Abstract
Chronic kidney disease (CKD) represents a leading cause of death in the United States. There is no cure for the disease, with current treatment strategies relying on blood pressure control through blockade of the renin angiotensin system and glycemic control. Such approaches only delay end stage kidney disease development, and can be associated with significant side effects. Recent identification of several novel mechanisms contributing to CKD development - including vascular changes, loss of podocytes and renal epithelial cells, matrix deposition, inflammation and metabolic dysregulation – has revealed new potential therapeutic approaches for CKD. This review will assess emerging strategies and agents for CKD treatment, highlighting associated challenges in their clinical development.
Introduction
Approximately 20 million people in the United States are currently affected by CKD, with half a million of these presenting with the most severe form; end stage renal disease (ESRD). The only treatment for ESRD is dialysis or transplantation. However, the mortality of patients on dialysis can be as high as 20% per year1,2 and transplantation is limited by organ shortage.
The most common cause of chronic and ESRD in the United States is diabetes, which accounts for roughly 50% of all cases, followed by hypertension (25%), with other causes including glomerulonephritides and polycystic kidney disease1,3. Cardiovascular disease (CVD) remains the leading cause of mortality in patients with CKD4. Despite tremendous progress in reducing CVD death in the general population, this has not translated into CKD patients. The reduction in CVD in the general population correlates strongly with serum cholesterol, smoking status and blood pressure, but the mortality of CKD of subjects is much higher than non-CKD subjects when compared by traditional CVD risk calculations5,6. So called “non-traditional risk factors” may result from the build-up of different toxins and metabolites which likely contributes to this increased mortality of patients with CKD.
The most commonly used definition for CKD is purely based on estimation of the glomerular filtration rate (eGFR)7, with a 40% decline to a GFR of less than 60ml/min/1.73m2 for more than 3 months being used to diagnose CKD. Different stages of CKD have been proposed, based on GFR criteria. These include stage G1 when GFR>90 cc/min, stage G2 when GFR is 90-60cc/min, stage G3 when GFR is 60-30 cc/min, stage G4 when GFR is 30–15 cc/min, while stage G5 is when GFR is below 15 cc/min. The stages were developed mostly for research purposes and at present we are not aware of any clear distinction between these stages, rather GFR decline represents a continuously increased risk for death. A broader definition of CKD is also in use, which takes structural, functional, pathological, laboratory or imaging abnormalities into consideration. Leakage of albumin or protein in the urine is one such functional abnormality that has gained more prominence in the new classification guidelines8,9. Most clinical studies use albuminuria and proteinuria interchangeably as albumin and its degradation products represent the overwhelming majority of urinary proteins. The presence of albuminuria correlates strongly with the development of ESRD, increased CVD and mortality, while reduction of albuminuria is usually associated with protection from functional decline10.
Sclerosis of the glomerulus and interstitial fibrosis are the common histopathological and structural features of CKD11. Glomerular changes are usually specific for disease etiology and as indicated above, are therefore used for diagnosis and disease classification12. Tubulointerstitial fibrosis strongly correlates with kidney function and represents a common complex architectural change in the kidney11,13, which includes matrix and collagen production by epithelial cells and activated myofibroblasts11. Mouse genetic studies have highlighted the key role of epithelial cells in the development of fibrosis14,15,16. Indeed, genetic overexpression of Notch, Wnt, KIM and HIF in tubule epithelial cells was sufficient to induce epithelial damage, dedifferentiation and the full spectrum of fibrosis14,15,16, while genetic deletion of these pathways protected from fibrosis development. Fibrosis is a reactive process that develops mostly in response to epithelial injury and is almost always accompanied by inflammation, manifested by increased cytokine expression and accumulation of macrophages and inflammatory cells17. Vascular injury and loss of capillaries exacerbates epithelial injury at later stages by limiting the nutrient availability to epithelial cells. Our understanding of fibrosis has significantly improved over the past several years, revealing new potential therapeutic targets.
At present there is no cure for most forms of chronic kidney disease. The list of conditions associated with reversible renal failure is short, comprising of decreased renal perfusion, nephrotoxic drugs and urinary obstruction. Although steroids and other immunosuppressive measurements can halt or reverse some diseases of the kidney, such as IgA neuropathy18, lupus nephritis, membranous nephropathy, focal segmental glomerulosclerosis, vasculitis and MCD, they have not shown benefit in kidney disease in patients with diabetes and hypertension19.
Hemodynamic changes play a critical role in CKD development and strategies to control high blood pressure have had a significant beneficial impact on disease20. An increase in systemic blood pressure can increase glomerular filtration, leading to hyperfiltration, an increase in glomerular size and glomerular cells must endure the increased stretch21. In addition, as the vessels that supply the kidney with nutrients originate from the efferent portion of the glomerulus, these changes reduce the post-glomerular blood flow leading to ischemic changes in the kidney22, 23. Inhibiting the renin angiotensin system, which is involved in the regulation of blood pressure and fluid balance19, using either angiotensin convertase enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARB), reduces glomerular hyperfiltration and albuminuria, and slows the decline in kidney function19. These agents have therefore become the mainstay of CKD treatment. However, this approach only slows the decline in kidney function and does not cure CKD. In addition these agents can be associated with significant side effects, such as an increase in serum potassium levels, which can limit their therapeutic use24.
There are several additional drugs in development that target hemodynamic changes in CKD. Smoking cessation also slows the rate of progression25. Many practitioners recommend protein restriction, statin therapy and the correction of metabolic acidosis, but at present the benefit of these measures have not been unequivocally demonstrated in large randomized trials26. As our understanding of CKD pathogenic mechanisms has improved, new therapeutic approaches, including targeting inflammation, fibrosis or podocytes, are emerging. In this Review, we will assess current and emerging strategies for the treatment of the major types of CKD - diabetic and hypertensive CKD -and address key challenges and considerations in the development of novel therapies and future clinical trial design.
Current treatments for CKD
Angiotensin converting enzyme inhibition (ACEi) and angiotensin receptor blockade (ARB) have been the mainstay of therapy for chronic kidney disease for over twenty years (Figure 1). The initial rationale for testing ACEi therapy was based on preclinical studies showing a reduction in intraglomerular hemodynamic pressure accompanied by proteinuria reduction in hyper-filtering rat kidney disease models, including diabetic nephropathy and partial renal ablation23,27. Clinical testing of the ACEi, captopril, in patients with type I diabetes and nephropathy revealed that captopril improved renal outcomes beyond that achieved with alternative methods of blood pressure reduction28, establishing captopril and ACEi as the standard of care for patients with diabetic nephropathy (DN). Subsequent studies extended the observed benefit to patients with other causes of kidney disease including those with late stage CKD29,30.
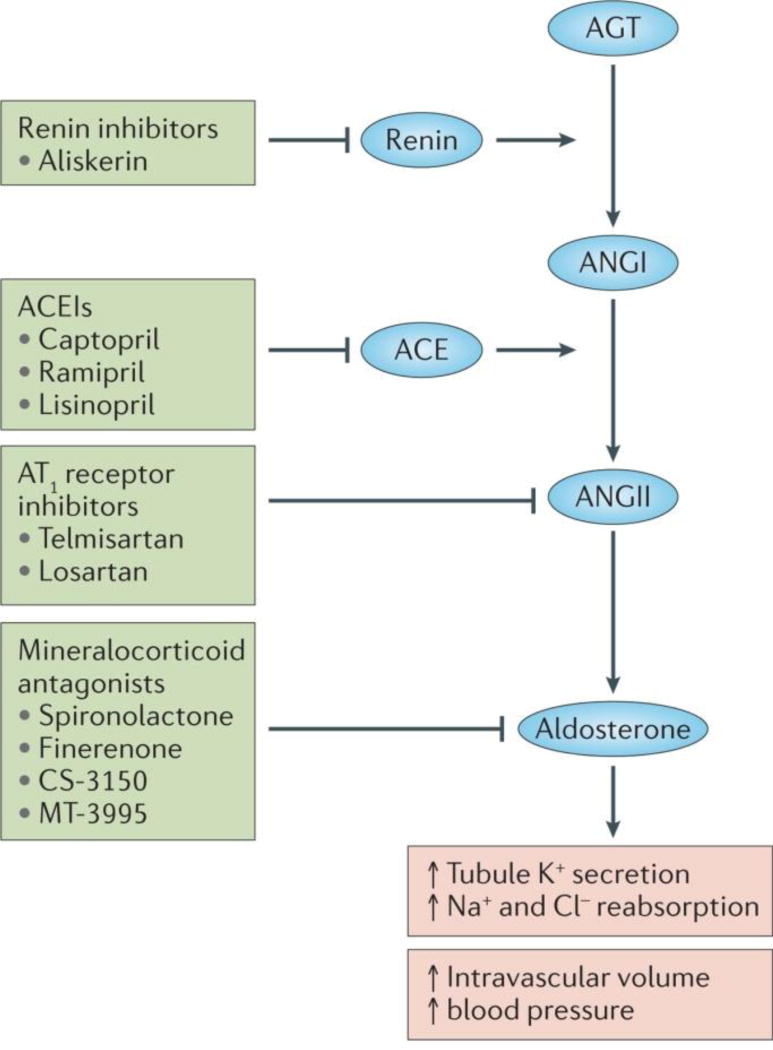
Figure 1. Targeting the renin angiotensin aldosterone system.
Schematic representation of the renin angiotensin aldosterone system. Renin converts angiotensinogen to Angiotensin I and the angiotensin converting enzyme converts angiotensin I to angiotensin II. Renin inhibitors block renin, ACEI block ACE, AT1R block angiotensin action on the receptor while mineralocorticoid antagonists inhibit aldosterone.
Similar beneficial effects of Angiotensin type 1 receptor blockade (ARB) were subsequently observed in patients with type 2 diabetes and nephropathy31–33, establishing the renin angiotensin pathway as a key target for the treatment of CKD. Notably, the therapeutic benefit of ACE/ARB therapy is closely associated with an early reduction in estimated GFR34, coupled with reduction in proteinuria (or albuminuria) (Figure 1)35,36, consistent with the capacity of these agents to reduce intra-glomerular filtration pressure as noted above27.
However, although ACEi/ARB therapy slows the progression of renal disease, it does not halt disease progression. In addition, such therapy has been associated with side effects - including cough for ACEi and serious hyperkalemia, that often limits their use37,38.
Although the introduction of ACEi and ARB therapy has decreased the incidence of renal failure in the US1, the prevalence of CKD has increased, with more than 100,000 patients developing renal failure each year1,39. Furthermore, the dramatic decrease in adverse cardiovascular outcomes, stroke and amputations, seen over the past twenty years in diabetes patients has not been accompanied by a similar reduction in diabetic ESRD39. At present there are no therapies that reverse the loss of renal function in CKD, in stark contrast to more acute inflammatory glomerulonephritis, where immunosuppression can potentially even cure disease.
Nevertheless ACEi or ARB therapy is now considered the standard of care for chronic proteinuric kidney disease, therefore any novel therapy must prove added benefit on the background of ACEi or ARB therapy.
New approaches to modulate blood pressure, hemodynamics
Combination of ACEi and ARB
Given the evident role of the renin angiotensin system (RAS) in the progression of renal disease, it was hypothesized that continued disease progression could be prevented by more complete RAS blockade38,40. To investigate this, several trials investigating the combination of both an ACEi and an ARB have been performed (discussed below).
The ONTARGET trial compared the benefit of the ACEi Ramipril, the ARB telmisartan or their combination in 25,920 patients with vascular disease and/or high-risk diabetes investigators41. Notably, most of the subjects enrolled in ONTARGET did not have microalbuminuria or macroalbuminuria at baseline, so renal benefit in proteinuric patients afforded by combination ACEi/ARB therapy could not be established41. During the study, 784 patients permanently discontinued randomized therapy because of hypotensive symptoms, with the majority of these being on combination therapy. The number of subjects achieving primary renal outcome of dialysis, doubling of serum creatinine, or death was significantly increased in the combination therapy group compared to those treated with monotherapy. Many of the dialysis events were due to acute renal failure, which was particularly increased in normotensive patients. These disappointing, but not entirely unpredictable results, underscore the safety risks associated with ACEi/ARB therapy.
Similarly, the VA-Nephron D trial studied whether the combination of an ACEi and ARB (Lisinopril and losartan, respectively) further improved renal outcomes in subjects with type 2 diabetes mellitus, but only enrolled patients with significant residual proteinuria37. Upon enrollment, subjects were all placed on Losartan. During the initial up-titration of losartan, there was a statistically significant decline in the median urinary albumin-to-creatinine ratio. Subjects were then randomized to additionally receive either Lisinopril or placebo. In the year following randomization, a further decline in albuminuria was observed, which was significantly greater in the combination-therapy group compared to patients receiving monotherapy. While there was a trend towards reduced renal failure events using the ACEi/ARB combination, this was not statistically significant. Moreover, the combination was associated with doubling of the risk for hyperkalemia and acute kidney injury compared to monotherapy37. Based on these results, combination therapy of ACEi/ARB cannot be widely recommended for use in clinical practice, although fastidious management of serum potassium might allow this combination to provide some benefit for select patients with residual proteinuria37.
Renin Inhibition
An alternative approach to RAS blockade is to inhibit the first step in the renin-angiotensin-aldosterone cascade, renin mediated cleavage of angiotensinogen to angiotensin-1 (Figure 1). The effect of the renin inhibitor, Aliskerin, on renal outcomes was tested in patients with diabetic nephropathy (ALTITUDE trial)42. Aliskerin added to either ACEi or ARB therapy resulted in greater albuminuria reduction than placebo, but did not decrease renal events or improve the rate of eGFR loss and was associated with increased hyperkalemia and stroke. The ALTITUDE trial was prematurely terminated due to increased adverse events and lack of benefit on renal function decline. Taken together these findings raise significant concerns regarding the safety of complete RAS inhibition as a strategy for treating progressive renal disease.
Mineralocorticoid receptor (MR) antagonists
The mineralocorticoid, aldosterone, is an important mediator of the effect of angiotensin, as angiotensin causes aldosterone to be released from the adrenal gland (Figure 1). Aldosterone increases tubule secretion of potassium and reabsorption of sodium and chloride, thereby expanding intravascular volume and increasing blood pressure43. Aldosterone also plays a role in directly regulating the expression of several profibrotic molecules; for example plasminogen activator inhibitor-144,45,46. Despite the preceding findings for the combination of ACEi plus ARB, and the known risks of hyperkalemia following mineralocorticoid receptor blockade47, trials of mineralocorticoid receptor inhibitors for the treatment of diabetic nephropathy are underway. An open label study investigating the aldosterone receptor antagonist spironolactone plus ARB, versus ACEi plus ARB, suggested superior ACR reduction in subjects with microalbuminuria and diabetic nephropathy taking spironolactone48, although the interpretation was confounded by lower blood pressures in this group49. Additional studies of MR antagonists are ongoing and include efficacy studies of Finerenone (Bayer)50, CS-3150 (Diachii Sankyo) and MT-3995 (Mitsubishi Tanabe), to determine the effects of these agents on ACR in subjects with diabetic nephropathy51. Whether appropriate dosing regimens can be identified which will provide efficacy yet circumvent the risk of hyperkalemia remains to be seen. It seems however that approaches that target pathways orthogonal to the RAAS should have the advantage of non-overlapping safety profile concerns.
Targeting the vasculature
Diabetic kidney disease is grouped under the microvascular complications of diabetes. Endothelial cell dysfunction, including proliferation and abnormal angiogenesis, can be observed in both experimental diabetes and human disease52. Similar endothelial pathology has been reported in the retina, which likely underlies the strong association between renal disease and retinopathy in diabetes53,54. Early phases of experimental diabetes are characterized by an increase in glomerular size, which may be a consequence of cellular endothelial hypertrophy and hyperplasia, as well as podocyte hypertrophy (Figure 2)55,56. Podocytes are a rich source of VEGF, angiopoetins and SDF, generating a bidirectional signaling pathway between endothelial and glomerular epithelial cells across the filtration barrier (Figure 2)57. This podocyte endothelial cross-talk plays a pivotal role in mediating filtration barrier perm-selectivity and albuminuria development58. The interaction between podocytes and endothelial cells has been the focus of robust preclinical experimentation, but most targets have not yet advanced into clinical development.
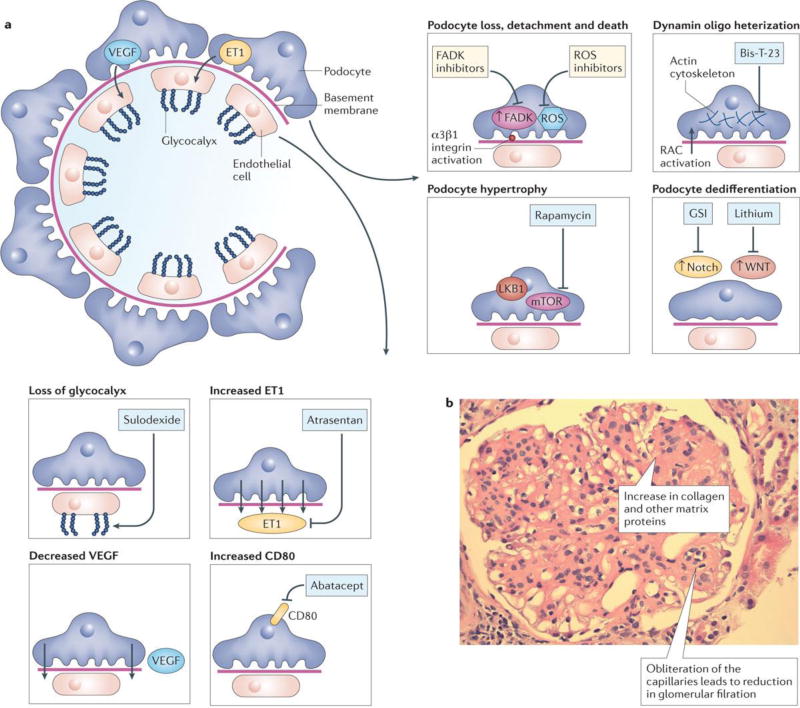
Figure 2. Targeting the glomerulus in the treatment of CKD.
A. The left of the figure depicts the normal glomerulus with endothelial cells, glycocalyx, basement membrane and podocytes shown. The boxes illustrate the different abnormalities observed in CKD and potential therapeutic strategies and agents to target them. For endothelial cells, these include: loss of glycocalyx targeted by sulodexide, increased endothelin 1A signaling blocked by Atrasertan, decreased VEGF expression and increased B7-1 expression targeted by Abatacept. For podocytes, these include podocyte loss by detachment and apoptosis targeted by ROS inhibitors, FAK inhibitors or integrin inhibitors, increased dynamin oligomerization and cytoskeletal changes targeted by BisT23, Podocyte hypertrophy by increased mTOR and LKB1 targeted by rapamycin, and podocyte dedifferentiation by increased Wnt and Notch signaling targeted by lithium and gamma secretase inhibitors. B. PAS stained kidney sections from a patient with diabetic glomerulosclerosis. Note the increased pink matrix materials, indicative of glomerulosclerosis and the obliterated glomerular capillaries.
Restoring the endothelial glycocalyx
The glycocalyx is a glycoprotein-polysaccharide rich coat that covers mammalian cells (Figure 2). Glomerular endothelial glycocalyx protects from proteinuria by limiting the entry of plasma proteins into the filtration barrier59,60. Perturbation and loss of the endothelial glycocalyx has been proposed to be an early event in diabetes61,62. Sulodexide is a highly purified mixture of glycosaminoglycans composed of 80% fast moving heparin and 20% dermatan sulphate, which exhibits anti-thrombotic and profibrinolytic properties and may inhibit endothelial glycocalyx breakdown63,64. However, while sulodexide reduced urine albumin excretion in patients with type 2 diabetic nephropathy, it failed to show benefit in a multicenter placebo-controlled double-blinded phase 3 study65. Although other approaches to restore the endothelial glycocalyx, such as the use of chemical heparanase inhibition, are being investigated, they have not yet been put into clinical development66.
Endothelin Receptor Antagonists
Preclinical studies of animal models with kidney disease have suggested that selective blockade of the endothelin A (ETA) receptor is associated with renal protection, when used in addition to existing therapies, such as RAS interventions (Figure 2)67. Several potential mechanisms for ETA receptor blockade providing renal protection have been proposed. ETA receptor blockade has vascular effects, which cause glomerular vasodilation68, and the ETA receptor antagonist atrasentan has been shown to reduce albuminuria (Table I)69. Second, endothelin has been associated with renal inflammation, which is reduced by ETA receptor blockade, perhaps by mitigating the inflammatory effects of albuminuria70–72. Finally, endothelin has been implicated in the deposition of collagen and fibrosis and, thus, ETA receptor antagonists may directly reduce fibrosis in the kidney72–74.
Table 1.
Selected ongoing Phase II and Phase III drug development programs for the treatment of kidney disease
| Drug (company) |
MOA | Hypothesized benefit |
ClinicalTrials.gov identifier (name) |
Trial information | Notes | Refs |
|---|---|---|---|---|---|---|
| Atrasentan (AbbVie) | ETA receptor antagonist | Haemodynamic | NCT01858532 (SONAR) |
|
Primary outcome measure: time to the first occurrence of a component of the composite creatinine doubling or ESRD | 77,263 |
| Canagliflozin (Janssen) | SGLT2 inhibitor | Haemodynamic | NCT02065791 (CREDENCE) |
|
Primary outcome measure: time to composite end point including ESRD, doubling of serum creatinine, renal or cardiovascular death | 189 |
| Pyridorin (NephroGenex) | Vitamin B6 analogue reducing advanced glycosylation end product protein modification | Antioxidant | NCT02156843 (PIONEER) |
|
Primary outcome measure:time to the composite end point (≥100% serum creatinine increase or ESRD) | 290 |
| ASP8232 (Astellas Pharma) | VAP1 inhibitor | Anti-inflammatory | NCT02358096 (ALBUM) |
|
Study aims to evaluate ASP8232 as an add-on therapy to an ACEI or an ARB in reducing albuminuria in patients withT2D and CKD | 139 |
| Baricitinib (Eli Lilly) | JAK1 and JAK2 inhibition | Anti-inflammatory | NCT01683409 |
|
Outcome showed an -30-40% decrease in UACR | 95,105,291 |
| CCX140 (ChemoCentryx) | CCR2 antagonist | Anti-inflammatory | NCT01447147 |
|
A significant (24%) reduction in first morning UACR at 12 weeks was observed | 104, 292 |
| CTP-499 (Concert Pharmaceuticals) | Deuterium-containing pentoxifylline metabolite, a multi-subtype PDE inhibitor | Anti-fibrotic | NCT01487109 |
|
Reportedly no significant change in UACR for patients taking CTP-499 compared with those on placebo at 24 weeks | 293 |
| Finerenone (Bayer) | MBA | Haemodynamic and anti-inflammatory | NCT01874431 |
|
Awaiting final data. Primary outcome change in UACR from that at baseline to that at 90 days | 294, 295 |
| GKT137831 (Genkyotex) | NOX1 and NOX4 inhibitor | Antioxidant | NCT02010242 |
|
Awaiting final data. Primary outcome 12-week change in UACR from baseline | 296 |
| VPI-2690B (Vascular Pharmaceuticals) | Monoclonal antibody to αvβ3 integrin | Inhibition of IGF1 signalling | NCT02251067 |
|
Albuminuria reduction and eGFR preservation during the 50-week trial duration | 230,232 |
| GS-4997 (Gilead Sciences) | ASK1 inhibitor | Protein kinase inhibitor and anti-inflammatory | NCT02177786 |
|
Change in eGFR from baseline at week 48. UACR reduction | 133 |
| PF-04634817 (Pfizer) | CCR2 and/or CCR5 antagonist | Anti-inflammatory | NCT01712061 |
|
Percent reduction from baseline in UACR at week 12 | - |
ACEI, angiotensin-converting enzyme inhibitor; ARB, type 1 angiotensin II receptor blocker: ASK1, apoptosis signal-regulating kinase 1; CCR2. CC-chemokine receptor 2; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; ETA receptor, endothelin A receptor; IGF1, insulin-like growth factor 1; JAK, Janus kinase; MOA, mechanism of action; MRA, mineralocorticoid receptor antagonist; NOX, NADPH oxidase; PDE, phosphodiesterase; SGLT2, sodium–glucose co-transporter 2; T2D, type 2 diabetes; UACR, urine albumin/creatinine ratio; VAP1, vascular adhesion protein 1.
However, a renal outcomes study using the ETA antagonist Avosentan was prematurely terminated because of a three-fold increase in fluid retention and heart failure compared with placebo75, and therefore it was no determined whether Avosentan slowed the progression of eGFR decline. It has been hypothesized that fluid retention is driven by the endothelin B receptor blocking capacity of the drugs, although there are also reports of sodium retention induced by ETA receptor blockade69,76. Based on this hypothesis, a hard renal outcome trial (SONAR) using the more selective ETA receptor antagonist Atrasentan, has been initiated. In this trial 4100 patients will be treated for 48 months with a primary outcome of creatinine doubling, ESRD or death. Because of the previous association of these drugs with congestive heart failure (CHF), the trial will specifically exclude patients with a previous history of CHF, pedal edema or a current BNP level >200pg/ml. The anticipated completion is in 201777.
Anti-inflammatory Therapy
An association between inflammation and diabetic renal failure has long been recognized (Figure 3)17,78. Bohle et. al. described the association of increased tubulointerstitial inflammatory cell infiltrate in diabetic nephropathy human kidney biopsies, with higher serum creatinine levels and longer duration of disease79. More recent mRNA profiling of renal biopsies from patients with diabetic nephropathy confirmed that inflammatory pathways are significantly increased in glomeruli and tubules17,78 and have provided evidence for stimulation of macrophage and dendritic cell maturation pathways and cytotoxic T lymphocyte mediated apoptosis, in glomeruli as well as in the tubulo-interstitial compartments. In later stages of kidney disease, leukocyte migration and complement system signatures were identified in glomeruli17. However, even patients with early stages of disease had evidence of activation of inflammatory pathways including the JAK/STAT and NFKB pathways78,80.
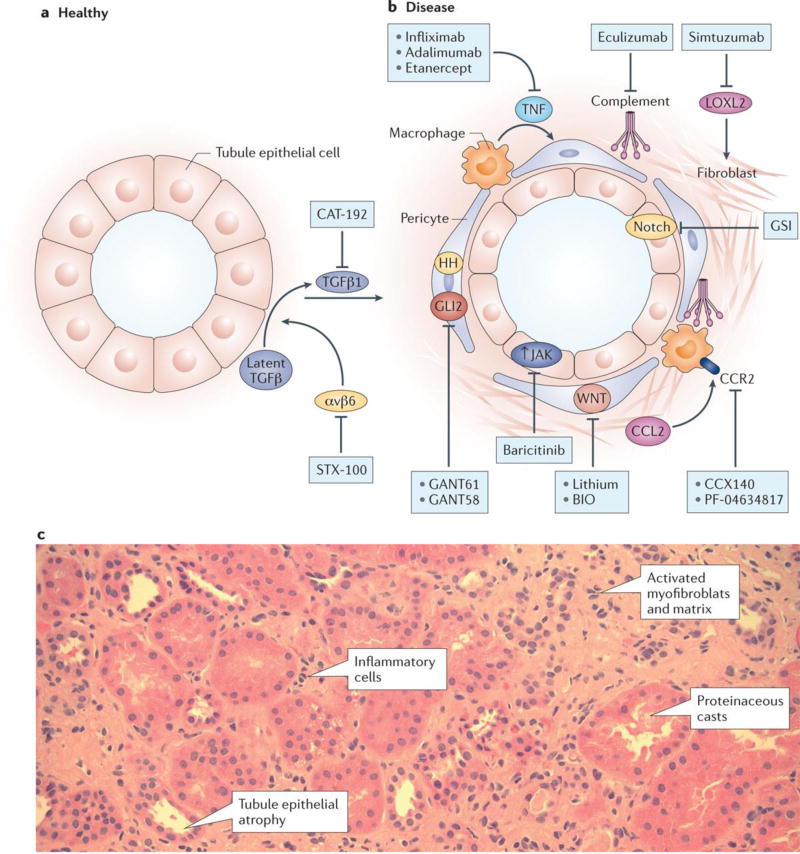
Figure 3. Targeting tubulointerstitial fibrosis in the treatment of CKD.
A. Normal (healthy) renal tubule cross-section versus B. tubulointerstitium in CKD. Several therapeutic targets are shown including: increased TGFb signaling blocked by neutralizing antibody CAT-192, blocking latent TGFb by inhibiting alphavbeta6 with STX 100 antibody, tubule dedifferentiation and proinflammatory phenotype by increasing JAK and Notch signaling (blocked by gamma secretase inhibitor or baricitinib), increased TNFa and MCP1 levels, increased complement activation, collagen cross-linking by LoxL2, and increased GLi2 and Wnt signaling in fibroblasts leading to activated myofibroblast accumulation. B. PAS stained human kidney section from a patient with diabetic kidney disease. Note the increased interstitial matrix, the increase in myofibroblasts and inflammatory cells and tubule cell dedifferentiation.
Tumor Necrosis Factor inhibition
The findings above are congruent with earlier studies suggesting hyperglycemia associated advanced glycosylation end-products (AGEs) activate TNFα production by macrophages in diabetes (Figure 3)81. Increased macrophage TNF and IL1 production were shown to be induced by glomerular basement membrane (GBM) from streptozotocin treated rats, compared to control GBM82,83. These findings are of particular note, since it has recently been demonstrated that circulating levels of soluble TNFR1 and TNFR2 (sTNFR1, sTNFR2) predict a higher rate of progression to ESRD over the following decade in patients with either type 1 or type 2 diabetes and nephropathy84,85. Membrane associated TNFR1 and TNFR2 play important roles in activating chemokine and cytokine release86. Interestingly, baseline circulating TNFα levels were only moderately increased in subjects who later developed ESRD, consistent with the possibility that membrane-associated TNF could serve as the activating ligand87,88. It is notable that both forms of the circulating sTNFRs are increased and predictive of progression89–91. TNFR2 appears to be selectively expressed in endothelium and leukocytes, whereas TNFR1 is widely expressed89,91. In addition TNFR1 and TNFR2 can be associated with different functions. A Renal protective role for TNFR1 is postulated, since deletion of this receptor is associated with higher systolic pressure and urinary albumin excretion, as well as an altered GFR92,93. TNFR2 has been shown to exert positive inotropic effects in cardiac myocytes via enhanced calcium handling94. Moreover, TNFR2 may play a role in increased susceptibility to albuminuria89
Whether TNF or its receptors play pathogenic roles in CKD progression or diabetic nephropathy remains uncertain. TNF neutralizing antibodies or TNF receptor knockouts have reduced severity in preclinical rodent models of kidney disease92,95. Nevertheless, despite anti-TNF agents having been utilized clinically for more than two decades, there have been few studies examining their clinical activity in kidney disease. Any studies have been relatively small and have mainly focused on lupus nephritis and FSGS, leaving their potential activity in other forms of CKD progression unaddressed89,92.
MCP1/CCR2 inhibition
Monocyte chemoattractant protein-1 (MCP-1), also known as chemokine (C-C motif) ligand 2 (CCL2), is a 99 amino acid residue secreted protein that interacts with the CCR2 receptor on T-cells and macrophages, recruiting these cells to sites of tissue injury (Figure 3)96,97. Renal expression of MCP1 is increased in patients with proteinuric diabetic nephropathy, being predominantly expressed in the tubulo-interstitium rather than the glomerulus98,99,100. Urinary excretion of MCP1 is also increased in patients with diabetic nephropathy and higher urine MCP1 appears to be predictive of worse renal outcomes101. Similarly, in animal models, blockade of CCR2, the receptor for MCP1, ameliorated progression of diabetic nephropathy in db/db mice as well as diabetic Ins2C96Y Akita mice102,103.
Several companies have established clinical programs to test the efficacy of CCR2 inhibitors in human diabetic nephropathy. Chemocentryx and Pfizer have ongoing clinical development programs of oral receptor antagonists for MCP1/CCR2 (CCX140) and CCR2/CCR5 (PF489791), respectively, and are testing them for their ability to reduce proteinuria in patients with diabetic nephropathy (Table I)104,105.
A 52-week phase II clinical trial of CCX140 in 332 patients with diabetic nephropathy106,107 met its primary endpoint, demonstrating that treatment with CCX140 added to an ACEi or ARB statistically significantly reduced the urinary albumin creatinine ratio (UACR), beyond that achieved with standard of care. The maximum treatment effect, an 18 percent reduction in UACR, was seen in the 5mg dose group at 12 weeks, and sustained reduction in albuminuria induced by CCX140 relative to SOC alone was observed over the full year106. Whether the reported reduction in albuminuria will translate into long term improvements in patient outcomes and reduce the rate of renal function deterioration remains to be tested. At the time of writing this review, results from Pfizer’s Phase II trial of the CCR2/5 antagonist PF489791 were not publically available (Table I).
JAK/STAT inhibition
Janus Kinase (JAK) and signal transducer and activators of transcription (STAT) are important intracellular mediators of receptors for erythopoetin, growth hormone, EGF, as well as inflammatory signaling interferons alpha, IL6, IL12, and IL23 (Figure 3)108,109. Ligand binding to their receptors leads to multimer or homodimer assembly, activating the auto-phosphorylation activity of JAK and subsequent STAT phosphorylation and its translocation to the nucleus110, resulting in the transcription of additional proinflammatory target genes including MCP1, as well as GATA3, IL24, LTB, and SOCS1111,112,113,114. Increased expression of these genes comprise a major genetic signature in diabetic nephropathy and lupus nephritis78,80,115,116. Evidence of JAK/STAT activation in experimental models of kidney disease, including diabetic mice and rats, has also been observed17,78,80 and the use of a non-selective JAK inhibitor (AG-490) significantly reduced urinary protein excretion in diabetic rats117.
Recently, JAK inhibitors including tofacitinib and baricitinib have been tested in the clinic and found to be efficacious in autoimmune inflammatory diseases, including rheumatoid arthritis and ulcerative colitis113,118,116,119,120. The documented clinical anti-inflammatory efficacy of JAK inhibitors, together with the gene pathway signature in diabetic nephropathy have prompted a phase 2 exploration to test the clinical efficacy of these JAK inhibitors in kidney disease96,121. The effects of 24 week treatment of the JAK1 and JAK2 inhibitor baricitinib (Eli Lilly/Incyte) on albuminuria has been investigated in 129 subjects with proteinuric diabetic nephropathy already taking ACEi or ARB121. Baricitinib treatment was associated with a 30–40% dose-dependent reduction in albuminuria. However, the side effect of reduced hemoglobin associated with this class of drugs was observed121. It will be necessary to determine whether or not these effects on albuminuria reduction translate into long-term benefit on kidney function and mortality.
Complement inhibition
Complement activation plays a well-established role in the pathogenesis of diverse renal disease, including membranoproliferative glomerulonephritis (MPGN), post-infectious glomerulonephritis, hemolytic uremic syndrome and IgA nephropathy107,122. Eculizumab (Alexion Pharmaceuticals) is a monoclonal antibody to C5, which blocks the cleavage of the C5 complement protein to C5a and C5b, thereby preventing the generation of the pro-inflammatory peptide C5a and the membrane-attack complex C5b-9 (Figure 3)123. Eculizumab treatment improved eGFR in patients with atypical hemolytic uremic syndrome and the associated thrombotic microangiopathy123,124. Although these were small phase two trials, the effects were sufficiently profound to support registration of eculizumab, for which it is now approved.
In addition to immune glomerulonephritides, complement activation has been proposed to contribute to the pathogenesis of diabetic nephropathy, possibly through glucose-associated production of neoepitopes activating the lectin complement pathway125,126. This hypothesis has received additional support from molecular profiling studies of human diabetic nephropathy kidney biopsies and plasma, which show complement to be among the most activated pathways17,127. C3 is robustly expressed in kidney biopsies from patients with diabetic nephropathy17. Although complement inhibition has not been clinically tested in diabetic nephropathy, inhibition of complement in animal models of the disease support potential therapeutic benefit128. However, the potential risk of increased infections with this approach must be considered124.
Apoptosis signaling kinase 1 (ASK1)
ASK1 is a mitogen activated protein kinase kinase kinase (MAPK3K) stress responsive kinase, that can be activated by multiple stimuli including reactive oxygen species (ROS) and TNFα activation of TNFR1129,130. ASK1 signals through a cascade of downstream kinases including p38 and c-Jun N-terminal kinase (JNK), leading to target gene expression including inflammatory cytokines131,132. A preliminary report suggests that ASK1 inhibition substantially improves glomerulosclerosis in a diabetic animal model133. Gilead Sciences has initiated a 300 patient phase 2 program to test the efficacy of the selective ASK1 inhibitor GS-4997 in diabetic nephropathy. The primary outcome is the change in eGFR following 48 weeks of treatment. A secondary outcome will determine the proportion of participants achieving at least a 30% reduction in albuminuria at week 48 from baseline134,135 .
Vascular adhesion protein 1 (VAP1) inhibition
Accumulating evidence pointing to renal inflammation in the pathogenesis of progressive kidney disease136 has generated interest in strategies to impede trans-capillary migration of inflammatory cells into the diseased kidney. Vascular adhesion protein 1 (VAP-1) is an endothelial sialoglycoprotein, whose cell surface expression is induced under inflammatory conditions137,138. VAP-1, also known as amine oxidase, copper containing 3 (AOC3), possesses mono-amine oxidase activity and also interacts with leukocyte adhesion molecules including Siglec-9 and 10139. Both these functions are important in facilitating leukocyte egress into inflamed tissue137. Based on these findings, Astellas has initiated a phase 2 study of ASP8232 - a small molecule inhibitor of AOC3 activity, in 110 patients with diabetic nephropathy140. The trial will explore urine ACR reduction following twelve weeks of treatment and is anticipated to read-out in June 2016 (Table I).
Targeting fibrosis
Tubulointerstitial fibrosis is the common histological manifestation of CKD, almost regardless of disease etiology (Figure 3). The degree of fibrosis is a strong predictor of future GFR decline and often an important reason why biopsies are performed79. Tubulointerstitial fibrosis shows similarities to other fibrotic conditions including lung fibrosis and liver cirrhosis141. Common mechanisms identified in fibrosis include: epithelial injury, fibroblast activation, matrix deposition and inflammation, which each provide potential therapeutic targets142.
TGFB1 inhibition
Cell culture and mouse model data strongly supporttransforming growth factor beta (TGFB1) as the key driver of collagen and activated myofibroblast accumulation, which is characteristic of fibrosis143. Inhibiting TGFB signaling therefore represents a promising antifibrotic therapeutic approach (Figure 3). However, clinical trials with CAT-192 (Genzyme), a recombinant human antibody that neutralizes TGFB1 in the treatment of early-stage diffuse cutaneous systemic sclerosis, failed to show benefit in diabetic kidney disease (Figure 3). Similarly, Phase II trials with a TGFB1 neutralizing antibody (Lilly, LY2382770) did not show clinically significant improvement in patients with diabetic kidney disease144. Systemic toxicity associated with TGFB inhibition may limit the maximal tolerated dose145. Further studies are needed to understand the complex role of TGFB in fibrosis and identify approaches that would avoid systemic inhibition.
One such approach that has recently emerged involves targeting the alpha(v)beta (6) integrin. αvβ6 is an epithelial restricted integrin that binds and activates latent TGFbeta146. An anti-αvβ6 neutralizing antibody developed by Biogen (STX100) has shown benefit in multiple fibrosis models146,147 and is currently undergoing Phase II trials in idiopathic pulmonary fibrosis (IPF) (Figure 3).
Pirfenidone
Pirfenidone has recently been approved for the treatment of IPF as it has shown statistically significant benefit in reducing pulmonary function decline in Phase III Trials148. The mechanism of action of pirfenidone is not fully understood. In cell culture studies it suppresses collagen expression and has an anti-inflammatory effect149,150,. It showed modest clinical benefit in a randomized, double-blinded, placebo-controlled Phase II study in 77 subjects with diabetic nephropathy who had elevated albuminuria and reduced eGFR. Among the 52 subjects who completed the study, a significant increase in the mean eGFR in the pirfenidone group compared to the placebo group was reported151.
Galectin-3
Galectin-3 is a member of the lectin family, with antimicrobial activity against bacteria and fungi. It has been shown to be involved in an array of biological processes including cell adhesion, growth, differentiation, fibrosis, heart disease, cancer and stroke (Figure 3)152. Human studies indicate a correlation between galectin 3 levels and ESRD, pulmonary fibrosis, heart disease and cancer153. Galecto Biotech is currently developing galectin-3 inhibitors for the treatment of fibrosis, specifically IPF154. It will be interesting to also investigate their effects on kidney disease progression.
Lysophosphatidic acid receptor (LPAR) antagonists
Fibrosis is associated with both increased lysophosphatidic (LPA) production as well as with increased expression of its receptor (mainly LPAR1) in a number of organs (Figure 3)155. LPA is a bioactive mediator which acts via specific G-protein coupled receptors156. Genetic (using knock-out mice) and pharmacological approaches demonstrated the contribution of the LPA1R subtype to the development of kidney, lung, vascular and dermal fibrosis157. To date, LPA1R antagonists have passed Phase I and Phase II clinical trials for IPF and systemic sclerosis158, and therefore may be tested in kidney disease in the future.
Tranilast
Tranilast (Kissei Pharmaceutical Co. Ltd.) has been marketed in Japan for the treatment of allergic diseases since 1982 and more recently for the treatment of keloid/hypertrophic scars (Figure 3)159,160. In pilot studies, one of its derivatives, 3- methoxy-4-propargyloxycinnamoyl anthranilate (FT011, Fibrotech Therapeutics) was shown to reduce albuminuria in a rat model of diabetic nephropathy161. In cell culture studies, FT011 inhibits both TGFB- and PDGF-induced collagen production162. At present we are not aware of an active development program for tranilast/FT011 in DKD.
Lysyl oxidase inhibition
Lysyl oxidase (LOX) is an extracellular copper-dependent amine oxidase that catalyzes the first step in the formation of crosslinks in collagens and elastin163,164. Collagen cross-linking is critical for fibrosis development (Figure 3). Gilead pharmaceuticals have developed a new humanized monoclonal antibody (simtuzumab) that binds and inhibits LOXL2, which is currently in clinical trials for lung and liver fibrosis. Based on its mechanism of action, it may also show benefit in kidney fibrosis165.
Targeting microRNAs
Mouse models of IPF have indicated decreased expression of microRNA-29 (miR29) in bleomycin-induced fibrosis166. Additional studies have indicated that miR-29 is an important regulator of TGFβ expression166. miRagen Therapeutics have developed miR-29 mimics that are now being tested for the treatment of IPF. Notably, miR29 has showed similar expression patterns in mouse models of kidney fibrosis167, indicating that this could be a potential therapeutic target. Another microRNA - miR-21 - might represent another important target in the kidney168 (see below).
Developmental pathways
Unbiased transcriptome analyses studies have highlighted the reactivation of multiple developmental pathways including Wnt/Notch and Hedgehog pathways (which play critical roles in kidney development) in patient samples and animal models of CKD (Figure 3)17,14,169,170. . While transient activation of any of these pathways is likely to be important in regeneration and repair after an acute injury, sustained activation of these pathways have been shown to induce fibrosis development in mouse models171. Increased chronic expression of Notch in tubule epithelial cells induced fibrosis development in mouse models, while genetic deletion of Notch from tubule cells or pharmacological inhibition of Notch ameliorated kidney disease development14. Notch seems to play an important role in mediating epithelial dedifferentiation, which is a key aspect of fibrosis. The Gli Hedgehog pathway on the other hand seems to play an important role in myofibroblast proliferation and transdifferentiation170. Genetic or pharmacological inhibition of Hedgehog signaling in fibroblasts was not only able to halt, but also to reverse fibrosis in a mouse model14,170,172. While these pathways may therefore represent new and potentially attractive therapeutic targets, at present, further studies are needed to better differentiate the profibrotic and regenerative activities.
Metabolic dysregulation in diabetes and CKD
Metabolic dysregulation, particularly poor glucose control, is associated with an increased rate of development of complications, including kidney disease, in patients with type 1 and type2 diabetes173,174. Indeed, cells incubated in high glucose or increased fatty acids show significant changes in their metabolic profiles175. Increased generation of reactive oxygen species from mitochondrial or plasma membrane oxidase has been proposed to be the unifying theme leading to cell damage (Figure 4)176.
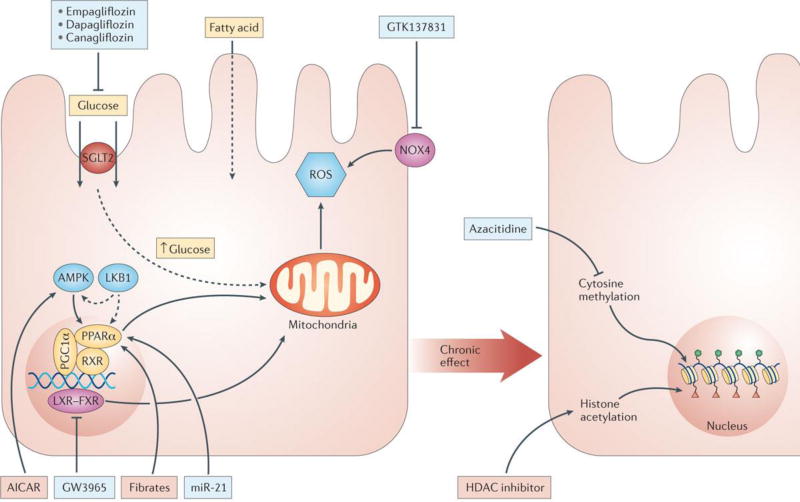
Figure 4. Metabolic alterations in CKD.
Metabolic dysregulation, particularly poor glucose control, is associated with the development of diabetic nephropathy. Key factors contributing to disease development include increased SGLT2 mediated glucose uptake and increased mitochondrial and plasma membrane ROS generation . In addition, fatty acid oxidation is decreased due to loss of nuclear receptor activity of PPARA, RXR, PPARGC1A, LXR/FXR and their upstream regulator AMPK, LKB1 and miR 21. Effects of metabolic fluctuations are long lasting as they can influence the epigenome; cytosine methylation and histone acetylation. Agents being investigated, which target many of these alterations, including: empagliflozin, dapaglifozin and canagliflozin that block SGLT1, GTK137831 that blocks NOX4, 5Aza that blocks cytosine methylation, HDAC inhibitors that block histone acyltransferases, miR21 and fibrates that activate PPARA, and GW3965 that blocks LXR/FXR, are shown in the red and green boxes.
However, recent studies exploring the effects of glucose normalization on diabetic kidney disease have presented unexpected observations. Findings from the Diabetes Complication Cohort Trial (DCCT) and United Kingdom Prospective Diabetes Study (UKPDS) indicate that subjects with initial poor glycemic control continue to develop a higher rate of kidney and cardiovascular disease despite several decades of improved glycemia173. Furthermore, we have also learned that in most patients with type2 diabetes and existing kidney disease, targeting a glycated hemoglobin level below 6.0% - almost to the degree of “normalization” of glucose levels - does not translate into reduced development of complications177,178. The term “metabolic memory” has been proposed to explain these results, indicating that cells are reprogrammed by prior episodes of metabolic derangements and continue to respond differently179. Epigenetic changes might provide the mechanistic basis for this metabolic memory effect180. Epigenetic enzymes require substrates of intermediate metabolism such as methyl and acetyl groups, which is the likely molecular mechanism by which environmental changes and nutrient availability can have a long-term influence on cellular behavior even after the insult is removed181. Animal model studies and some human observations indicate epigenetic differences in patients with DKD and CKD179,182. Targeting metabolism therefore represents a promising therapeutic strategy as: a) metabolic dysregulation is likely one of the most proximal mechanisms in diabetes and known to play a role in kidney disease development b) acute metabolic derangements likely have a long-term impact on the development of complications by epigenetic reprogramming and c) at later stages, insufficient energy availability is an important mechanism contributing to CKD development180,183.
SGLT2 inhibition
The sodium/glucose cotransporter 2 (SGLT2) is the major glucose renal re-uptake mechanism in the apical membrane of the proximal tubule (Figure 4)184. SGLT2 inhibitors have entered clinical practice as glucose control agents for diabetics, through their activity to inhibit renal glucose absorption and reduce hyperglycemia185,186. Studies in type 1 and type 2 diabetic mice show that SGLT2 inhibition not only reduces hyperglycemia but also has beneficial effects in DKD, including reduced glomerular hyperfiltration, renal hypertrophy and albuminuria, as well as decreased systolic blood pressure187,188. These findings were interpreted as being consistent with a critical role for suppression of tubulo-glomerular feedback in hyperfiltration in diabetes. By blocking glucose-coupled Na+ reabsorption, SGLT2 inhibition is thought to increase Na+ delivery to the macula densa, activating constrictor signals to the afferent arteriole, thereby reducing glomerular filtration pressure189. This proposed effect of SGLT2 inhibitors to reduce glomerular filtration pressure therefore overlaps with the mechanisms attributed to ACEi/ARB, although SGLT2 inhibition may target the afferent arteriole more selectively.
Like ACEi/ARB, a phase 2 trial of the SGLT2 inhibitor canagliflozin in type 2 diabetics with CKD showed an acute reduction in eGFR, followed by eGFR stabilization190. This was accompanied by a significant and dose-dependent reduction in albuminuria. To determine whether the observed decrease in albuminuria translates to improved outcomes in subjects with diabetic nephropathy, Janssen has initiated a phase 3 study of canagliflozin. The CREDENCE trial will enroll 3700 subjects at 531 sites globally191. The primary composite endpoint of the study includes end-stage kidney disease, doubling of serum creatinine, and renal or cardiovascular death. Results from this trial are expected in early 2020 (Table I).
Lipid metabolism
Renal tubular and glomerular epithelial cells are high energy demanding cells with dense mitochondria. Their primary source of energy comes from fatty acid oxidation. Genetic mutations in mitochondrial enzymes have been shown to result in severe tubulointerstitial fibrosis and glomerulosclerosis (Figure 4)192,193. As non-transformed cells do not use glucose in high amounts, decreased fatty acid oxidation induces accelerated cell death and dedifferentiation of the cells183. Nuclear receptors, including PPARA, PPARGC1A and LXR pathways have been shown to be key regulators of fatty acid metabolism in tubule cells194. Indeed, transgenic expression of PPARA was able to ameliorate CKD development in animal models195. Pharmacological activators of PPARA (mostly fibrates) have been in clinical use for treatment of serum lipid abnomalities for many years. Two clinical trials - FIELD and ACCORD - have examined the effectiveness of a PPARA agonist (fibrates) on kidney disease development, both reporting a statistically significant reduction in albuminuria development in diabetic patients (Figure 4)196,197. Unfortunately, fibrates raise serum creatinine levels, possibly by interfering with creatinine secretion, therefore determining their effectiveness in GFR decline based on serum creatinine levels cannot be directly studied. The follow-up wash-out study indicates that the GFR decline was statistically slower in the fibrate treated group198,199. Upstream targeting of the pathway at the level of AMPK or liver kinase B1 (LKB1), which is an important regulator of AMPK and downstream metabolism, might also offer therapeutic benefits (Figure 4)200. An AMP kinase agonist has shown significant benefit in mouse models of diabetic kidney disease and other forms of CKD201.
miR-21 antagonism
miR-21 is one of the first discovered and fairly abundant microRNAs, which like many other mRNAs, has pleotropic functions associated with PTEN, TGFβ and tumor suppression202. Mouse model studies have reported a protective role for miR21 antagonism in heart fibrosis development (Figure 4)203,204. Moreover, small molecule antagonism of miR21205 has shown a marked protective effect in several mouse fibrosis models168. MiR-21 in the kidney affects multiple metabolic pathways converging on PPARA and ultimately improving mitochondrial function and metabolism205. However, it is interesting to note that genetic deletion of miR-21 does not seem to recapitulate the effect of pharmacological inhibition206. Further studies are therefore needed to determine whether miR-21 could potentially be modulated in the treatment of kidney disease.
Reactive oxygen species: NADPH oxidase inhibition
Increased generation of reactive oxygen species from mitochondrial sources has been proposed as a unifying hypothesis for the development of diabetic complications207. Both mitochondrial and plasma membrane NADPH oxidase contribute to ROS increase in diabetes175. In addition, inhibition of the plasma membrane NADPH oxidase has shown significant benefit in different rodent models of fibrosis175. The kidney specific NADPH isoform, NOX4, has recently received significant attention as specific NOX4 inhibitors appear to show benefit in mouse models of diabetic kidney disease (Figure 4)208. NOX4 inhibitors seem to be broadly effective in fibrosis as recent studies have also indicated beneficial effects in models of pulmonary and liver fibrosis209–211. In 2012, Genkyotex announced the successful completion of a Phase Ia study with GKT137831, a first in class inhibitor targeting NOX1 and NOX4 enzymes (Table 1)208. GKT137831 appears to be safe and well tolerated following oral administration. Phase II studies for diabetic kidney disease with GTK-137831 have been completed but results remain to be reported. Pyridorin (pyridoxamine) (NephroGenex) is a novel compound that is a derivative of vitamin B6, shown to target and reduce pathogenic oxidative chemistries212. However, a phase 2 efficacy study failed to show a robust effect in subjects with diabetic nephropathy213. A Phase 3 trial (PIONEER) of pyridorin in patients with earlier stage diabetic nephropathy is ongoing (Table 1).
Epigenetic targets/HDAC and cytosine methylation
The effect of acute metabolic changes often may not be detected until later stages of disease, as they can result in long-term epigenetic modifications (Figure 4). Several groups have reported differences in cytosine methylation levels between control and disease human kidney samples214. Increased methylation of the RASAL1 gene, which encodes an inhibitor of the Ras oncoprotein, has been associated with fibroblast activation and fibrogenesis in the kidney214. RASAL1 hypermethylation is mediated by the methyltransferase Dnmt1 and in mouse models of kidney fibrosis, genetic deletion of the Dnmt1 or pharmacological inhibition of the enzyme protected animals from kidney fibrosis development214. Studies from our laboratory have also shown that methylation differences target key profibrotic pathways, indicating that they are functionally important182.
Histone modifications represent another important epigenetic regulator of gene transcription. Inhibitors of histone deacetylases (HDACs) have been reproducibly shown to ameliorate fibrosis development in different models (Figure 4)215,216. However, as these drugs broadly interfere with transcription, extended safety studies are needed to understand their side effect profiles.
Targeting podocytes and albuminuria
The presence of albuminuria in a patient with diabetes and CKD currently fulfills the diagnostic criteria for diabetic CKD (Figure 2). Albuminuria used in conjunction with gender, GFR and serum phosphorus level is an excellent predictor of future glomerular function decline, therefore most Phase 2 studies use albuminuria as an intermediate outcome7. Human genetic studies of patients with rare monogenic diseases, Focal Segmental Glomerulosclerosis (FSGS) and Minimal Change Disease (MCD)193 indicate that podocytes play a critical role in albuminuria and glomerulosclerosis, as almost all monogenic mutations are podocyte specific proteins193. Mouse genetic models have further substantiated the role of podocytes in proteinuria169,217. Furthermore, podocytes are unable to divide and they have limited (if any) capacity to be replaced218. The slit diaphragm which is a junction between podocyte foot processes, the glomerular basement membrane and the fenestrated endothelial cells, represent the key barriers to albumin leakage into the urine (Figure 2)219. Over the years, we have learned that the slit diaphragm acts as a signaling platform220. Proteinuric disease conditions are almost uniformly associated with foot process simplification of podocytes, caused by reorganization of the actin cytoskeleton193. These changes are identified as foot process effacement by histopathological examination. Nephrin clustering and endocytosis is associated with changes in the actin cytoskeleton, cell adhesion and endocytosis221–223, playing a key role in proteinuria development. Although proteinuria and foot process effacement are reversible conditions, the critical point of no return is reached upon 20% podocyte loss, due to apoptosis, detachment or dedifferentiation224,225. At this point glomerulosclerosis develops leading to a decrease in GFR.
Abatacept
B7 is a membrane protein found on activated antigen presenting cells that can produce a costimulatory signal to enhance the activity of the T cell226. Interestingly, B7 expression is increased in podocytes in a subset of patients with FSGS, a relatively rare glomerular disease (Figure 2)227. Abatacept is a clinically approved inhibitor of B7 signaling for the treatment of rheumatoid arthritis, which acts by blocking its interaction with its receptors228. Abatacept treatment of 5 subjects with B7-positive FSGS resulted in a significant reduction of proteinuria, but the results were not replicated in other cohort229. Mouse model studies indicate that a similar mechanism might be present in models of diabetic kidney disease230. Larger clinical studies will be needed to determine the clinical benefit in these disease conditions, which are currently hindered by difficulties of reliable detection of B7 expression in kidney tissue samples229.
Actin-cytoskeleton
Recent reports indicate that Bis-T-23, a compound that promotes actin-dependent dynamin oligomerization and thus increases actin polymerization in injured podocytes, improves renal health both in transient and CKD models (Figure 2)231. Bis-T-23 has been reported to interact with the GTPase dynamin222, which has been implicated in the maintenance of cellular architecture in podocytes, through its direct interaction with actin. Dynamin oligomerization appears to play an important role in actin polymerization and global organization of the actin cytoskeleton in the cell231. Further studies into the potential of this approach for the treatment of kidney disease are therefore warranted.
Integrin inhibition
VPI-2690B (Vascular Pharmaceuticals) is a monoclonal antibody which binds to the C-loop domain sequence of integrin αVβ3232. This antibody has been shown to reduce albuminuria in diabetic rats and atherosclerosis in diabetic pigs233,234. It has been proposed that these effects are mediated by blocking the action of integrin αVβ3 to stimulate insulin-like growth factor-I (IGF-I) signaling in vascular smooth muscle cells and mesangial cells232–234. αVβ3 might also play role in podocyte and matrix interaction. VPI-2690B is currently in phase 2 clinical testing for the treatment of diabetic nephropathy235. This placebo-controlled trial will enroll 300 diabetic subjects and is projected to complete in August 2017 (Table 1).
Additional agents in development
There are several other agents, which do not fall into the classes discussed above, that are also being clinically investigated for the treatment of chronic kidney disease.
Phosphodiesterase inhibitors
Pentoxifylline, a methylxanthine derivative and non-specific phosphodiesterase inhibitor, has long been proposed as a therapy for diabetic nephropathy236. The recently completed PREDIAN study tested the effects of 24 months of pentoxyfylline treatment on proteinuria and eGFR decline in 160 subjects with type 2 diabetes and nephropathy237. Subjects randomized to pentoxifylline experienced a marked reduction in albuminuria, but only a relatively small effect on eGFR. Similarly, in a preliminary report, Pfizer carried out a Phase 2 trial of the PDE4/5 inhibitor, PF-00489781, in 256 subjects with diabetic nephropathy238. Following 12 weeks of treatment, the urine albumin to creatinine ratio was significantly reduced compared to placebo. In addition, phase 2 testing of phosphodiesterase inhibition with CTP-499, a deuterated analog of 1-(S)-5-hydroxyhexyl-3,7-dimethylxanthine (an active metabolite of pentoxifylline), in patients with type 2 diabetes mellitus and nephropathy has been undertaken by CoNCERT pharma (Table 1). Although this trial has been completed, the full results have not yet been reported. Thus, while phosphodiesterase inhibition appears to have beneficial effects on albuminuria in ACEi/ARB treated patients, it remains to be determined whether these observations will translate into a benefit in renal outcomes and slowing of dialysis progression.
Allopurinol
Clinical observational studies indicate a strong correlation between serum uric acid levels and the loss of kidney function in diabetic patients239. However, whether high uric acid levels actually cause renal function decline remains unclear. Two small clinical trials have provided proof of concept for using allopurinol to lower the risk of kidney function decline. One of the studies included only 51 subjects with high serum urate levels and although the other study had more than 100 participants, the average age in that cohort was unusually high240–242. It is therefore not yet clear whether these results can be generalized to all patients with DKD. A large NIH-funded clinical trial - Preventing Early Renal Function Loss (PERL) Consortium - has been formed to test this hypothesis243. This study specifically targets patients with type 1 diabetes and relatively preserved kidney function, who are at high risk for progression (Table 1).
Sodium bicarbonate
One key function of the kidney is to excrete acids that are generated after burning proteins. In the urine, most protons are excreted as ammonia. Ammonia-genesis is decreased in early stages of CKD and many patients with CKD develop metabolic acidosis26. As most enzymes operate within a narrow pH range, even small changes could have a significant effect. Metabolic acidosis can activate the complement pathway, leading to low-grade persistent complement activation and inflammation244. Small clinical trials aiming to correct acidosis have indicated a benefit of treating patients with sodium bicarbonate245. Larger double blinded placebo controlled trials are currently being conducted to determine whether sodium bicarbonate treatment may slow progression of renal disease.
Challenges in the development of novel therapies for chronic kidney disease
There are a number of challenges facing the development of new treatments for CKD. In particular, several factors must be considered in the design of future clinical trials, including issues regarding patient recruitment and selection, as well as the identification of appropriate endpoints and efficacy biomarkers. It must be noted that testing of novel therapies aimed at slowing progression of proteinuric CKD must be performed in subjects already receiving the standard of care, including angiotensin converting enzyme inhibition or angiotensin receptor blockers.
Disease awareness and clinical trial recruitment
The number of randomized controlled trials in nephrology is lower than that for almost any other medical sub-specialty246. The specific factors contributing to this gap are complex, but certainly7,9 include lack of patient disease awareness and self-advocacy247. Research has shown that disease awareness in patients with CKD stages 1–3 is as low as 5% even in patients that have seen a physician within the last year247–250. Physicians may neglect mentioning to patients the fact that they have kidney disease, perhaps in part because they don’t believe they can offer effective therapeutic options.
These issues undoubtedly contribute to major operational difficulties in executing trials designed to slow the progression of kidney disease to dialysis - especially low recruitment rates251,252. Overall recruitment rates for diabetic kidney disease trials approximate ~0.20 patients per site per month which is about a quarter of the number of patients enrolled per month for a diabetes trial. While the size of clinical trials may be similar to diabetes mellitus studies, the rate at which patients enroll into diabetic kidney disease trials significantly delays timelines, increases cost, and negatively impacts the willingness of major pharmaceutical companies to invest in these trials. Clinical trial networks and patient registries can significantly facilitate patient identification and recruitment rate in clinical trials for testing therapies. Although clinical trial networks for acute kidney injury have been established, chronic kidney disease trial networks including the two major causes of CKD (diabetic nephropathy and hypertension) are just now being initiated in Canada and Italy but have not included the US, the rest of Europe or Asia248,253.
Patient selection
Pre-screening of patients and clear inclusion and exclusion criteria, should balance the desire to eliminate likely biological non-responders that decrease trial efficiency with the need to rapidly accrue patients who are representative of the disease population. These design choices require a sophisticated understanding of the pathophysiology of the disease process. In some circumstances, biomarker-based target engagement can help drive such study enrichment, as exemplified by the significant impact of precision medicine approaches being implemented in the oncology field.
There is a growing appreciation of patient heterogeneity with respect to the rate of loss of renal function. Accordingly, efficient identification and enrollment of patients who are most likely to progress and benefit from the candidate therapeutic intervention remains a significant challenge. Recent results of the large Chronic Renal Insufficiency Cohort (CRIC) have indicated that when using a general patient population, it could take more than a decade to achieve hard end-points used in clinical trials48. This has raised the question as to whether trials should be focused on a subset of patients who follow a more rapid kidney function decline, considered “rapid progressors” (Box 1).
Box 1: Should clinical studies focus on rapid progressors?
Kidney function decline in patients follows a highly heterogeneous pattern. Recent results of the large Chronic Renal Insufficiency Cohort (CRIC) Indicates that the mean GFR decline in patients with stage 3 diabetic CKD is around 1.5-3 cc/year268. However, a subset of patients follows a more rapid kidney function decline and can be considered “rapid progressors”. Unfortunately, at present there is no consensus definition for “rapid progression”. Expert consensus suggested the use of >5 cc/min/1.73m2/year decline269. Results from the CRIC cohort indicate that >3cc/min/1.73m2 is already 2 standard deviations above the mean GFR decline, therefore can be used to identify patients with rapid functional decline268.
Multiple studies have been undertaken to identify clinical, biochemical and genetic markers that can detect rapid progressors. Recent studies indicate that in the stage 3–5 CKD group, the 4 variables: creatinine, proteinuria, phosphate and sex have C-statistics of 0.91 to predict ESRD270,271. This means that while it is interesting to identify new biomarkers, it will be challenging to meaningfully add to the identification of people who progress to ESRD especially in disease conditions associated with proteinuria.
Levels of plasma soluble TNFR1 and TNFR2 may potentially be used as predictors of renal failure in patients with either type 1 or type 2 diabetes84,85,272. An association between increased circulating TNFR levels and poor renal disease prognosis has also been observed in both IgA nephropathy and idiopathic membranous nephropathy273,274. Data suggests that circulating sTNFR could derive from exosomes shed into plasma86, however the precise tissue and cells responsible for the increased circulating levels remain unclear. Increased urinary excretion and blood levels of kidney injury molecule-1 (KIM-1), also known as TIM1 and hepatitis A virus cellular receptor 1 (HAVCR1), have been associated with acute kidney injury, IgA nephropathy and diabetic nephropathy275,77,78. KIM-1 is relatively selectively expressed in liver and renal proximal tubule cells, and appears to play a role in modulating T cell immunity276,277,278. Interestingly, treatment with the angiotensin receptor antagonist irbesartan reduces u-KIM-1 in patients with diabetic nephropathy279. Notably, recent studies have demonstrated that plasma KIM-1 also increases with AKI and importantly, plasma KIM-1 levels are predictive of progression of CKD in patients with type 1 diabetes mellitus275. The predictive value of these plasma inflammatory markers supports potential involvement of specific inflammatory pathways in the pathogenesis of diabetic nephropathy.
While identification of rapid progressors is an important endeavor, some critically important questions remain to be addressed. First, and most importantly, what drives rapid progression in these subjects? Are the pathways that drive rapid progression the same as those that drive moderate or slow progression, just with an increased magnitude or activation of different pathways that are responsible for rapid progression? Will trials actually benefit from enrichment strategies or will we be simply selecting for resistant, potentially less compliant subjects, where intervention will make less of an impact.
One key inclusion criteria used to enrich for CKD patients at high risk for progression has been a high level of proteinuria. Baseline proteinuria is a strong predictor of poor renal outcomes254–256. Furthermore the extent of treatment associated proteinuria reduction in Phase 2 studies was highly predictive of a favorable outcome257,258. These observations have driven trials of new therapeutics to recruit patients with high baseline levels of proteinuria. However, with more widespread use of ACEi/ARB therapy, proteinuria levels are already decreased in many patients, and recruiting subjects with overt proteinuria is increasingly challenging in the implementation of renal trials.
Furthermore, clinical experience working with patients with minimal change disease indicates that proteinuria can be reversible and is not always associated with progressive kidney function decline259. There are increasing numbers of DKD patients who progress without developing overt albuminuria – perhaps due to ACEi/ARB induced proteinuria reduction. More than a decade ago, a study showed that close to 25% of subjects do not follow the “classic” paradigm for DKD progression, where micro albuminuria is followed by macroalbuminuria and progressive GFR decline260.
The development of novel biomarkers that supplement proteinuria in predicting progression of renal disease in patients already taking ACEi/ARB comprises a new challenge for the renal research community. Recent studies indicate that levels of plasma inflammatory markers may have some predictive value72, 73, 76 (Box 1).
Clinical Trial Endpoints
Traditional drug registration trial endpoints in kidney disease include doubling of the serum creatinine, initiation of renal replacement therapy (dialysis, transplant) or death. Using the CDK-EPI equation, doubling of serum creatinine level is equivalent to a 57% decline in estimated glomerular filtration rate (eGFR) (Box 2)261. Several recent studies have explored the predictive validity of using lesser eGFR decrements (i.e. −30% or −40%) of ESRD events in CKD trials7,262–264. These analyses support the strong predictive value of these intermediate eGFR decrements as surrogates of the currently accepted endpoints. Because there are greater numbers of these intermediate events, use of these surrogate endpoints could enable smaller and/or shorter trials265. However, adopting this design would have to match with the appropriate mechanism of action as many drugs (ACE and ARB) acutely reduce GFR while providing protection from ESRD after a longer-term follow-up32.
Box 2: Trial Efficacy: measured versus estimated GFR.
Although almost all-current treatment trials have used serum creatinine based estimated GFR (eGFR) to evaluate the beneficial effect of drugs32, several studies have shown that estimated GFR does not correlate perfectly with tracer based measured GFR (mGFR)280. This correlation is thought to be particularly insufficient at higher GFR ranges, where creatinine based estimations performs very poorly. Several researchers therefore advocate for mGFR in clinical trials as a way to improve precision and thereby reduce cost281. While small single center studies reported variable results282,283, investigation of the Chronic Renal Insufficiency Cohort (CRIC) cohort –a large multi-center trial that resembles most Phase 3 treatment studies282,284,285 - showed that one-time measures of mGFR do not have stronger cross–sectional associations with concurrent metabolic consequences of decreased renal function (such as hyperphosphatemia or hyperkalemia) than eGFR284, indicating that in this population mGFR is not a superior surrogate for CKD. Longitudinal measures of mGFR were also not more strongly associated with future clinical sequelae of decreased renal function than repeated measures of eGFR285. Considering the cost, the laborious nature of measuring GFR and the invasiveness of such techniques, mGFR should ideally provide superior prediction of clinically relevant CKD outcomes compared with estimated measures of renal function. However, iGFR did not outperform eGFR in this study or others. These results therefore do not appear to support the use of mGFR in order to improve kidney function change estimation, at GFR levels below 60 cc/min/1.73m2. Future studies might be needed to study this at higher GFR levels, however high GFR groups are not typically targeted for intervention trials.
While the data supporting the use of these intermediate surrogates is clear, the acceptability of these endpoints to regulatory agencies and payers has not yet been tested. Ultimately payers shoulder the disproportionate economic burden of CKD patients as they transition onto dialysis, however simply shifting the costs from dialysis (ESRD) to pre-dialysis care is not an attractive proposition. New therapies will not only need to demonstrate reduced ESRD events in the treated population, but that the patient number-needed-to-treat (NNT) to prevent one patient per year from going on to dialysis justifies the cost of that therapy. Perhaps the medical community has grown accustomed to accepting the wrong type of CKD therapeutic –one that slows disease, but does not remit disease.
Conclusions and future directions
The need for development of novel therapeutics for CKD development has never been greater. While mortality has been dropping from most disease conditions, it has actually increased for patients with chronic kidney disease. No new drug has been registered for the treatment of chronic kidney disease since 2001. However, recent research has identified several potential novel therapeutic targets, including inflammation, fibrosis, cellular metabolism, vascular and podocyte alterations, with some related agents already undergoing Phase II and III clinical trials.
At present, most renal clinical trials continue to enroll subjects with stage 3 CKD and a relatively high level of albuminuria, as these are subjects who will likely reach trial end-point (death, dialysis or doubling of serum creatinine) the fastest. The SONAR study is the only study that uses a more individualized approach and enriches for subjects that are more likely to respond to treatment and less likely to develop side effects77,266. The targeted approach is only slowly gaining popularity. For example, drugs that target metabolism would likely need to be introduced early, which is a difficult issue as these trials require large cohorts studied for long durations.188. Other strategies, such as targeting inflammation, are likely to be effective even in later stages of the disease267. Therapeutics that specifically target fibrosis but not albuminuria are especially challenging as clinically validated biomarkers linked to decreased fibrosis are not available to assess efficacy in short phase II studies
Once an individual drug exhibits clinical benefit by delaying the progression of functional decline, it will be critical to determine whether drug combination strategies could achieve not only prevention but remission. In this regard combining hemodynamic targeting with, metabolic targets, podocyte specific targets and an anti-inflammatory might provide benefits above and beyond single drug treatments.
Opportunities in the field of “genetic medicine” are also emerging in the treatment of kidney disease. Two genetic diseases for which this represents a promising approach are polycystic kidney disease (PKD) and kidney disease associated with a mutation in Apolipoprotein L1 (APOL1) (Box 3). Tailoring of specific therapy combinations towards these genetically defined diseases may help optimize efficacy and reduce the number of patients needed to treat.
Box 3: Genetic stratification or precision medicine: are the nephrons ready?
The “genetic revolution” has transformed several areas of medicine, of which oncology has been the most prominent. Large collaborative efforts such as The Cancer Genome Atlas (TCGA), is enabling the systematic analysis of the genetics, genomics, epigenetics and proteomics of all cancer types, collecting hundreds of patient samples and associated clinical information286.
Implementing such a collaborative team approach could be highly relevant for CKD, which is unlikely to be a homogenous disease and diagnosis is almost exclusively based on clinical description. Although kidney biopsy is not performed on everyone with CKD, the procedure appears to be increasingly safe and tissue samples are reasonably accessible.
Even though the genetic effect size is expected to be small in CKD, this does not mean that they do not highlight critically important and potentially targetable pathways. Two genetic diseases offer an important potential therapeutic opportunity in nephrology. The first is polycystic kidney disease (PKD), which is caused by a relatively common dominant mutation287. The mutation leads to epithelial cell proliferation and fluid secretion causing a cystic appearance of the kidney. Arginine vasopressin and its second messenger cAMP act as important promoters of cyst cell proliferation and fluid secretion into cysts288.Recent clinical studies indicate that treatment with tolvaptan, which is a vasopressin antagonist, shows some clinical benefit in patients with PKD289. The second is kidney disease associated with a mutation in Apolipoprotein L1 (APOL1). APOL1 mutation has a high allele frequency in West African population and its diaspora, including African Americans. People with 2 risk alleles have 3–100 times increased risk of developing CKD, indicating a strong effect size, further highlighting its clinical significance290. As of yet, studies were unable to demonstrate increased disease risk in patients with only single APOL1 risk allele, indicating a recessive mode of inheritance268,290. Although most recessive mutations are loss of function variants, people who have APOL1 null mutations are phenotypically normal and do present with kidney disease291, suggesting a recessive gain of function variant. While the mechanism of APOL1 induced cytotoxicity remains to be explored, we are presented with a unique therapeutic opportunity, whereby simply reducing APOL1 levels would likely be associated with therapeutic benefit. In this aspect it is important to understand the transcriptional regulation of APOL1. APOL1 appears to be part of the innate immune system and transcriptionally regulated by bacterial toxins and interferon292. As compounds that target those pathways are already in clinical development, some of these could potentially be repurposed for APOL1 associated kidney disease.
Cost remains a key limitation in the development of novel CKD therapies. Foremost among the development costs is the barriers to rapidly identifying and enrolling the appropriate patients into these trials. Patient registries and new trial designs may help address some of the issues discussed above, as well as validation of novel trial end-points. Adaptive clinical trial design - which evaluates patients' reactions to a drug beginning early in a clinical trial and modifies the trial in accord with those findings - might also be beneficial in CKD. Indeed, according to industry estimation this design can reduce the cost of entering a drug to the market to one third.
In summary, care of patients with CKD is emerging as one of most costly and rapidly increasing disease burdens facing society. Although drug development for CKD has lagged behind other therapeutic areas, recent activity in this area has increased, due in part to recognition of this unmet medical need. Nevertheless systemic engagement of the pharmaceutical industry has been lacking, partly due to lack of success, operational difficulties (e.g. slow patient enrollment) and long duration outcomes trials. Early patient centric discovery approaches and broad academic, industry and patient advocacy partnerships can provide avenues to enhance patient therapeutic response, patient/physician disease awareness, and improve clinical trial efficiencies. Integrated engagement of scientists and stakeholders across the spectrum of medical delivery and payer systems is achievable but critical to stem the tide of kidney disease in the twenty first century.
Acknowledgments
The work was supported by NIH R01 DK076077, DK087635 DK105821 and DP3 108220 to K. Susztak.
References
- 1.Collins AJ, Foley RN, Gilbertson DT, Chen SC. United States Renal Data System public health surveillance of chronic kidney disease and end-stage renal disease. Kidney Int Suppl (2011) 2015;5:2–7. doi: 10.1038/kisup.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kovesdy CP, et al. Outcomes associated with microalbuminuria: effect modification by chronic kidney disease. Journal of the American College of Cardiology. 2013;61:1626–1633. doi: 10.1016/j.jacc.2012.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foley RN, Collins AJ. The USRDS: what you need to know about what it can and can't tell us about ESRD. Clinical journal of the American Society of Nephrology : CJASN. 2013;8:845–851. doi: 10.2215/CJN.06840712. [DOI] [PubMed] [Google Scholar]
- 4.Weiner DE, et al. Kidney function and risk of cardiovascular disease and mortality in kidney transplant recipients: the FAVORIT trial. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12:2437–2445. doi: 10.1111/j.1600-6143.2012.04101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herzog CA, et al. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney international. 2011;80:572–586. doi: 10.1038/ki.2011.223. [DOI] [PubMed] [Google Scholar]
- 6.Foley RN, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. Journal of the American Society of Nephrology : JASN. 2005;16:489–495. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- 7.Coresh J, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. Jama. 2014;311:2518–2531. doi: 10.1001/jama.2014.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levey AS, et al. Proteinuria as a surrogate outcome in CKD: report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2009;54:205–226. doi: 10.1053/j.ajkd.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 9.Inker LA, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014;63:713–735. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- 10.Rahman M, et al. Association between chronic kidney disease progression and cardiovascular disease: results from the CRIC Study. American journal of nephrology. 2014;40:399–407. doi: 10.1159/000368915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. The Journal of clinical investigation. 2014;124:2299–2306. doi: 10.1172/JCI72267. Recent comprehensive review on fibrosis development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reidy K, Kang HM, Hostetter T, Susztak K. Molecular mechanisms of diabetic kidney disease. The Journal of clinical investigation. 2014;124:2333–2340. doi: 10.1172/JCI72271. Excellent review on molecular pathways that contribute to diabetic kidney disease development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou D, Liu Y. Renal fibrosis in 2015: Understanding the mechanisms of kidney fibrosis. Nature reviews. Nephrology. 2015 doi: 10.1038/nrneph.2015.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bielesz B, et al. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. The Journal of clinical investigation. 2010;120:4040–4054. doi: 10.1172/JCI43025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins DF, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. The Journal of clinical investigation. 2007;117:3810–3820. doi: 10.1172/JCI30487. Important paper describing the role of hypoxia inducible factor in kidney fibrosis development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Humphreys BD, et al. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. The Journal of clinical investigation. 2013;123:4023–4035. doi: 10.1172/JCI45361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woroniecka KI, et al. Transcriptome analysis of human diabetic kidney disease. Diabetes. 2011;60:2354–2369. doi: 10.2337/db10-1181. Comprehensive transcript prolifing dataset from a human control and diabetic kidney disease dataset. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou YH, et al. Steroids in the treatment of IgA nephropathy to the improvement of renal survival: a systematic review and meta-analysis. PloS one. 2011;6:e18788. doi: 10.1371/journal.pone.0018788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perico N, Benigni A, Remuzzi G. Present and future drug treatments for chronic kidney diseases: evolving targets in renoprotection. Nature reviews. Drug discovery. 2008;7:936–953. doi: 10.1038/nrd2685. [DOI] [PubMed] [Google Scholar]
- 20.Denker M, et al. Chronic Renal Insufficiency Cohort Study (CRIC): Overview and Summary of Selected Findings. Clinical journal of the American Society of Nephrology : CJASN. 2015;10:2073–2083. doi: 10.2215/CJN.04260415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simons JL, et al. Modulation of glomerular hypertension defines susceptibility to progressive glomerular injury. Kidney international. 1994;46:396–404. doi: 10.1038/ki.1994.287. [DOI] [PubMed] [Google Scholar]
- 22.Dunn BR, et al. Prevention of glomerular capillary hypertension in experimental diabetes mellitus obviates functional and structural glomerular injury. J Hypertens Suppl. 1986;4:S251–254. [PubMed] [Google Scholar]
- 23.Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Journal of the American Society of Nephrology : JASN. 2001;12:1315–1325. doi: 10.1681/ASN.V1261315. [DOI] [PubMed] [Google Scholar]
- 24.MacKinnon M, et al. Combination therapy with an angiotensin receptor blocker and an ACE inhibitor in proteinuric renal disease: a systematic review of the efficacy and safety data. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2006;48:8–20. doi: 10.1053/j.ajkd.2006.04.077. [DOI] [PubMed] [Google Scholar]
- 25.Hallan S, et al. Obesity, smoking, and physical inactivity as risk factors for CKD: are men more vulnerable? American journal of kidney diseases : the official journal of the National Kidney Foundation. 2006;47:396–405. doi: 10.1053/j.ajkd.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 26.Dobre M, Rahman M, Hostetter TH. Current status of bicarbonate in CKD. Journal of the American Society of Nephrology : JASN. 2015;26:515–523. doi: 10.1681/ASN.2014020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zatz R, et al. Prevention of diabetic glomerulopathy by pharmacological amelioration of glomerular capillary hypertension. The Journal of clinical investigation. 1986;77:1925–1930. doi: 10.1172/JCI112521. Important study defining the role of glomerular capillary hyperfiltration. Controlling capillary presssure can provide therapeutic benefit in controlling glomerulosclerosis development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. The New England journal of medicine. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 29.Maschio G, et al. Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. The New England journal of medicine. 1996;334:939–945. doi: 10.1056/NEJM199604113341502. [DOI] [PubMed] [Google Scholar]
- 30.Hou FF, et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. The New England journal of medicine. 2006;354:131–140. doi: 10.1056/NEJMoa053107. [DOI] [PubMed] [Google Scholar]
- 31.Lewis EJ, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. The New England journal of medicine. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 32.Brenner BM, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. The New England journal of medicine. 2001;345:861–869. doi: 10.1056/NEJMoa011161. Critical studies demonstrating the effectiveness of ARB in slowing kidney function decline. [DOI] [PubMed] [Google Scholar]
- 33.Parving HH, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. The New England journal of medicine. 2001;345:870–878. doi: 10.1056/NEJMoa011489. Critical studies demonstrating the effectiveness of ARB in slowing kidney function decline. [DOI] [PubMed] [Google Scholar]
- 34.Schoolwerth AC, et al. Renal considerations in angiotensin converting enzyme inhibitor therapy: a statement for healthcare professionals from the Council on the Kidney in Cardiovascular Disease and the Council for High Blood Pressure Research of the American Heart Association. Circulation. 2001;104:1985–1991. doi: 10.1161/hc4101.096153. [DOI] [PubMed] [Google Scholar]
- 35.de Zeeuw D, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney international. 2004;65:2309–2320. doi: 10.1111/j.1523-1755.2004.00653.x. [DOI] [PubMed] [Google Scholar]
- 36.Heerspink HJ. Therapeutic approaches in lowering albuminuria: travels along the renin-angiotensin-aldosterone-system pathway. Advances in chronic kidney disease. 2011;18:290–299. doi: 10.1053/j.ackd.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Fried LF, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. The New England journal of medicine. 2013;369:1892–1903. doi: 10.1056/NEJMoa1303154. [DOI] [PubMed] [Google Scholar]
- 38.Marquez DF, Ruiz-Hurtado G, Ruilope LM, Segura J. An update of the blockade of the renin angiotensin aldosterone system in clinical practice. Expert opinion on pharmacotherapy. 2015;16:2283–2292. doi: 10.1517/14656566.2015.1079623. [DOI] [PubMed] [Google Scholar]
- 39.Gregg EW, et al. Changes in diabetes-related complications in the United States, 1990–2010. The New England journal of medicine. 2014;370:1514–1523. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 40.Xie X, et al. Renin-Angiotensin System Inhibitors and Kidney and Cardiovascular Outcomes in Patients With CKD: A Bayesian Network Meta-analysis of Randomized Clinical Trials. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2015 doi: 10.1053/j.ajkd.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 41.Investigators O, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. The New England journal of medicine. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 42.Parving HH, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. The New England journal of medicine. 2012;367:2204–2213. doi: 10.1056/NEJMoa1208799. The effect of renin inhibitors on renal end-points. [DOI] [PubMed] [Google Scholar]
- 43.Pearce D, et al. Collecting duct principal cell transport processes and their regulation. Clinical journal of the American Society of Nephrology : CJASN. 2015;10:135–146. doi: 10.2215/CJN.05760513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Azibani F, Fazal L, Chatziantoniou C, Samuel JL, Delcayre C. Aldosterone mediates cardiac fibrosis in the setting of hypertension. Current hypertension reports. 2013;15:395–400. doi: 10.1007/s11906-013-0354-3. [DOI] [PubMed] [Google Scholar]
- 45.Azibani F, et al. Aldosterone inhibits antifibrotic factors in mouse hypertensive heart. Hypertension. 2012;59:1179–1187. doi: 10.1161/HYPERTENSIONAHA.111.190512. [DOI] [PubMed] [Google Scholar]
- 46.Ma J, et al. Plasminogen activator inhibitor-1 deficiency protects against aldosterone-induced glomerular injury. Kidney international. 2006;69:1064–1072. doi: 10.1038/sj.ki.5000201. [DOI] [PubMed] [Google Scholar]
- 47.Juurlink DN, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. The New England journal of medicine. 2004;351:543–551. doi: 10.1056/NEJMoa040135. [DOI] [PubMed] [Google Scholar]
- 48.Saito M, Takada M, Hirooka K, Isobe F, Yasumura Y. Serum concentration of potassium in chronic heart failure patients administered spironolactone plus furosemide and either enalapril maleate, losartan potassium or candesartan cilexetil. J Clin Pharm Ther. 2005;30:603–610. doi: 10.1111/j.1365-2710.2005.00694.x. [DOI] [PubMed] [Google Scholar]
- 49.Esteghamati A, et al. Long-term effects of addition of mineralocorticoid receptor antagonist to angiotensin II receptor blocker in patients with diabetic nephropathy: a randomized clinical trial. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2013;28:2823–2833. doi: 10.1093/ndt/gft281. [DOI] [PubMed] [Google Scholar]
- 50.Bakris GL, et al. Effect of Finerenone on Albuminuria in Patients With Diabetic Nephropathy: A Randomized Clinical Trial. Jama. 2015;314:884–894. doi: 10.1001/jama.2015.10081. [DOI] [PubMed] [Google Scholar]
- 51.Health, U. S. N. I. o. A Study to Evaluate Efficacy and Safety of CS-3150 in Japanese Type 2 Diabetic Subjects With Microalbuminuria. 2015 < https://clinicaltrials.gov/show/NCT02345057>.
- 52.Dessapt-Baradez C, et al. Targeted glomerular angiopoietin-1 therapy for early diabetic kidney disease. Journal of the American Society of Nephrology : JASN. 2014;25:33–42. doi: 10.1681/ASN.2012121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mogensen CE. Progression of nephropathy in long-term diabetics with proteinuria and effect of initial anti-hypertensive treatment. Scand J Clin Lab Invest. 1976;36:383–388. doi: 10.1080/00365517609055274. [DOI] [PubMed] [Google Scholar]
- 54.Mogensen CE. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. The New England journal of medicine. 1984;310:356–360. doi: 10.1056/NEJM198402093100605. [DOI] [PubMed] [Google Scholar]
- 55.Osterby R. Renal pathology in diabetes mellitus. Current opinion in nephrology and hypertension. 1993;2:475–483. [PubMed] [Google Scholar]
- 56.Osterby R, et al. Neovascularization at the vascular pole region in diabetic glomerulopathy. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1999;14:348–352. doi: 10.1093/ndt/14.2.348. [DOI] [PubMed] [Google Scholar]
- 57.Eremina V, et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. The Journal of clinical investigation. 2003;111:707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scott RP, Quaggin SE. Review series: The cell biology of renal filtration. The Journal of cell biology. 2015;209:199–210. doi: 10.1083/jcb.201410017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dane MJC, et al. A microscopic view on the renal endothelial glycocalyx. American Journal of Physiology - Renal Physiology. 2015;308:F956–F966. doi: 10.1152/ajprenal.00532.2014. [DOI] [PubMed] [Google Scholar]
- 60.Perrin RM, Harper SJ, Bates DO. A role for the endothelial glycocalyx in regulating microvascular permeability in diabetes mellitus. Cell Biochem Biophys. 2007;49:65–72. doi: 10.1007/s12013-007-0041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nieuwdorp M, et al. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes. 2006;55:1127–1132. doi: 10.2337/diabetes.55.04.06.db05-1619. [DOI] [PubMed] [Google Scholar]
- 62.Nussbaum C, et al. Early microvascular changes with loss of the glycocalyx in children with type 1 diabetes. The Journal of pediatrics. 2014;164:584–589. doi: 10.1016/j.jpeds.2013.11.016. e581. [DOI] [PubMed] [Google Scholar]
- 63.Heerspink HL, et al. Effects of sulodexide in patients with type 2 diabetes and persistent albuminuria. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2008;23:1946–1954. doi: 10.1093/ndt/gfm893. [DOI] [PubMed] [Google Scholar]
- 64.Kristova V, Liskova S, Sotnikova R, Vojtko R, Kurtansky A. Sulodexide improves endothelial dysfunction in streptozotocin-induced diabetes in rats. Physiol Res. 2008;57:491–494. doi: 10.33549/physiolres.931506. [DOI] [PubMed] [Google Scholar]
- 65.Lewis EJ, et al. Sulodexide for kidney protection in type 2 diabetes patients with microalbuminuria: a randomized controlled trial. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011;58:729–736. doi: 10.1053/j.ajkd.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 66.Basche M, et al. A phase I biological and pharmacologic study of the heparanase inhibitor PI-88 in patients with advanced solid tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:5471–5480. doi: 10.1158/1078-0432.CCR-05-2423. [DOI] [PubMed] [Google Scholar]
- 67.Barton M, Mullins JJ, Bailey MA, Kretzler M. Role of endothelin receptors for renal protection and survival in hypertension: waiting for clinical trials. Hypertension. 2006;48:834–837. doi: 10.1161/01.HYP.0000245138.09687.8a. [DOI] [PubMed] [Google Scholar]
- 68.Daehn I, et al. Endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis. The Journal of clinical investigation. 2014;124:1608–1621. doi: 10.1172/JCI71195. Important publication indicating the critical role of endothelial cells in glomerulosclerosis development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Zeeuw D, et al. The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. Journal of the American Society of Nephrology : JASN. 2014;25:1083–1093. doi: 10.1681/ASN.2013080830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saleh MA, Boesen EI, Pollock JS, Savin VJ, Pollock DM. Endothelin receptor A-specific stimulation of glomerular inflammation and injury in a streptozotocin-induced rat model of diabetes. Diabetologia. 2011;54:979–988. doi: 10.1007/s00125-010-2021-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saleh MA, Boesen EI, Pollock JS, Savin VJ, Pollock DM. Endothelin-1 increases glomerular permeability and inflammation independent of blood pressure in the rat. Hypertension. 2010;56:942–949. doi: 10.1161/HYPERTENSIONAHA.110.156570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kohan DE, Barton M. Endothelin and endothelin antagonists in chronic kidney disease. Kidney international. 2014;86:896–904. doi: 10.1038/ki.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boffa JJ, Tharaux PL, Dussaule JC, Chatziantoniou C. Regression of renal vascular fibrosis by endothelin receptor antagonism. Hypertension. 2001;37:490–496. doi: 10.1161/01.hyp.37.2.490. [DOI] [PubMed] [Google Scholar]
- 74.Hocher B, et al. Effects of endothelin receptor antagonists on the progression of diabetic nephropathy. Nephron. 2001;87:161–169. doi: 10.1159/000045906. [DOI] [PubMed] [Google Scholar]
- 75.Mann JF, et al. Avosentan for overt diabetic nephropathy. Journal of the American Society of Nephrology : JASN. 2010;21:527–535. doi: 10.1681/ASN.2009060593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ritz E, Wenzel RR. Endothelin antagonist as add-on treatment for proteinuria in diabetic nephropathy: is there light at the end of the tunnel? Journal of the American Society of Nephrology : JASN. 2011;22:593–595. doi: 10.1681/ASN.2011020158. [DOI] [PubMed] [Google Scholar]
- 77.Health, U. S. N. I. o. Study Of Diabetic Nephropathy With Atrasentan (SONAR) 2015 < https://clinicaltrials.gov/show/NCT01858532>.
- 78.Hodgin JB, et al. Identification of cross-species shared transcriptional networks of diabetic nephropathy in human and mouse glomeruli. Diabetes. 2013;62:299–308. doi: 10.2337/db11-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bohle A, et al. The pathogenesis of chronic renal failure in diabetic nephropathy. Investigation of 488 cases of diabetic glomerulosclerosis. Pathol Res Pract. 1991;187:251–259. doi: 10.1016/s0344-0338(11)80780-6. [DOI] [PubMed] [Google Scholar]
- 80.Berthier CC, et al. Enhanced expression of Janus kinase-signal transducer and activator of transcription pathway members in human diabetic nephropathy. Diabetes. 2009;58:469–477. doi: 10.2337/db08-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vlassara H, Brownlee M, Manogue KR, Dinarello CA, Pasagian A. Cachectin/TNF and IL-1 induced by glucose-modified proteins: role in normal tissue remodeling. Science. 1988;240:1546–1548. doi: 10.1126/science.3259727. [DOI] [PubMed] [Google Scholar]
- 82.Hasegawa G, et al. Possible role of tumor necrosis factor and interleukin-1 in the development of diabetic nephropathy. Kidney international. 1991;40:1007–1012. doi: 10.1038/ki.1991.308. [DOI] [PubMed] [Google Scholar]
- 83.Goldwich A, et al. Podocytes are nonhematopoietic professional antigen-presenting cells. Journal of the American Society of Nephrology : JASN. 2013;24:906–916. doi: 10.1681/ASN.2012020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Niewczas MA, et al. Serum concentrations of markers of TNFalpha and Fas-mediated pathways and renal function in nonproteinuric patients with type 1 diabetes. Clinical journal of the American Society of Nephrology : CJASN. 2009;4:62–70. doi: 10.2215/CJN.03010608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Niewczas MA, et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. Journal of the American Society of Nephrology : JASN. 2012;23:507–515. doi: 10.1681/ASN.2011060627. Recent publications indicating that TNFR1 and TNFR2 levels can predict nephropathy development in patients with diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hawari FI, et al. Release of full-length 55-kDa TNF receptor 1 in exosome-like vesicles: a mechanism for generation of soluble cytokine receptors. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:1297–1302. doi: 10.1073/pnas.0307981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tartaglia LA, Pennica D, Goeddel DV. Ligand passing: the 75-kDa tumor necrosis factor (TNF) receptor recruits TNF for signaling by the 55-kDa TNF receptor. The Journal of biological chemistry. 1993;268:18542–18548. [PubMed] [Google Scholar]
- 88.Pober JS, Min W, Bradley JR. Mechanisms of Endothelial Dysfunction, Injury, and Death. Annual Review of Pathology: Mechanisms of Disease. 2009;4:71–95. doi: 10.1146/annurev.pathol.4.110807.092155. [DOI] [PubMed] [Google Scholar]
- 89.Al-Lamki RS, et al. TNFR1- and TNFR2-mediated signaling pathways in human kidney are cell type-specific and differentially contribute to renal injury. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19:1637–1645. doi: 10.1096/fj.05-3841com. [DOI] [PubMed] [Google Scholar]
- 90.Tartaglia LA, Rothe M, Hu YF, Goeddel DV. Tumor necrosis factor's cytotoxic activity is signaled by the p55 TNF receptor. Cell. 1993;73:213–216. doi: 10.1016/0092-8674(93)90222-c. [DOI] [PubMed] [Google Scholar]
- 91.Godwin P, et al. Targeting nuclear factor-kappa B to overcome resistance to chemotherapy. Frontiers in oncology. 2013;3:120. doi: 10.3389/fonc.2013.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Al-Lamki RS, Mayadas TN. TNF receptors: signaling pathways and contribution to renal dysfunction. Kidney international. 2015;87:281–296. doi: 10.1038/ki.2014.285. [DOI] [PubMed] [Google Scholar]
- 93.Rastogi S, Rizwani W, Joshi B, Kunigal S, Chellappan SP. TNF-alpha response of vascular endothelial and vascular smooth muscle cells involve differential utilization of ASK1 kinase and p73. Cell death and differentiation. 2012;19:274–283. doi: 10.1038/cdd.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tsai CT, et al. TNF-alpha down-regulates sarcoplasmic reticulum Ca(2)(+) ATPase expression and leads to left ventricular diastolic dysfunction through binding of NF-kappaB to promoter response element. Cardiovascular research. 2015;105:318–329. doi: 10.1093/cvr/cvv008. [DOI] [PubMed] [Google Scholar]
- 95.Moriwaki Y, et al. Effect of TNF-alpha inhibition on urinary albumin excretion in experimental diabetic rats. Acta diabetologica. 2007;44:215–218. doi: 10.1007/s00592-007-0007-6. [DOI] [PubMed] [Google Scholar]
- 96.Matsushima K, Larsen CG, DuBois GC, Oppenheim JJ. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. The Journal of experimental medicine. 1989;169:1485–1490. doi: 10.1084/jem.169.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marisa C, et al. MCP-1 and MIP-2 expression and production in BB diabetic rat: effect of chronic hypoxia. Molecular and cellular biochemistry. 2005;276:105–111. doi: 10.1007/s11010-005-3556-4. [DOI] [PubMed] [Google Scholar]
- 99.Mine S, et al. Increased expression levels of monocyte CCR2 and monocyte chemoattractant protein-1 in patients with diabetes mellitus. Biochemical and biophysical research communications. 2006;344:780–785. doi: 10.1016/j.bbrc.2006.03.197. [DOI] [PubMed] [Google Scholar]
- 100.Wada T, et al. Up-regulation of monocyte chemoattractant protein-1 in tubulointerstitial lesions of human diabetic nephropathy. Kidney international. 2000;58:1492–1499. doi: 10.1046/j.1523-1755.2000.00311.x. [DOI] [PubMed] [Google Scholar]
- 101.Titan SM, et al. Urinary MCP-1 and RBP: independent predictors of renal outcome in macroalbuminuric diabetic nephropathy. Journal of diabetes and its complications. 2012;26:546–553. doi: 10.1016/j.jdiacomp.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 102.Seok SJ, et al. Blockade of CCL2/CCR2 signalling ameliorates diabetic nephropathy in db/db mice. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2013;28:1700–1710. doi: 10.1093/ndt/gfs555. [DOI] [PubMed] [Google Scholar]
- 103.Awad AS, et al. Monocyte/macrophage chemokine receptor CCR2 mediates diabetic renal injury. American journal of physiology. Renal physiology. 2011;301:F1358–1366. doi: 10.1152/ajprenal.00332.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Health, U. S. N. I. o. A Phase 2 Multi-Center Study To Evaluate The Efficacy And Safety Of A Chemokine CCR2/5 Receptor Antagonist In Adults With Type 2 Diabetes And Overt Nephropathy. 2014 < https://www.clinicaltrials.gov/ct2/show/NCT01712061>.
- 105.Health, U. S. N. I. o. A Study to Evaluate the Safety and Efficacy of CCX140-B in Subjects With Diabetic Nephropathy. 2014 < https://clinicaltrials.gov/show/NCT01447147>.
- 106.de Zeeuw D, et al. The effect of CCR2 inhibitor CCX140-B on residual albuminuria in patients with type 2 diabetes and nephropathy: a randomised trial. The lancet. Diabetes & endocrinology. 2015;2 doi: 10.1016/S2213-8587(15)00261-2. [DOI] [PubMed] [Google Scholar]
- 107.Berger S, Daha M. Complement in glomerular injury. Seminars in immunopathology. 2007;29:375–384. doi: 10.1007/s00281-007-0090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stark George R, Darnell James E., Jr The JAK-STAT Pathway at Twenty. Immunity. 2012;36:503–514. doi: 10.1016/j.immuni.2012.03.013. doi: http://dx.doi.org/10.1016/j.immuni.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thacker SG, et al. The detrimental effects of IFN-alpha on vasculogenesis in lupus are mediated by repression of IL-1 pathways: potential role in atherogenesis and renal vascular rarefaction. Journal of immunology. 2010;185:4457–4469. doi: 10.4049/jimmunol.1001782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Alexander JJ, et al. Mouse podocyte complement factor H: the functional analog to human complement receptor 1. Journal of the American Society of Nephrology : JASN. 2007;18:1157–1166. doi: 10.1681/ASN.2006101125. [DOI] [PubMed] [Google Scholar]
- 111.O'Shea John J, Plenge R. JAK and STAT Signaling Molecules in Immunoregulation and Immune-Mediated Disease. Immunity. 2012;36:542–550. doi: 10.1016/j.immuni.2012.03.014. doi: http://dx.doi.org/10.1016/j.immuni.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Durant L, et al. Diverse Targets of the Transcription Factor STAT3 Contribute to T Cell Pathogenicity and Homeostasis. Immunity. 2010;32:605–615. doi: 10.1016/j.immuni.2010.05.003. doi: http://dx.doi.org/10.1016/j.immuni.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sandborn WJ, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. The New England journal of medicine. 2012;367:616–624. doi: 10.1056/NEJMoa1112168. [DOI] [PubMed] [Google Scholar]
- 114.Vyas D, O'Dell KM, Bandy JL, Boyce EG. Tofacitinib: The First Janus Kinase (JAK) inhibitor for the treatment of rheumatoid arthritis. Ann Pharmacother. 2013;47:1524–1531. doi: 10.1177/1060028013512790. [DOI] [PubMed] [Google Scholar]
- 115.Neusser MA, et al. Human nephrosclerosis triggers a hypoxia-related glomerulopathy. The American journal of pathology. 2010;176:594–607. doi: 10.2353/ajpath.2010.090268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Banes AK, et al. Angiotensin II blockade prevents hyperglycemia-induced activation of JAK and STAT proteins in diabetic rat kidney glomeruli. American journal of physiology. Renal physiology. 2004;286:F653–659. doi: 10.1152/ajprenal.00163.2003. [DOI] [PubMed] [Google Scholar]
- 117.Marrero MB, Banes-Berceli AK, Stern DM, Eaton DC. Role of the JAK/STAT signaling pathway in diabetic nephropathy. American journal of physiology. Renal physiology. 2006;290:F762–768. doi: 10.1152/ajprenal.00181.2005. [DOI] [PubMed] [Google Scholar]
- 118.Fleischmann R, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. The New England journal of medicine. 2012;367:495–507. doi: 10.1056/NEJMoa1109071. [DOI] [PubMed] [Google Scholar]
- 119.Sawano A, et al. Flt-1, vascular endothelial growth factor receptor 1, is a novel cell surface marker for the lineage of monocyte-macrophages in humans. Blood. 2001;97:785–791. doi: 10.1182/blood.v97.3.785. [DOI] [PubMed] [Google Scholar]
- 120.Santulli G. Angiopoietin-like proteins: a comprehensive look. Frontiers in endocrinology. 2014;5 doi: 10.3389/fendo.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.TUTTLE KR, et al. Baricitinib in Diabetic Kidney Disease: Results from a Phase 2, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study. 75th annual meeting of the American Diabetes Association. 2015 114-LB — 2015. [Google Scholar]
- 122.Mathern DR, Heeger PS. Molecules Great and Small: The Complement System. Clinical journal of the American Society of Nephrology : CJASN. 2015;10:1636–1650. doi: 10.2215/CJN.06230614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rother RP, Rollins SA, Mojcik CF, Brodsky RA, Bell L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotech. 2007;25:1256–1264. doi: 10.1038/nbt1344. [DOI] [PubMed] [Google Scholar]
- 124.Licht C, et al. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney international. 2015;87:1061–1073. doi: 10.1038/ki.2014.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Flyvbjerg A. Diabetic angiopathy, the complement system and the tumor necrosis factor superfamily. Nat Rev Endocrinol. 2010;6:94–101. doi: 10.1038/nrendo.2009.266. [DOI] [PubMed] [Google Scholar]
- 126.Shao Z, Schwarz H. CD137 ligand, a member of the tumor necrosis factor family, regulates immune responses via reverse signal transduction. Journal of leukocyte biology. 2011;89:21–29. doi: 10.1189/jlb.0510315. [DOI] [PubMed] [Google Scholar]
- 127.Hansen HG, et al. Finding diabetic nephropathy biomarkers in the plasma peptidome by high-throughput magnetic bead processing and MALDI-TOF-MS analysis. Proteomics Clin Appl. 2010;4:697–705. doi: 10.1002/prca.200900169. [DOI] [PubMed] [Google Scholar]
- 128.Li L, et al. C3a Receptor Antagonist Ameliorates Inflammatory and Fibrotic Signals in Type 2 Diabetic Nephropathy by Suppressing the Activation of TGF-β/smad3 and IKBα Pathway. PloS one. 2014;9:e113639. doi: 10.1371/journal.pone.0113639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhao Y, Conze DB, Hanover JA, Ashwell JD. Tumor necrosis factor receptor 2 signaling induces selective c-IAP1-dependent ASK1 ubiquitination and terminates mitogen-activated protein kinase signaling. The Journal of biological chemistry. 2007;282:7777–7782. doi: 10.1074/jbc.M609146200. [DOI] [PubMed] [Google Scholar]
- 130.Zhang C, et al. TNF-alpha suppresses prolyl-4-hydroxylase alpha1 expression via the ASK1-JNK-NonO pathway. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:1760–1767. doi: 10.1161/ATVBAHA.107.144881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang M, et al. ASK1-JNK signaling cascade mediates Ad-ST13-induced apoptosis in colorectal HCT116 cells. Journal of cellular biochemistry. 2010;110:581–588. doi: 10.1002/jcb.22551. [DOI] [PubMed] [Google Scholar]
- 132.Nishitoh H, et al. ASK1 is essential for JNK/SAPK activation by TRAF2. Molecular cell. 1998;2:389–395. doi: 10.1016/s1097-2765(00)80283-x. [DOI] [PubMed] [Google Scholar]
- 133.Tesch GH, et al. ASK1 Inhibitor Halts Progression of Diabetic Nephropathy in Nos3-Deficient Mice. Diabetes. 2015;64:3903–3913. doi: 10.2337/db15-0384. [DOI] [PubMed] [Google Scholar]
- 134.Health, U. S. N. I. o. Efficacy, Safety, and Tolerability of GS-4997 in Participants With Diabetic Kidney Disease. 2015 < https://clinicaltrials.gov/ct2/show/NCT02177786?term=GS-4997&rank=3>.
- 135.Lin JH, Zhang JJ, Lin SL, Chertow GM. Design of a Phase 2 Clinical Trial of an ASK1 Inhibitor, GS-4997, in Patients with Diabetic Kidney Disease. Nephron. Experimental nephrology. 2015;129:29–33. doi: 10.1159/000369152. [DOI] [PubMed] [Google Scholar]
- 136.Ledo N, et al. Functional Genomic Annotation of Genetic Risk Loci Highlights Inflammation and Epithelial Biology Networks in CKD. Journal of the American Society of Nephrology : JASN. 2014 doi: 10.1681/ASN.2014010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Salmi M, Kalimo K, Jalkanen S. Induction and function of vascular adhesion protein-1 at sites of inflammation. The Journal of experimental medicine. 1993;178:2255–2260. doi: 10.1084/jem.178.6.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Smith DJ, et al. Cloning of Vascular Adhesion Protein 1 Reveals a Novel Multifunctional Adhesion Molecule. The Journal of experimental medicine. 1998;188:17–27. doi: 10.1084/jem.188.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Koskinen K, et al. Granulocyte transmigration through the endothelium is regulated by the oxidase activity of vascular adhesion protein-1 (VAP-1) Blood. 2004;103:3388–3395. doi: 10.1182/blood-2003-09-3275. [DOI] [PubMed] [Google Scholar]
- 140.Health, U. S. N. I. o. A Study to Evaluate ASP8232 as Add-On Therapy to Angiotensin Converting Enzyme Inhibitor (ACEi) or Angiotensin Receptor Blocker (ARB) in Reducing Albuminuria in Patients With Type 2 Diabetes and Chronic Kidney Disease (ALBUM) 2015 < https://clinicaltrials.gov/ct2/show/NCT02358096?term=NCT02358096&rank=1>.
- 141.Friedman SL, Sheppard D, Duffield JS, Violette S. Therapy for fibrotic diseases: nearing the starting line. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3004700. 167sr161. [DOI] [PubMed] [Google Scholar]
- 142.Huang S, Susztak K. Epithelial Plasticity versus EMT in Kidney Fibrosis. Trends in molecular medicine. 2015 doi: 10.1016/j.molmed.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kopp JB, et al. Transgenic mice with increased plasma levels of TGF-beta 1 develop progressive renal disease. Laboratory investigation; a journal of technical methods and pathology. 1996;74:991–1003. [PubMed] [Google Scholar]
- 144.Voelker JR, et al. American Society of Nephrology. Vol. 25. Philadelphia: 2014. Abstract Edition. [Google Scholar]
- 145.Varga J, Pasche B. Antitransforming growth factor-beta therapy in fibrosis: recent progress and implications for systemic sclerosis. Current opinion in rheumatology. 2008;20:720–728. doi: 10.1097/BOR.0b013e32830e48e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Porte J, Jenkins G. Assessment of the effect of potential antifibrotic compounds on total and alphaVbeta6 integrin-mediated TGF-beta activation. Pharmacol Res Perspect. 2014;2:e00030. doi: 10.1002/prp2.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Eberlein C, et al. A human monoclonal antibody 264RAD targeting alphavbeta6 integrin reduces tumour growth and metastasis, and modulates key biomarkers in vivo. Oncogene. 2013;32:4406–4416. doi: 10.1038/onc.2012.460. [DOI] [PubMed] [Google Scholar]
- 148.Karimi-Shah BA, Chowdhury BA. Forced vital capacity in idiopathic pulmonary fibrosis--FDA review of pirfenidone and nintedanib. The New England journal of medicine. 2015;372:1189–1191. doi: 10.1056/NEJMp1500526. [DOI] [PubMed] [Google Scholar]
- 149.Xiang XH, et al. Pirfenidone inhibits proliferation, arrests the cell cycle, and downregulates heat shock protein47 and collagen type I in rat hepatic stellate cells in vitro. Molecular medicine reports. 2015;12:309–314. doi: 10.3892/mmr.2015.3403. [DOI] [PubMed] [Google Scholar]
- 150.RamachandraRao SP, et al. Pirfenidone is renoprotective in diabetic kidney disease. Journal of the American Society of Nephrology : JASN. 2009;20:1765–1775. doi: 10.1681/ASN.2008090931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sharma K, et al. Pirfenidone for diabetic nephropathy. Journal of the American Society of Nephrology : JASN. 2011;22:1144–1151. doi: 10.1681/ASN.2010101049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pugliese G, Iacobini C, Blasetti Fantauzzi C, Menini S. The dark and bright side of atherosclerotic calcification. Atherosclerosis. 2015;238:220–230. doi: 10.1016/j.atherosclerosis.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 153.Drechsler C, et al. Galectin-3, Renal Function, and Clinical Outcomes: Results from the LURIC and 4D Studies. Journal of the American Society of Nephrology : JASN. 2015 doi: 10.1681/ASN.2014010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Matsumoto N, et al. A possible role of galectin-9 in the pulmonary fibrosis of patients with interstitial pneumonia. Lung. 2013;191:191–198. doi: 10.1007/s00408-012-9446-0. [DOI] [PubMed] [Google Scholar]
- 155.Tager AM, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nature medicine. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 156.Aikawa S, Hashimoto T, Kano K, Aoki J. Lysophosphatidic acid as a lipid mediator with multiple biological actions. Journal of biochemistry. 2015;157:81–89. doi: 10.1093/jb/mvu077. [DOI] [PubMed] [Google Scholar]
- 157.Castelino FV, et al. Amelioration of dermal fibrosis by genetic deletion or pharmacologic antagonism of lysophosphatidic acid receptor 1 in a mouse model of scleroderma. Arthritis and rheumatism. 2011;63:1405–1415. doi: 10.1002/art.30262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Llona-Minguez S, Ghassemian A, Helleday T. Lysophosphatidic acid receptor (LPAR) modulators: The current pharmacological toolbox. Progress in lipid research. 2015;58:51–75. doi: 10.1016/j.plipres.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 159.Shigeki S, Murakami T, Yata N, Ikuta Y. Treatment of keloid and hypertrophic scars by iontophoretic transdermal delivery of tranilast. Scand J Plast Reconstr Surg Hand Surg. 1997;31:151–158. doi: 10.3109/02844319709085482. [DOI] [PubMed] [Google Scholar]
- 160.Yamada H, Tajima S, Nishikawa T. Tranilast inhibits collagen synthesis in normal, scleroderma and keloid fibroblasts at a late passage culture but not at an early passage culture. J Dermatol Sci. 1995;9:45–47. doi: 10.1016/0923-1811(94)00355-i. [DOI] [PubMed] [Google Scholar]
- 161.Lau X, Zhang Y, Kelly DJ, Stapleton DI. Attenuation of Armanni-Ebstein lesions in a rat model of diabetes by a new anti-fibrotic, anti-inflammatory agent, FT011. Diabetologia. 2013;56:675–679. doi: 10.1007/s00125-012-2805-9. [DOI] [PubMed] [Google Scholar]
- 162.Gilbert RE, et al. A purpose-synthesised anti-fibrotic agent attenuates experimental kidney diseases in the rat. PloS one. 2012;7:e47160. doi: 10.1371/journal.pone.0047160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Decitre M, et al. Lysyl oxidase-like protein localizes to sites of de novo fibrinogenesis in fibrosis and in the early stromal reaction of ductal breast carcinomas. Laboratory investigation; a journal of technical methods and pathology. 1998;78:143–151. [PubMed] [Google Scholar]
- 164.Kim Y, Peyrol S, So CK, Boyd CD, Csiszar K. Coexpression of the lysyl oxidase-like gene (LOXL) and the gene encoding type III procollagen in induced liver fibrosis. Journal of cellular biochemistry. 1999;72:181–188. doi: 10.1002/(sici)1097-4644(19990201)72:2<181::aid-jcb3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 165.Van Bergen T, et al. The role of LOX and LOXL2 in scar formation after glaucoma surgery. Invest Ophthalmol Vis Sci. 2013;54:5788–5796. doi: 10.1167/iovs.13-11696. [DOI] [PubMed] [Google Scholar]
- 166.Yang T, et al. miR-29 mediates TGFbeta1-induced extracellular matrix synthesis through activation of PI3K–AKT pathway in human lung fibroblasts. Journal of cellular biochemistry. 2013;114:1336–1342. doi: 10.1002/jcb.24474. [DOI] [PubMed] [Google Scholar]
- 167.Qin W, et al. TGF-beta/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. Journal of the American Society of Nephrology : JASN. 2011;22:1462–1474. doi: 10.1681/ASN.2010121308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gomez IG, et al. Anti-microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. The Journal of clinical investigation. 2015;125:141–156. doi: 10.1172/JCI75852. Important paper demonstrating that miR21 interferes with metabolic changes in the kidney and could represent and new therapeutic target for CKD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Niranjan T, et al. The Notch pathway in podocytes plays a role in the development of glomerular disease. Nature medicine. 2008;14:290–298. doi: 10.1038/nm1731. First demonstration that developmental morphogens such as Notch plays a key role in kidney disease development. [DOI] [PubMed] [Google Scholar]
- 170.Kramann R, et al. Pharmacological GLI2 inhibition prevents myofibroblast cell-cycle progression and reduces kidney fibrosis. The Journal of clinical investigation. 2015 doi: 10.1172/JCI74929. Defines the critical role of hedgehog and Gli2 in interstitial fibroblasts. This could be a novel therapeutic target for CKD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sirin Y, Susztak K. Notch in the kidney: development and disease. The Journal of pathology. 2012;226:394–403. doi: 10.1002/path.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kawakami T, Ren S, Duffield JS. Wnt signalling in kidney diseases: dual roles in renal injury and repair. The Journal of pathology. 2013;229:221–231. doi: 10.1002/path.4121. [DOI] [PubMed] [Google Scholar]
- 173.Nathan DM, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. The New England journal of medicine. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Adler AI, et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64) Kidney international. 2003;63:225–232. doi: 10.1046/j.1523-1755.2003.00712.x. [DOI] [PubMed] [Google Scholar]
- 175.Susztak K, Raff AC, Schiffer M, Bottinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. One of the first papers indicating the critical role of podocytes and podocyte ROS in diabetic kidney disease development. [PubMed] [Google Scholar]
- 176.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 177.Shurraw S, et al. Association between glycemic control and adverse outcomes in people with diabetes mellitus and chronic kidney disease: a population-based cohort study. Archives of internal medicine. 2011;171:1920–1927. doi: 10.1001/archinternmed.2011.537. [DOI] [PubMed] [Google Scholar]
- 178.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. The New England journal of medicine. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. This paper demonstrates that patients with early strict glycemic control continue to have lower complications even after 25 years and establishes the concept of “metabolic memory”. [DOI] [PubMed] [Google Scholar]
- 179.Miao F, et al. Evaluating the role of epigenetic histone modifications in the metabolic memory of type 1 diabetes. Diabetes. 2014;63:1748–1762. doi: 10.2337/db13-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Susztak K. Understanding the epigenetic syntax for the genetic alphabet in the kidney. Journal of the American Society of Nephrology : JASN. 2014;25:10–17. doi: 10.1681/ASN.2013050461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.El-Osta A, et al. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. The Journal of experimental medicine. 2008;205:2409–2417. doi: 10.1084/jem.20081188. One of the first demonstrations that high glucose leads to persistent epigenetic changes, potentially providing the mechanistic basis for long term “memory” effect for metabolic alterations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ko YA, et al. Cytosine methylation changes in enhancer regions of core pro-fibrotic genes characterize kidney fibrosis development. Genome biology. 2013;14:R108. doi: 10.1186/gb-2013-14-10-r108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kang HM, et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nature medicine. 2015;21:37–46. doi: 10.1038/nm.3762. Combined systems biology and mechanistic studies showing the dysregulation of metabolic pathways in patients with CKD. They could provide a new therapeutic target for CKD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ly JP, et al. The Sweet Pee model for Sglt2 mutation. Journal of the American Society of Nephrology : JASN. 2011;22:113–123. doi: 10.1681/ASN.2010080888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yale JF, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab. 2014;16:1016–1027. doi: 10.1111/dom.12348. [DOI] [PubMed] [Google Scholar]
- 186.Zinman B, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. The New England journal of medicine. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. Reduction of cardiovascular disease in patients treated with SGLT2 inhibitors. [DOI] [PubMed] [Google Scholar]
- 187.Vallon V, et al. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Renal physiology. 2014;306:F194–204. doi: 10.1152/ajprenal.00520.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gembardt F, et al. The SGLT2 inhibitor empagliflozin ameliorates early features of diabetic nephropathy in BTBR ob/ob type 2 diabetic mice with and without hypertension. Renal physiology. 2014;307:F317–325. doi: 10.1152/ajprenal.00145.2014. The above 2 papers demonstrates that SGLT2 inhibitors reduce albuminuria and other features of diabetic kidney disease. [DOI] [PubMed] [Google Scholar]
- 189.Cherney DZ, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587–597. doi: 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
- 190.Yale JF, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab. 2014;16:1016–1027. doi: 10.1111/dom.12348. [DOI] [PubMed] [Google Scholar]
- 191.Health, U. S. N. I. o. Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy (CREDENCE) 2015 < https://clinicaltrials.gov/ct2/show/NCT02065791>.
- 192.Hallman TM, et al. The mitochondrial and kidney disease phenotypes of kd/kd mice under germfree conditions. Journal of autoimmunity. 2006;26:1–6. doi: 10.1016/j.jaut.2005.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Malaga-Dieguez L, Susztak K. ADCK4 “reenergizes” nephrotic syndrome. The Journal of clinical investigation. 2013;123:4996–4999. doi: 10.1172/JCI73168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang Z, et al. Regulation of renal lipid metabolism, lipid accumulation, and glomerulosclerosis in FVBdb/db mice with type 2 diabetes. Diabetes. 2005;54:2328–2335. doi: 10.2337/diabetes.54.8.2328. [DOI] [PubMed] [Google Scholar]
- 195.Li S, et al. Proximal tubule PPARalpha attenuates renal fibrosis and inflammation caused by unilateral ureteral obstruction. American journal of physiology. Renal physiology. 2013;305:F618–627. doi: 10.1152/ajprenal.00309.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Davis TM, et al. Effects of fenofibrate on renal function in patients with type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) Study. Diabetologia. 2011;54:280–290. doi: 10.1007/s00125-010-1951-1. [DOI] [PubMed] [Google Scholar]
- 197.Ting RD, et al. Benefits and safety of long-term fenofibrate therapy in people with type 2 diabetes and renal impairment: the FIELD Study. Diabetes care. 2012;35:218–225. doi: 10.2337/dc11-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cefalu WT, Watson K. Intensive glycemic control and cardiovascular disease observations from the ACCORD study: now what can a clinician possibly think? Diabetes. 2008;57:1163–1165. doi: 10.2337/db08-0220. [DOI] [PubMed] [Google Scholar]
- 199.Noto D, Cefalu AB, Averna MR. Beyond statins: new lipid lowering strategies to reduce cardiovascular risk. Current atherosclerosis reports. 2014;16:414. doi: 10.1007/s11883-014-0414-4. [DOI] [PubMed] [Google Scholar]
- 200.Han SH, et al. Deletion of Lkb1 in Renal Tubular Epithelial Cells Leads to CKD by Altering Metabolism. Journal of the American Society of Nephrology : JASN. 2015 doi: 10.1681/ASN.2014121181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Dugan LL, et al. AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. The Journal of clinical investigation. 2013;123:4888–4899. doi: 10.1172/JCI66218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Cancer Genome Atlas Research, N. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499:43–49. doi: 10.1038/nature12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Xu HF, et al. MicroRNA21 regulation of the progression of viral myocarditis to dilated cardiomyopathy. Molecular medicine reports. 2014;10:161–168. doi: 10.3892/mmr.2014.2205. [DOI] [PubMed] [Google Scholar]
- 204.Thum T, Lorenzen JM. Cardiac fibrosis revisited by microRNA therapeutics. Circulation. 2012;126:800–802. doi: 10.1161/CIRCULATIONAHA.112.125013. [DOI] [PubMed] [Google Scholar]
- 205.Chau BN, et al. MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003205. 121ra118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lai JY, et al. MicroRNA-21 in Glomerular Injury. Journal of the American Society of Nephrology : JASN. 2015;26:805–816. doi: 10.1681/ASN.2013121274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Nishikawa T, Edelstein D, Brownlee M. The missing link: a single unifying mechanism for diabetic complications . Kidney international supplement. 2000;77:S26–30. doi: 10.1046/j.1523-1755.2000.07705.x. [DOI] [PubMed] [Google Scholar]
- 208.Gorin Y, et al. Targeting NADPH oxidase with a novel dual Nox1/Nox4 inhibitor attenuates renal pathology in type 1 diabetes. American journal of physiology. Renal physiology. 2015 doi: 10.1152/ajprenal.00396.2014. ajprenal 00396 02014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Maalouf RM, et al. Nox4-derived reactive oxygen species mediate cardiomyocyte injury in early type 1 diabetes. American journal of physiology. Cell physiology. 2012;302:C597–604. doi: 10.1152/ajpcell.00331.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Aoyama T, et al. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology. 2012;56:2316–2327. doi: 10.1002/hep.25938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hecker L, et al. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3008182. 231ra247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lewis EJ, et al. Pyridorin in type 2 diabetic nephropathy. Journal of the American Society of Nephrology : JASN. 2012;23:131–136. doi: 10.1681/ASN.2011030272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Dwyer JP, et al. Pyridoxamine dihydrochloride in diabetic nephropathy (PIONEER-CSG-17): lessons learned from a pilot study. Nephron. 2015;129:22–28. doi: 10.1159/000369310. [DOI] [PubMed] [Google Scholar]
- 214.Bechtel W, et al. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nature medicine. 2010;16:544–550. doi: 10.1038/nm.2135. First paper demonstating that epigenetic changes mediate fibrosis development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Gilbert RE, et al. Histone deacetylase inhibition attenuates diabetes-associated kidney growth: potential role for epigenetic modification of the epidermal growth factor receptor. Kidney international. 2011;79:1312–1321. doi: 10.1038/ki.2011.39. [DOI] [PubMed] [Google Scholar]
- 216.Van Beneden K, et al. Valproic acid attenuates proteinuria and kidney injury. Journal of the American Society of Nephrology : JASN. 2011;22:1863–1875. doi: 10.1681/ASN.2010111196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kos CH, et al. Mice deficient in alpha-actinin-4 have severe glomerular disease. The Journal of clinical investigation. 2003;111:1683–1690. doi: 10.1172/JCI17988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiological reviews. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 219.Pavenstädt H, Kriz W, Kretzler M. Cell Biology of the Glomerular Podocyte. Physiological reviews. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 220.Ly J, Alexander M, Quaggin SE. A podocentric view of nephrology. Current opinion in nephrology and hypertension. 2004;13:299–305. doi: 10.1097/00041552-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 221.George B, et al. Crk1/2-dependent signaling is necessary for podocyte foot process spreading in mouse models of glomerular disease. The Journal of clinical investigation. 2012;122:674–692. doi: 10.1172/JCI60070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Tian X, et al. Podocyte-associated talin1 is critical for glomerular filtration barrier maintenance. The Journal of clinical investigation. 2014;124:1098–1113. doi: 10.1172/JCI69778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Soda K, et al. Role of dynamin, synaptojanin, and endophilin in podocyte foot processes. The Journal of clinical investigation. 2012;122:4401–4411. doi: 10.1172/JCI65289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Rutkowski JM, et al. Adiponectin promotes functional recovery after podocyte ablation. Journal of the American Society of Nephrology : JASN. 2013;24:268–282. doi: 10.1681/ASN.2012040414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wharram BL, et al. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. Journal of the American Society of Nephrology : JASN. 2005;16:2941–2952. doi: 10.1681/ASN.2005010055. [DOI] [PubMed] [Google Scholar]
- 226.Greaves P, Gribben JG. The role of B7 family molecules in hematologic malignancy. Blood. 2013;121:734–744. doi: 10.1182/blood-2012-10-385591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Yu CC, et al. Abatacept in B7-1-positive proteinuric kidney disease. The New England journal of medicine. 2013;369:2416–2423. doi: 10.1056/NEJMoa1304572. First paper demonstrating the therapeutic efficacy of Abatacept in patients with focal glomerulosclerosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Korhonen R, Moilanen E. Abatacept, a novel CD80/86-CD28 T cell co-stimulation modulator, in the treatment of rheumatoid arthritis. Basic & clinical pharmacology & toxicology. 2009;104:276–284. doi: 10.1111/j.1742-7843.2009.00375.x. [DOI] [PubMed] [Google Scholar]
- 229.Benigni A, Gagliardini E, Remuzzi G. Abatacept in B7-1-positive proteinuric kidney disease. The New England journal of medicine. 2014;370:1261–1263. doi: 10.1056/NEJMc1400502. [DOI] [PubMed] [Google Scholar]
- 230.Fiorina P, et al. Role of podocyte B7-1 in diabetic nephropathy. Journal of the American Society of Nephrology : JASN. 2014;25:1415–1429. doi: 10.1681/ASN.2013050518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Schiffer M, et al. Pharmacological targeting of actin-dependent dynamin oligomerization ameliorates chronic kidney disease in diverse animal models. Nature medicine. 2015 doi: 10.1038/nm.3843. This paper demonstrates that targeting the actin dependent dynamin oligomerization in podocytes provided a new potential avenue to treat proteinuria and glomerulosclerosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Maile LA, et al. Insulin-Like Growth Factor-I Signaling in Smooth Muscle Cells Is Regulated by Ligand Binding to the 177CYDMKTTC184 Sequence of the β3-Subunit of αVβ3. Molecular endocrinology. 2006;20:405–413. doi: 10.1210/me.2005-0241. [DOI] [PubMed] [Google Scholar]
- 233.Maile LA, et al. A Monoclonal Antibody Against αVβ3 Integrin Inhibits Development of Atherosclerotic Lesions in Diabetic Pigs. Science Translational Medicine. 2010;2 doi: 10.1126/scitranslmed.3000476. 18ra11-18ra11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Maile LA, et al. Blocking aVb3 Integrin Ligand Occupancy Inhibits the Progression of Albuminuria in Diabetic Rats. Journal of Diabetes Research. 2014;2014:10. doi: 10.1155/2014/421827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Health, U. S. N. I. o. Phase 2 Study to Evaluate Safety & Efficacy of VPI-2690B in Diabetic Nephropathy Patients. 2015 < https://clinicaltrials.gov/ct2/show/NCT02251067?term=NCT02251067&rank=1>.
- 236.Afsar B, et al. Phosphodiesterase type 5 inhibitors and kidney disease. International urology and nephrology. 2015;47:1521–1528. doi: 10.1007/s11255-015-1071-4. [DOI] [PubMed] [Google Scholar]
- 237.Crawford A, et al. Molecular and Transcriptional Basis of CD4+ T Cell Dysfunction during Chronic Infection. Immunity. 2014;40:289–302. doi: 10.1016/j.immuni.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Takata Y, et al. PPARdelta-mediated antiinflammatory mechanisms inhibit angiotensin II-accelerated atherosclerosis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4277–4282. doi: 10.1073/pnas.0708647105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ito H, et al. Hyperuricemia is independently associated with coronary heart disease and renal dysfunction in patients with type 2 diabetes mellitus. PloS one. 2011;6:e27817. doi: 10.1371/journal.pone.0027817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Goicoechea M, et al. Allopurinol and Progression of CKD and Cardiovascular Events: Long-term Follow-up of a Randomized Clinical Trial. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2015;65:543–549. doi: 10.1053/j.ajkd.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 241.Goicoechea M, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clinical journal of the American Society of Nephrology : CJASN. 2010;5:1388–1393. doi: 10.2215/CJN.01580210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2006;47:51–59. doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 243.Maahs DM, et al. Uric acid lowering to prevent kidney function loss in diabetes: the preventing early renal function loss (PERL) allopurinol study. Current diabetes reports. 2013;13:550–559. doi: 10.1007/s11892-013-0381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Nath KA, Hostetter MK, Hostetter TH. Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3. The Journal of clinical investigation. 1985;76:667–675. doi: 10.1172/JCI112020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Dobre M, et al. Persistent high serum bicarbonate and the risk of heart failure in patients with chronic kidney disease (CKD): A report from the Chronic Renal Insufficiency Cohort (CRIC) study. Journal of the American Heart Association. 2015;4 doi: 10.1161/JAHA.114.001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Strippoli GF, Craig JC, Schena FP. The number, quality, and coverage of randomized controlled trials in nephrology. Journal of the American Society of Nephrology : JASN. 2004;15:411–419. doi: 10.1097/01.asn.0000100125.21491.46. [DOI] [PubMed] [Google Scholar]
- 247.Szczech LA, et al. Primary care detection of chronic kidney disease in adults with type-2 diabetes: the ADD-CKD Study (awareness, detection and drug therapy in type 2 diabetes and chronic kidney disease) PloS one. 2014;9:e110535. doi: 10.1371/journal.pone.0110535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Tuot DS, et al. The Kidney Awareness Registry and Education (KARE) study: protocol of a randomized controlled trial to enhance provider and patient engagement with chronic kidney disease. BMC nephrology. 2015;16:166. doi: 10.1186/s12882-015-0168-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Nitta K, Okada K, Yanai M, Takahashi S. The Kidney Early Evaluation Program (KEEP) of Japan. Clin Nephrol. 2015;83:52–55. doi: 10.5414/cnp83s052. [DOI] [PubMed] [Google Scholar]
- 250.Narva AS, Norton JM, Boulware LE. Educating Patients about CKD: The Path to Self-Management and Patient-Centered Care. Clinical journal of the American Society of Nephrology : CJASN. 2015 doi: 10.2215/CJN.07680715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Sutton AJ, et al. Methods Used in Economic Evaluations of Chronic Kidney Disease Testing - A Systematic Review. PloS one. 2015;10:e0140063. doi: 10.1371/journal.pone.0140063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Fox CH, et al. Improving evidence-based primary care for chronic kidney disease: study protocol for a cluster randomized control trial for translating evidence into practice (TRANSLATE CKD) Implementation science : IS. 2013;8:88. doi: 10.1186/1748-5908-8-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Levin A, Stevens PE, Coresh J, Levey A. Screening, monitoring, and treatment of stage 1 to 3 chronic kidney disease. Annals of internal medicine. 2014;161:81–82. doi: 10.7326/L14-5013-2. [DOI] [PubMed] [Google Scholar]
- 254.Hemmelgarn BR, et al. Nephrology visits and health care resource use before and after reporting estimated glomerular filtration rate. Jama. 2010;303:1151–1158. doi: 10.1001/jama.2010.303. [DOI] [PubMed] [Google Scholar]
- 255.Hemmelgarn BR, et al. Relation between kidney function, proteinuria, and adverse outcomes. Jama. 2010;303:423–429. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- 256.Zhang Z, et al. Importance of baseline distribution of proteinuria in renal outcomes trials: lessons from the reduction of endpoints in NIDDM with the angiotensin II antagonist losartan (RENAAL) study. Journal of the American Society of Nephrology : JASN. 2005;16:1775–1780. doi: 10.1681/ASN.2004080632. [DOI] [PubMed] [Google Scholar]
- 257.Eijkelkamp WB, et al. Albuminuria is a target for renoprotective therapy independent from blood pressure in patients with type 2 diabetic nephropathy: post hoc analysis from the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial. Journal of the American Society of Nephrology : JASN. 2007;18:1540–1546. doi: 10.1681/ASN.2006050445. [DOI] [PubMed] [Google Scholar]
- 258.Holtkamp FA, et al. Albuminuria and blood pressure, independent targets for cardioprotective therapy in patients with diabetes and nephropathy: a post hoc analysis of the combined RENAAL and IDNT trials. European heart journal. 2011;32:1493–1499. doi: 10.1093/eurheartj/ehr017. [DOI] [PubMed] [Google Scholar]
- 259.Chou YH, et al. Clinical outcomes and predictors for ESRD and mortality in primary GN. Clinical journal of the American Society of Nephrology : CJASN. 2012;7:1401–1408. doi: 10.2215/CJN.04500511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Perkins BA, et al. Regression of microalbuminuria in type 1 diabetes. The New England journal of medicine. 2003;348:2285–2293. doi: 10.1056/NEJMoa021835. [DOI] [PubMed] [Google Scholar]
- 261.Levey AS, et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014;64:821–835. doi: 10.1053/j.ajkd.2014.07.030. Statistical comparison of 50% reduction in kidney function with smaller changes in kidney function. [DOI] [PubMed] [Google Scholar]
- 262.Inker LA, et al. GFR decline as an alternative end point to kidney failure in clinical trials: a meta-analysis of treatment effects from 37 randomized trials. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014;64:848–859. doi: 10.1053/j.ajkd.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 263.Lambers Heerspink HJ, et al. GFR decline and subsequent risk of established kidney outcomes: a meta-analysis of 37 randomized controlled trials. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014;64:860–866. doi: 10.1053/j.ajkd.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 264.Lambers Heerspink HJ, et al. Estimated GFR decline as a surrogate end point for kidney failure: a post hoc analysis from the Reduction of End Points in Non-Insulin-Dependent Diabetes With the Angiotensin II Antagonist Losartan (RENAAL) study and Irbesartan Diabetic Nephropathy Trial (IDNT) American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014;63:244–250. doi: 10.1053/j.ajkd.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 265.Greene T, et al. Utility and validity of estimated GFR-based surrogate time-to-event end points in CKD: a simulation study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014;64:867–879. doi: 10.1053/j.ajkd.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 266.Schievink B, et al. Prediction of the effect of atrasentan on renal and heart failure outcomes based on short-term changes in multiple risk markers. Eur J Prev Cardiol. 2015 doi: 10.1177/2047487315598709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Figarola JL, Shanmugam N, Natarajan R, Rahbar S. Anti-inflammatory effects of the advanced glycation end product inhibitor LR-90 in human monocytes. Diabetes. 2007;56:647–655. doi: 10.2337/db06-0936. [DOI] [PubMed] [Google Scholar]
- 268.Parsa A, et al. APOL1 risk variants, race, and progression of chronic kidney disease. The New England journal of medicine. 2013;369:2183–2196. doi: 10.1056/NEJMoa1310345. Important paper showing that patients with G1 and G2 APOL1 variants have faster functional decline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Chapter 1: Definition and classification of CKD. Kidney Int Suppl (2011) 2013;3:19–62. doi: 10.1038/kisup.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Tangri N, et al. A predictive model for progression of chronic kidney disease to kidney failure. Jama. 2011;305:1553–1559. doi: 10.1001/jama.2011.451. Critial paper showing that only 4–6 variables can predict future kidney function decline with high precision. [DOI] [PubMed] [Google Scholar]
- 271.Landray MJ, et al. Prediction of ESRD and death among people with CKD: the Chronic Renal Impairment in Birmingham (CRIB) prospective cohort study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2010;56:1082–1094. doi: 10.1053/j.ajkd.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Gohda T, et al. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. Journal of the American Society of Nephrology : JASN. 2012;23:516–524. doi: 10.1681/ASN.2011060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Sonoda Y, et al. Circulating TNF receptors 1 and 2 are associated with the severity of renal interstitial fibrosis in IgA nephropathy. PloS one. 2015;10:e0122212. doi: 10.1371/journal.pone.0122212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Lee SM, et al. Circulating TNF receptors are significant prognostic biomarkers for idiopathic membranous nephropathy. PloS one. 2014;9:e104354. doi: 10.1371/journal.pone.0104354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Sabbisetti VS, et al. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. Journal of the American Society of Nephrology : JASN. 2014;25:2177–2186. doi: 10.1681/ASN.2013070758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Fufaa GD, et al. Association of urinary KIM-1, L-FABP, NAG and NGAL with incident end-stage renal disease and mortality in American Indians with type 2 diabetes mellitus. Diabetologia. 2015;58:188–198. doi: 10.1007/s00125-014-3389-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Kwon SH, et al. KIM-1 expression predicts renal outcomes in IgA nephropathy. Clinical and experimental nephrology. 2013;17:359–364. doi: 10.1007/s10157-012-0707-2. [DOI] [PubMed] [Google Scholar]
- 278.Hosohata K, et al. Urinary Kim-1 is a sensitive biomarker for the early stage of diabetic nephropathy in Otsuka Long-Evans Tokushima Fatty rats. Diabetes & vascular disease research : official journal of the International Society of Diabetes and Vascular Disease. 2014;11:243–250. doi: 10.1177/1479164114531299. [DOI] [PubMed] [Google Scholar]
- 279.Nielsen SE, et al. The effect of RAAS blockade on markers of renal tubular damage in diabetic nephropathy: u-NGAL, u-KIM1 and u-LFABP. Scand J Clin Lab Invest. 2012;72:137–142. doi: 10.3109/00365513.2011.645055. [DOI] [PubMed] [Google Scholar]
- 280.Budoff MJ, et al. Relationship of estimated GFR and coronary artery calcification in the CRIC (Chronic Renal Insufficiency Cohort) Study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011;58:519–526. doi: 10.1053/j.ajkd.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Hsu CY, Bansal N. Measured GFR as "gold standard"–all that glitters is not gold? Clinical journal of the American Society of Nephrology : CJASN. 2011;6:1813–1814. doi: 10.2215/CJN.06040611. [DOI] [PubMed] [Google Scholar]
- 282.Foster MC, et al. Filtration Markers as Predictors of ESRD and Mortality in Southwestern American Indians With Type 2 Diabetes. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2015 doi: 10.1053/j.ajkd.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Lafayette RA, Lai G. Examining chronic kidney disease management in a single center. Clin Nephrol. 2004;62:260–266. doi: 10.5414/cnp62260. [DOI] [PubMed] [Google Scholar]
- 284.Hsu CY, et al. Measured GFR does not outperform estimated GFR in predicting CKD-related complications. Journal of the American Society of Nephrology : JASN. 2011;22:1931–1937. doi: 10.1681/ASN.2010101077. Important paper showing that measured GFR does not outperform estimated GFR in large cohorts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Ku E, et al. Change in Measured GFR Versus eGFR and CKD Outcomes. Journal of the American Society of Nephrology : JASN. 2015 doi: 10.1681/ASN.2015040341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Davis CF, et al. The somatic genomic landscape of chromophobe renal cell carcinoma. Cancer cell. 2014;26:319–330. doi: 10.1016/j.ccr.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Antignac C, et al. The Future of Polycystic Kidney Disease Research-As Seen By the 12 Kaplan Awardees. Journal of the American Society of Nephrology : JASN. 2015 doi: 10.1681/ASN.2014121192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Hopp K, et al. Tolvaptan plus pasireotide shows enhanced efficacy in a PKD1 model. Journal of the American Society of Nephrology : JASN. 2015;26:39–47. doi: 10.1681/ASN.2013121312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Patel V, Chowdhury R, Igarashi P. Advances in the pathogenesis and treatment of polycystic kidney disease. Current opinion in nephrology and hypertension. 2009;18:99–106. doi: 10.1097/MNH.0b013e3283262ab0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Genovese G, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. This paper shows that coding variants (G1 and G2) in APOL1 is associated with kidney disease development. Due to a positive selection pressure these variants are present with high allele frequency amongst people with West African descent. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Johnstone DB, et al. APOL1 null alleles from a rural village in India do not correlate with glomerulosclerosis. PloS one. 2012;7:e51546. doi: 10.1371/journal.pone.0051546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Nichols B, et al. Innate immunity pathways regulate the nephropathy gene Apolipoprotein L1. Kidney international. 2015;87:332–342. doi: 10.1038/ki.2014.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Health, U. S. N. I. o. Pyridorin in Diabetic Nephropathy (PIONEER) 2015 < https://clinicaltrials.gov/ct2/show/NCT02156843?term=NCT02156843&rank=1>.
- 294.Health, U. S. N. I. o. A Study to Test Safety and Efficacy of Baricitinib in Participants With Diabetic Kidney Disease. 2015 < https://clinicaltrials.gov/show/NCT01683409>.
- 295.de Zeeuw D, et al. CCR2 inhibitor CCX140 effective in phase 2 clinical trial in diabetic nephropathy. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2015;30(suppl 3):iii531. [Google Scholar]
- 296.Health, U. S. N. I. o. A Phase 2 Study to Evaluate the Safety and Efficacy of CTP-499 in Type 2 Diabetic Nephropathy Patients. 2015 < https://clinicaltrials.gov/ct2/show/NCT01487109?term=NCT01487109&rank=1>.
- 297.Health, U. S. N. I. o. Safety and Efficacy of Different Oral Doses of BAY94-8862 in Subjects With Type 2 Diabetes Mellitus and the Clinical Diagnosis of Diabetic Nephropathy (ARTS-DN) 2014 < https://clinicaltrials.gov/show/NCT01874431>.
- 298.Ruilope LM, et al. Rationale, design, and baseline characteristics of ARTS-DN: a randomized study to assess the safety and efficacy of finerenone in patients with type 2 diabetes mellitus and a clinical diagnosis of diabetic nephropathy. American journal of nephrology. 2014;40:572–581. doi: 10.1159/000371497. [DOI] [PubMed] [Google Scholar]
- 299.Health, U. S. N. I. o. Safety and Efficacy of Oral GKT1377831 in Patient with Type 2 diabetes and Albuminuria. 2015 < https://clinicaltrials.gov/ct2/show/NCT02010242?term=NCT02010242&rank=1>.