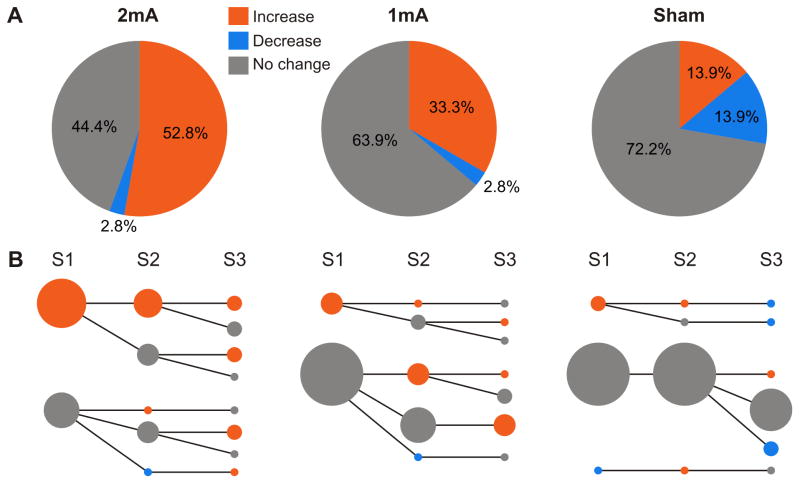
After we classified tDCS sessions by using the VBTsham, we found that 52.8% of 2mA-sessions showed an increase of MEP amplitude induced by tDCS, whereas only 33.3% of sessions have an increased response in the 1mA-condition (Fig. 5A). To compare our VBTsham technique with conventional methods, we additionally represented our data using a two-step cluster analysis and choosing an arbitrary threshold (‘increase’ > 1mV, Suppl. Material 1).
Figure 5.
Pie charts of post-tDCS responses across sessions classified with the Sham Variability-Based Threshold. A) Responses were classified as ‘increase’ (> 1.3), ‘no change’ (0.7 < ratio < 1.3) and ‘decrease’ (< 0.7). B) Individual response tracking across sessions for each condition. Same classification scheme as in A but separated by session. Dot sizes are linearly proportional to the number of subjects (n = 12). The biggest dot size represents 9 subjects and the smallest dot size represents 1 subject.
To track individual responses based on VBTsham across multiple sessions, we performed a session specific analysis (Fig. 5B). Here, bigger dots depict greater number of subjects. For the 2mA-condition a clear response pattern could be observed, where most subjects with an ‘increase’ response in the first session showed a similar effect in at least one more session (Fig. 5B, 2mA). This response pattern was not observed in the 1mA-condition (Fig. 5B, 1mA).
Discussion
Previous studies assessing consistency of atDCS effects on corticomotor excitability present their results based on mean group responses from a single tDCS session [2, 5, 7, 42, 43]. Surprisingly, most of these studies have been performed without an appropriate control condition (see for review [23]). Here we examined the variability of atDCS-induced effects on cortical excitability at the inter- and intra-individual level across multiple sessions applying different tDCS intensities and controlling for the variability introduced by the measuring tool (i.e., TMS) by establishing a sham condition.
Consistency of tDCS effects at group level
Supporting previous findings [2–4, 30, 44], we reproduced at the group level the classical atDCS effect represented by an increase of MEP amplitude after applying the stimulation. First, we found no significant difference in tDCS effects across sessions within each condition. Therefore, we present our results as the mean of responses combining all three sessions for each condition. A current intensity of 2mA induced a significant increase in MEP amplitude for the entire duration (30 min) of post-tDCS measurements, whereas 1mA-tDCS revealed a trend towards increased MEP in all post-tDCS measures, but only significant at Post30 compared to pre-values.
In agreement with our results, previous findings showed consistent group effects for a repeated tDCS session (i.e., no session effect) with a significant mean time effect up to minute 25 post-stimulation using 1mA intensity (electrode size: 35cm2; current density: 0.029 mA/cm2; tDCS duration: 13min) [29]. Accordingly, Dyke and colleagues (2016) showed that 2mA-atDCS increased M1 excitability consistently across four sessions (electrode size: 35cm2; current density: 0.058 mA/cm2; tDCS duration: 20min), whereas no change was induced by cathodal or sham stimulation [34]. Another recent study also performed three different tDCS conditions (anodal, cathodal, sham) repeating each condition three times (electrode size: 35cm2; current density: 0.029 mA/cm2; tDCS duration: 10min). In accord with our findings, the results of this study show no differences between sessions. However, in their study not only the anodal condition, but also the cathodal and sham condition induced a significant overall increase in MEP amplitude compared to baseline [33]. The authors interpreted the variability of the MEPs as a potential cause for their findings. In contrast to these studies and ours, Chew and colleagues found inconsistent effects of atDCS (electrode size: 16cm2; current density: 0.031 mA/cm2; tDCS duration: 10min) tested across two repeated sessions. Specifically, the first session revealed a main increase of MEP amplitude compared to baseline, whereas the second session did not show significant changes in amplitude [27].
Several methodological differences are present between the stated studies, which may promote different findings. For instance, it is known that MEP amplitude changes induced by tDCS is affected by the size of electrodes [43–45] and by the duration of stimulation [2, 5, 31]. On the other side the different time periods between sessions (6 to 12 months [29]; 1 to 7 weeks [27]; 2 to 9 days [33]; 3 to 4 days [34]; 2 to 31 days - present study) may also have an impact on ultimate results. Current density is another critical parameter directly related to the applied electric field strength contributing to the effects of tDCS on brain excitability [46]. We applied current densities of 0.04mA/cm2 and 0.08mA/cm2 corresponding to 1mA and 2mA, respectively. No significant differences were found when we compared the after-effects of condition 2mA vs. 1mA. However, in accordance with previous investigations [43, 47] we observed higher MEP amplitudes after 2mA for all three sessions. Consequently, we concluded that 2mA-atDCS induces a more consistent group excitatory response than 1mA-atDCS across repeated sessions when applied with at least 48 hours apart. In contrast to previous studies [2, 7, 48], we induced significant long-lasting after-effects with 7 min atDCS, confirming results by Cantarero and colleagues [39]. This discrepancy between studies might be due to different electrode size (35cm2 vs. 25cm2), and therefore different applied current densities.
Individual response variability to tDCS
The mean group results do not offer much information about the within-subject response variability across sessions. For this reason we calculated the ICC as a measure of reliability. Considering every post-tDCS assessment separately we found that ICCs revealed a fair to high degree of reliability.
If one were to perform the ICC analysis using a mean value of all the post-tDCS measures per session, i.e., Grand Mean (as done in [27, 29, 33]), our calculated ICCs would show only poor to fair individual reliability across sessions. This difference is due to the ICC being largely influenced by sample heterogeneity [49]. Specifically, the Grand Mean values are more narrowly distributed than the values of the three single time points. The ICC method has more difficulty to discriminate between subjects resulting in a lower between-subject variance and hence a lower ICC value. Thus, the ICC is not powerful enough to check for intra-individual variability when Grand Mean values are used. We think this is the reason why four recent studies found different results when they assessed tDCS test-retest reliability using Grand Mean values. Particularly, two studies tested the intra-individual consistency of M1-atDCS for repeated sessions showing a fair to good degree of reliability (ICC = 0.565 [29]; ICC = 0.738 [35]) of tDCS effects; in other words individual effects were consistent on different days. However, fairly worse results of intra-individual reliability have been reported by other investigations indicating a lack of reproducibility (ICC = −0.500 [27]; ICC = 0.062 [33]) for repeated tDCS sessions. In addition to the different approaches to the ICC analysis, it is important to note that some of these investigations also lack a control condition [27, 29] and have differences in the tDCS parameters (e.g.; duration and current density of tDCS).
Defining a threshold to classify tDCS responses
Similar to previous publications, we classified tDCS effects as ‘increase’, ‘no change’ or ‘decrease’. Prior research established classification thresholds based on cluster analysis [26, 28, 29], or choosing an arbitrary value [26, 27, 33]. While a recent study based the classification of post-tDCS responses on the SEM of the baseline MEP amplitude for each subject [31], we defined the sham SD (1σ) as a threshold (VBTsham) for our group classification. The purpose was to exclude as much as possible changes in MEP amplitudes that are not directly caused by atDCS to establish a more representative demonstration of the actual response to tDCS. Supporting the efficiency of this novel method, we also calculated the response in our data using the mentioned traditional techniques. We found that, albeit the cluster analysis showed comparable results, it does not provide an ideal classification if the resulting number of clusters is inconsistent. Furthermore, it becomes clear that choosing an arbitrary threshold could exhibit clearly fictitious tDCS effects, especially when investigations lack control groups (Suppl. Material 1).
The noticeable overlap of sham ‘increase’ responses with active-tDCS responses points to the fact that other sources of variability have been present during the execution of our experiments that need to be considered. Surprisingly, only few studies exploring tDCS effects based on MEP measures have employed a control condition (see for review [23]), and those who performed this control generally do not present individual data [5, 35, 50–53]. To date, only two studies that reported reliability of tDCS effects across multiple sessions included a sham condition and presented as well individual responses [33, 34]. In agreement with our results Horvath and colleagues (2016) showed variable MEP responses for the sham condition at the individual level. However, the results of that study present noticeably higher variability compared to our results. Specifically, if we use our VBTsham value (‘increase’ > 1.3) on their individual data, ~ 38% of the sham sessions present an MEP amplitude increase compared to baseline values, and two of their participants actually showed an excitatory response to all three sessions [33]. In contrast, we found that only ~ 14 % of all sham sessions exhibit an ‘increase’ effect. Since it is well known that the MEP measurement presents a possible source of variability [54–56], we assume that nearly all the sham-variability we report has been introduced by the TMS measures. The same reason could explain that Dyke and colleagues (2016) observed in some of their individual’s TMS recruitment curves slope values different from baseline after applying sham stimulation [34].
Once we established our VBTsham numerous sessions became excluded from an excitatory or inhibitory atDCS effect. Consequently, only the 2mA-condition showed a clear and consistent response pattern when performing individual tracking of tDCS responses across sessions (Fig 5). This analysis revealed that most individuals who present an ‘increase’ in the first session also experience a similar response in at least one more tDCS session. López-Alonso and colleagues (2015) presented a comparable pattern with 1mA-tDCS applied on repeated sessions. Most of their ‘responders’ from the 1st session also acted as a ‘responder’ in the 2nd session. Nonetheless, this study set a considerable lower threshold (‘responder’ if ratio >1) and did not include a sham condition [29].
How can we improve reliability?
Our results confirm that besides the known sources of variability related to tDCS, other sources (e.g., the TMS measures) can cause inconsistency at the intra- and inter-individual level (see for review [57]). As previously mentioned, it is known that MEP measurement is a possible source of variability [54–56]. The use of minimum 10 pulses as outcome measures of TMS [38] and the use of frameless neuronavigation systems [58, 59] can help decrease MEP variability. While we applied these methods in the present study, a recent report indicated that 18 single TMS pulses delivered better reliability when measuring MEP amplitudes [60]. Despite this potential limitation of our study, we found significant effects when comparing anodal vs. sham tDCS. Nonetheless, future studies should consider increasing the number of TMS trials in order to improve reliability. Another approach to reduce TMS variability reported by Meincke and colleagues (2016) is using a fully automated hotspot-search procedure based on an algorithm that takes into account the RMT instead of MEP amplitudes [61].
Finally, the use of a single-blinded study design may have biased our results, though experimental conditions were maintained equal for all sessions. While a fixed SI1mV to assess corticospinal excitability might represent another potential limitation of the present study, since this method eliminates between-session baseline variability, it allowed us to focus on the question of whether tDCS results in consistent effects. Moreover, some subjects might display highly variable MEPs responses due to a steep slope in their recruitment curve. Therefore, another consideration to reduce intra-individual variability in the future would be to define the TMS intensity for each subject based on the individual’s TMS recruitment curve.
Conclusions
We found that atDCS at 2mA intensity delivered for 7 minutes over M1 results in consistent increase of MEP amplitudes at the group level across multiple sessions, as well as a fair to high reliability at the individual level when the ICC is calculated based on all post-tDCS time-points (instead of Grand Mean values). The use of the VBTsham represents an appropriate method to classify tDCS effects. Using this approach 2mA current intensity seems a better choice than 1mA to induce predictable responses across multiple sessions.
Supplementary Material
Suppl. Material 1. Pie charts of post-tDCS responses across sessions for each condition classified with A) two-step cluster analysis using MEP ratios from Post0 to Post30 (3 levels) as cluster predictors, and B) choosing an arbitrary threshold where overall MEP ratios >1mV were classified as ‘increase. Note that cluster analysis resulted in an inconsistent number of clusters across conditions and >1mV analysis is inefficient in representing the real tDCS-induced effect on excitability.
Highlights.
2mA tDCS applied for 7min induced increased cortical excitability in all sessions.
A fair to high degree of reliability of individual responses was observed.
A new method to classify tDCS responses controlling for TMS variability is proposed.
2mA tDCS, but not 1mA, resulted as a better choice to induce predictable responses.
Acknowledgments
Funding: This work was supported by the National Institutes of Health (R01HD073147).
The authors thank Mr. Shintaro Uehara for his help with the data analysis.
Footnotes
Additional Information: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Priori A, Berardelli A, Rona S, Accornero N, Manfredi M. Polarization of the human motor cortex through the scalp. NeuroReport. 1998;9:2257–60. doi: 10.1097/00001756-199807130-00020. [DOI] [PubMed] [Google Scholar]
- 2.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(3):633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 2002;125:2238–47. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- 4.Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, et al. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003;553:293–301. doi: 10.1113/jphysiol.2003.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batsikadze G, Moliadze V, Paulus W, Kuo MF, Nitsche MA. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J Physiol. 2013;591:1987–2000. doi: 10.1113/jphysiol.2012.249730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuo HI, Bikson M, Datta A, Minhas P, Paulus W, Kuo MF, et al. Comparing cortical plasticity induced by conventional and high-definition 4 × 1 ring tDCS: a neurophysiological study. Brain Stimul. 2013;6:644–8. doi: 10.1016/j.brs.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- 8.Boggio PS, Castro LO, Savagim EA, Braite R, Cruz VC, Rocha RR, et al. Enhancement of non-dominant hand motor function by anodal transcranial direct current stimulation. Neurosci Lett. 2006;404:232–6. doi: 10.1016/j.neulet.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 9.Galea JM, Celnik P. Brain polarization enhances the formation and retention of motor memories. J Neurophysiol. 2009;102:294–301. doi: 10.1152/jn.00184.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galea JM, Vazquez A, Pasricha N, de Xivry JJ, Celnik P. Dissociating the roles of the cerebellum and motor cortex during adaptive learning: the motor cortex retains what the cerebellum learns. Cereb Cortex. 2011;21:1761–70. doi: 10.1093/cercor/bhq246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A. 2009;106:1590–5. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hummel FC, Heise K, Celnik P, Flöel A, Gerloff C, Cohen LG. Facilitating skilled right hand motor function in older subjects by anodal polarization over the left primary motor cortex. Neurobiol Aging. 2010;31:2160–8. doi: 10.1016/j.neurobiolaging.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jayaram G, Tang B, Pallegadda R, Vasudevan EV, Celnik P, Bastian A. Modulating locomotor adaptation with cerebellar stimulation. J Neurophysiol. 2012;107:2950–7. doi: 10.1152/jn.00645.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardwick RM, Celnik PA. Cerebellar direct current stimulation enhances motor learning in older adults. Neurobiol Aging. 2014;35:2217–21. doi: 10.1016/j.neurobiolaging.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cantarero G, Spampinato D, Reis J, Ajagbe L, Thompson T, Kulkarni K, et al. Cerebellar direct current stimulation enhances on-line motor skill acquisition through an effect on accuracy. J Neurosci. 2015;35:3285–90. doi: 10.1523/JNEUROSCI.2885-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fregni F, Boggio PS, Nitsche MA, Bermpohl F, Antal A, Feredoes E, et al. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp Brain Res. 2005;166:23–30. doi: 10.1007/s00221-005-2334-6. [DOI] [PubMed] [Google Scholar]
- 17.Marshall L, Molle M, Siebner HR, Born J. Bifrontal transcranial direct current stimulation slows reaction time in a working memory task. BMC Neurosci. 2005;6:23. doi: 10.1186/1471-2202-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrews SC, Hoy KE, Enticott PG, Daskalakis ZJ, Fitzgerald PB. Improving working memory: the effect of combining cognitive activity and anodal transcranial direct current stimulation to the left dorsolateral prefrontal cortex. Brain Stimul. 2011;4:84–9. doi: 10.1016/j.brs.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Antal A, Nitsche MA, Kruse W, Kincses TZ, Hoffmann KP, Paulus W. Direct current stimulation over V5 enhances visuomotor coordination by improving motion perception in humans. J Cogn Neurosci. 2004;16:521–7. doi: 10.1162/089892904323057263. [DOI] [PubMed] [Google Scholar]
- 20.Ragert P, Vandermeeren Y, Camus M, Cohen LG. Improvement of spatial tactile acuity by transcranial direct current stimulation. Clin Neurophysiol. 2008;119:805–11. doi: 10.1016/j.clinph.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barbieri M, Negrini M, Nitsche MA, Rivolta D. Anodal-tDCS over the human right occipital cortex enhances the perception and memory of both faces and objects. Neuropsychologia. 2016;81:238–44. doi: 10.1016/j.neuropsychologia.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 22.Jacobson L, Koslowsky M, Lavidor M. tDCS polarity effects in motor and cognitive domains: a meta-analytical review. Exp Brain Res. 2012;216:1–10. doi: 10.1007/s00221-011-2891-9. [DOI] [PubMed] [Google Scholar]
- 23.Horvath JC, Carter O, Forte JD. Transcranial direct current stimulation: five important issues we aren’t discussing (but probably should be) Front Syst Neurosci. 2014;8:2. doi: 10.3389/fnsys.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fricke K, Seeber AA, Thirugnanasambandam N, Paulus W, Nitsche MA, Rothwell JC. Time course of the induction of homeostatic plasticity generated by repeated transcranial direct current stimulation of the human motor cortex. J Neurophysiol. 2011;105:1141–9. doi: 10.1152/jn.00608.2009. [DOI] [PubMed] [Google Scholar]
- 25.Tremblay S, Beaule V, Lepage JF, Theoret H. Anodal transcranial direct current stimulation modulates GABAB-related intracortical inhibition in the M1 of healthy individuals. Neuroreport. 2013;24:46–50. doi: 10.1097/WNR.0b013e32835c36b8. [DOI] [PubMed] [Google Scholar]
- 26.Wiethoff S, Hamada M, Rothwell JC. Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimul. 2014;7:468–75. doi: 10.1016/j.brs.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Chew T, Ho KA, Loo CK. Inter- and Intra-individual Variability in Response to Transcranial Direct Current Stimulation (tDCS) at Varying Current Intensities. Brain Stimul. 2015;8:1130–7. doi: 10.1016/j.brs.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 28.López-Alonso V, Cheeran B, Rio-Rodriguez D, Fernandez-Del-Olmo M. Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimul. 2014;7:372–80. doi: 10.1016/j.brs.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 29.López-Alonso V, Fernandez-Del-Olmo M, Costantini A, Gonzalez-Henriquez JJ, Cheeran B. Intra-individual variability in the response to anodal transcranial direct current stimulation. Clin Neurophysiol. 2015;126:2342–7. doi: 10.1016/j.clinph.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 30.Strube W, Bunse T, Malchow B, Hasan A. Efficacy and interindividual variability in motor-cortex plasticity following anodal tDCS and paired-associative stimulation. Neural Plast. 2015;2015:530423. doi: 10.1155/2015/530423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tremblay S, Larochelle-Brunet F, Lafleur LP, El Mouderrib S, Lepage JF, Theoret H. Systematic assessment of duration and intensity of anodal tDCS on primary motor cortex excitability. Eur J Neurosci. 2016;44(5):2184–90. doi: 10.1111/ejn.13321. [DOI] [PubMed] [Google Scholar]
- 32.Nuzum ND, Hendy AM, Russell AP, Teo WP. Measures to Predict The Individual Variability of Corticospinal Responses Following Transcranial Direct Current Stimulation. Front Hum Neurosci. 2016;10:487. doi: 10.3389/fnhum.2016.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horvath JC, Vogrin SJ, Carter O, Cook MJ, Forte JD. Effects of a common transcranial direct current stimulation (tDCS) protocol on motor evoked potentials found to be highly variable within individuals over 9 testing sessions. Exp Brain Res. 2016;234(9):2629–42. doi: 10.1007/s00221-016-4667-8. [DOI] [PubMed] [Google Scholar]
- 34.Dyke K, Kim S, Jackson GM, Jackson SR. Intra-Subject Consistency and Reliability of Response Following 2 mA Transcranial Direct Current Stimulation. Brain Stimul. 2016;9:819–25. doi: 10.1016/j.brs.2016.06.052. [DOI] [PubMed] [Google Scholar]
- 35.Jamil A, Batsikadze G, Kuo HI, Labruna L, Hasan A, Paulus W, et al. Systematic evaluation of the impact of stimulation intensity on neuroplastic after-effects induced by transcranial direct current stimulation. J Physiol. 2016;10:1–16. doi: 10.1113/JP272738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alonzo A, Brassil J, Taylor JL, Martin D, Loo CK. Daily transcranial direct current stimulation (tDCS) leads to greater increases in cortical excitability than second daily transcranial direct current stimulation. Brain Stimul. 2012;5:208–13. doi: 10.1016/j.brs.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Gálvez V, Alonzo A, Martin D, Loo CK. Transcranial direct current stimulation treatment protocols: should stimulus intensity be constant or incremental over multiple sessions? Int J Neuropsychopharmacol. 2013;16:13–21. doi: 10.1017/S1461145712000041. [DOI] [PubMed] [Google Scholar]
- 38.Bastani A, Jaberzadeh S. A higher number of TMS-elicited MEP from a combined hotspot improves intra- and inter-session reliability of the upper limb muscles in healthy individuals. PLoS One. 2012;7:e47582. doi: 10.1371/journal.pone.0047582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cantarero G, Tang B, O’Malley R, Salas R, Celnik P. Motor learning interference is proportional to occlusion of LTP-like plasticity. J Neurosci. 2013;33:4634–41. doi: 10.1523/JNEUROSCI.4706-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychological bulletin. 1979;86:420–8. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 41.Poreisz C, Boros K, Antal A, Paulus W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull. 2007;72:208–14. doi: 10.1016/j.brainresbull.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Lang N, Nitsche MA, Paulus W, Rothwell JC, Lemon RN. Effects of transcranial direct current stimulation over the human motor cortex on corticospinal and transcallosal excitability. Exp Brain Res. 2004;156:439–43. doi: 10.1007/s00221-003-1800-2. [DOI] [PubMed] [Google Scholar]
- 43.Bastani A, Jaberzadeh S. Differential modulation of corticospinal excitability by different current densities of anodal transcranial direct current stimulation. PLoS One. 2013;8:e72254. doi: 10.1371/journal.pone.0072254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ho KA, Taylor JL, Chew T, Gálvez V, Alonzo A, Bai S, et al. The Effect of Transcranial Direct Current Stimulation (tDCS) Electrode Size and Current Intensity on Motor Cortical Excitability: Evidence From Single and Repeated Sessions. Brain Stimul. 2016;9:1–7. doi: 10.1016/j.brs.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Nitsche MA, Doemkes S, Karakose T, Antal A, Liebetanz D, Lang N, et al. Shaping the effects of transcranial direct current stimulation of the human motor cortex. J Neurophysiol. 2007;97:3109–17. doi: 10.1152/jn.01312.2006. [DOI] [PubMed] [Google Scholar]
- 46.Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008;1:206–23. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Murray LM, Edwards DJ, Ruffini G, Labar D, Stampas A, Pascual-Leone A, et al. Intensity dependent effects of transcranial direct current stimulation on corticospinal excitability in chronic spinal cord injury. Archives of physical medicine and rehabilitation. 2015;96:S114–21. doi: 10.1016/j.apmr.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nitsche MA, Roth A, Kuo MF, Fischer AK, Liebetanz D, Lang N, et al. Timing-dependent modulation of associative plasticity by general network excitability in the human motor cortex. J Neurosci. 2007;27:3807–12. doi: 10.1523/JNEUROSCI.5348-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Vet HCW, Terwee CB, Knol DL, Bouter LM. When to use agreement versus reliability measures. J Clin Epidemiol. 2006;59:1033–9. doi: 10.1016/j.jclinepi.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 50.Siebner HR, Lang N, Rizzo V, Nitsche MA, Paulus W, Lemon RN, et al. Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: evidence for homeostatic plasticity in the human motor cortex. J Neurosci. 2004;24:3379–85. doi: 10.1523/JNEUROSCI.5316-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lang N, Siebner HR, Ernst D, Nitsche MA, Paulus W, Lemon RN, et al. Preconditioning with transcranial direct current stimulation sensitizes the motor cortex to rapid-rate transcranial magnetic stimulation and controls the direction of after-effects. Biol Psychiatry. 2004;56:634–9. doi: 10.1016/j.biopsych.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 52.Power HA, Norton JA, Porter CL, Doyle Z, Hui I, Chan KM. Transcranial direct current stimulation of the primary motor cortex affects cortical drive to human musculature as assessed by intermuscular coherence. J Physiol. 2006;577:795–803. doi: 10.1113/jphysiol.2006.116939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schabrun SM, Chipchase LS, Zipf N, Thickbroom GW, Hodges PW. Interaction between simultaneously applied neuromodulatory interventions in humans. Brain Stimul. 2013;6:624–30. doi: 10.1016/j.brs.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 54.Kiers LCD, Chiappa KH, Fang J. Variability of motor potentials evoked by transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1993;89:415–23. doi: 10.1016/0168-5597(93)90115-6. [DOI] [PubMed] [Google Scholar]
- 55.Kamen G. Reliability of Motor-Evoked Potentials during Resting and Active Contraction Conditions. Med Sci Sports Exerc. 2004;36:1574–9. doi: 10.1249/01.mss.0000139804.02576.6a. [DOI] [PubMed] [Google Scholar]
- 56.Malcolm MP, Triggs WJ, Light KE, Shechtman O, Khandekar G, Gonzalez Rothi LJ. Reliability of motor cortex transcranial magnetic stimulation in four muscle representations. Clin Neurophysiol. 2006;117:1037–46. doi: 10.1016/j.clinph.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 57.Li LM, Uehara K, Hanakawa T. The contribution of interindividual factors to variability of response in transcranial direct current stimulation studies. Front Cell Neurosci. 2015;9:181. doi: 10.3389/fncel.2015.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ahdab R, Ayache SS, Brugieres P, Goujon C, Lefaucheur JP. Comparison of “standard” and “navigated” procedures of TMS coil positioning over motor, premotor and prefrontal targets in patients with chronic pain and depression. Clin Neurophysiol. 2010;40:27–36. doi: 10.1016/j.neucli.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 59.Sparing R, Buelte D, Meister IG, Paus T, Fink GR. Transcranial magnetic stimulation and the challenge of coil placement: a comparison of conventional and stereotaxic neuronavigational strategies. Hum Brain Mapp. 2008;29:82–96. doi: 10.1002/hbm.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang WH, Fried PJ, Saxena S, Jannati A, Gomes-Osman J, Kim YH, et al. Optimal number of pulses as outcome measures of neuronavigated transcranial magnetic stimulation. Clin Neurophysiol. 2016;127:2892–7. doi: 10.1016/j.clinph.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meincke J, Hewitt M, Batsikadze G, Liebetanz D. Automated TMS hotspot-hunting using a closed loop threshold-based algorithm. Neuroimage. 2016;124:509–17. doi: 10.1016/j.neuroimage.2015.09.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Material 1. Pie charts of post-tDCS responses across sessions for each condition classified with A) two-step cluster analysis using MEP ratios from Post0 to Post30 (3 levels) as cluster predictors, and B) choosing an arbitrary threshold where overall MEP ratios >1mV were classified as ‘increase. Note that cluster analysis resulted in an inconsistent number of clusters across conditions and >1mV analysis is inefficient in representing the real tDCS-induced effect on excitability.