Abstract
Introduction:
Serum uric acid (SUA) is the final product of purine metabolism in humans.
Aim:
The present study aimed to identify a potential association between serum UA and cardiac troponin I (cTnI) levels and to find out whether uric acid could differentiate patients presenting with the acute myocardial infarction (AMI) and unstable angina pectoris (UAP) in hyperuricemic and normouricemic acute coronary syndrome (ACS) patients.
Methods:
Eighty ACS patients, aged 50-83 years, were enrolled in the study, 40 of them presenting with AMI and 40 with UAP. Frequency of patients with serum uric level over threshold for hyperuricemia was investigated and two groups of patients were formed such as hyperuricemic and normouricemic groups (A and B groups, respectively) independently of type of ACS. Those groups of patients were also subjected to cTnI measurement.
Results:
Levels of SUA are associated with the type of ACS in the hyperuricemic ACS patients (AMI versus UAP, 499(458-590), 425(400-447) mmol/L, p=0.007, respectively). Uric acid correlated significantly with cTnI, moderate positively in the group A (rho=0.358, p=0.038) and moderate negatively in the group B (r=-0.309, p=0.037) of ACS patients. Multiple logistic regression analysis revealed that cTnI and age were independently associated with the SUA levels in the group A of ACS patients.
Conclusions:
Serum uric acid differentiates AIM and UAP patients in hyperuricemic group of acute coronary syndrome. Therefore it can be used as nonspecific parameter for evaluation of the myocardial lesion extent only in hyperuricemic ACS patients. This is supported by finding that cTnI along with age predicts SUA level in hyperuricemic ACS patients.
Keywords: angina, unstable, myocardial infarction, uric acid
1. INTRODUCTION
Acute coronary syndrome (ACS) as a complex syndrome with a heterogeneous etiology consists of a group of clinical symptoms caused by acute myocardial ischemia (1). The most common cause of the ACS is atherosclerotic coronary artery disease. The highly sensitive and specific cardiac troponin (cTn) is the preferred biomarker of myocardial necrosis according to the Third Universal Definition of Myocardial Infarction expert consensus document, published in October 2012 by the global Myocardial Infarction Task Force (2). Uric acid in serum is the final product of purine metabolism in the body produced by action of xanthine oxidase. This metabolic pathway is source of production of oxygen-free radicals (3). Several recent studies supported the association between serum uric acid (SUA) and cardiovascular diseases (CVDs) (4-8). Increased SUA concentrations are associated with hypertension, atherogenic dyslipidemia, diabetes, age, alcohol consumption and renal failure. All of these conditions predispose to atherosclerosis. On the other hand, SUA may be proatherogenic per se (9). According to study of Karim and associates, hyperuricemia represents a risk factor for major adverse cardiac events in ACS patients (10). As serum uric acid levels are in relation with cardiac dysfunction we have hypothesized that SUA is produced in a greater or less amount as result of myocardial necrosis or ischemia.
2. AIM
In order to test our hypothesis, this study aim was to identify a potential association between SUA and cardiac troponin I (cTnI) levels as gold standard for diagnosis of ACS and to find out whether SUA could differentiate patients presenting with the acute myocardial infarction (AMI) and unstable angina pectoris (UAP) in hyperuricemic and normouricemic patients.
3. PATIENTS AND METHODS
Patients with ACS, attending the Intensive Care Unit of Cardiology Clinic, Clinical Centre University of Sarajevo were included in this observational study. The data were collected using patients’ medical charts and the hospital investigation database. Patients were required to have had evidence of diagnosis of ACS on the basis of ECG changes, clinical symptoms and elevated levels of serum cTnI to be eligible for inclusion. Information with respect to smoking status and pre-existing diabetes mellitus, hypertension and dyslipidemia were obtained. Patients with stable angina pectoris, malignant, liver and kidneys diseases, acute or chronic systematic inflammatory diseases, infectious or septic states, patients treated with allopurinol and chronic alcohol consumption were excluded from the study. The final sample size included 80 participants of both genders (40 males, 40 females), aged 50-83 years and consisted of 40 AMI and 40 UAP patients. According to the SUA values, ACS patients were distributed into two groups: group A represented patients with hyperuricemia and group B represented patients with normouricemia (uric acid cut of for males 416 mmol/L and 357 mmol/L for women)(11).
Peripheral venous blood samples for biochemical analyses were obtained from patients within 48 hours since the hospital admission. Blood samples were centrifuged and serum analyzed at the Clinical Chemistry and Biochemistry, Clinical Centre University of Sarajevo. The patient’s serum cTnI were measured by AxSYM Troponin-I ADV Immunoassay using AxSym analyzer (Abbott Laboratories, Abbott Park, IL, USA). Spectrophotometric method for SUA measurements for all participants was used for analysis on Dimension Xpand Plus (Siemens, Munich, Germany). Standardization, calibration of instruments and processing of samples were done according to manufacturer’s instructions. The study was performed according to the principles outlined in the Declaration of Helsinki. This investigation was approved by Faculty of Medicine, University of Sarajevo, Bosnia and Herzegovina.
Statistical analysis
Normality of distribution of numerical variables analyzed by Shapiro-Wilk test was not satisfied, so values were presented by median with interquartile range (25-75 percentiles). Categorical variables were presented as frequencies and percentages. Statistical significances were calculated using Mann-Withney U and χ2 test. The degree of correlations was examined by the test according to Spearman and Pearson. Multiple linear regression was used to assess the combined association between all independent variables and SUA, as dependent variable. P values <0.05 were considered statistically significant. All statistical tests were performed using SPSS software version 19.0 (SPSS, Inc., Chicago, IL, USA).
4. RESULTS
A total of 80 ACS patients fulfilling the selection criteria included 40 (out of 80, 50%) females and 40 (out of 80, 50%) males. Table 1 describes the demographic characteristics and risk factors for CVDs frequency distributions in ACS patients enrolled in the study.
Table 1.
Population characteristics. ACS- acute coronary syndrome; n- subjects’ number; %–percentage
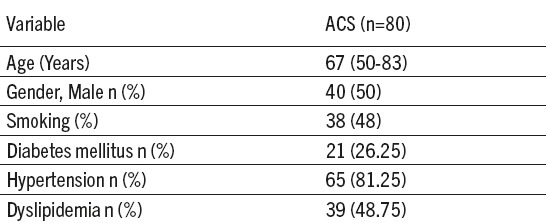
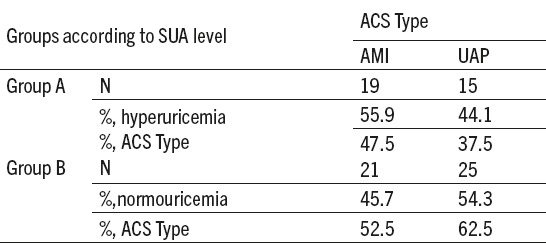
Highest frequency (40%) of ACS patients was with two, out of four listed CVDs risk factors and lowest (6.25%), without any of these. There were 7.50% patients who had all of the listed CVDs risk factors. Of the total number of ACS patients, according to their SUA levels, 34 (out of 80, 42.5%) were in the group A and 46 (out of 80, 57.5%) in the group B. Hyperuricemic AMI patients were more numerous than UAP but insignificantly more patients were diagnosed as UAP in normouremic ACS group (Table 2). No difference in frequency of hyperuricemic and normouricemic ACS subjects depending on type of ACS was found (χ2 (1, n=80)=0.460, p=0.5).
Table 2.
Frequency of hyperuricemic and normouricemic subjects depending on the type of acute coronary syndrome (ACS). SUA-serum uric acid level; ACS- acute coronary syndrome; AMI- acute myocardial infarction; UAP- unstable angina pectoris; Group A-hyperuricemic acute coronary syndrome patients; Group B-noromouricemic acute coronary syndrome patients; n-subjects’ number. %–percentage
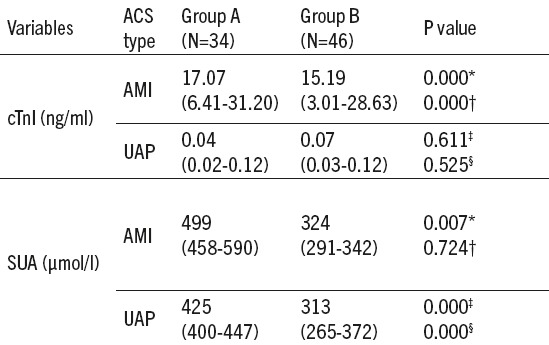
Median level of SUA in the ACS patients was 364 (312-437.75) µmol/L and significant difference between SUA values depending on the type of ACS (AMI patients (357.50 (323.25-498.25) µmol/L, UAP patients (379 (290.75-414.50)) was not found (p=0.118). Serum cardiac troponin I of ACS patients was 0,33(0,06-18,84) with significant difference in level of AMI and UAP patients (16.22 (4.38-29.97) vs 0.06 (0.027-0.12) ng/m; p<0.01). No difference was found between median values of cTnI in the group A (4.61 (0.04-24.27) ng/mL) and the group B (0.20 (0.063-12.86) ng/mL), p=0.430.
We found significant differences in cTnI values between AMI and UAP patients both in the group A and in the group B (p<0.01). Value of SUA was higher in AMI compared to UAP patients (p=0.007) in the group A, while, in the group B, that difference was not significant (p=0.724). Regarding SUA status, no difference in serum levels of cTnI in AIM and UAP groups was found (p=0.611 and p=0.525, respectively). Values of SUA both in AIM and UAP patients were significantly higher in the group A compared to the group B (p<0.01) (Table 3).
Table 3.
Serum values of cardiac troponin I (cTnI) and uric acid in hyperuricemic and normouricemic subjects according to the type of acute coronary syndrome (ACS). cTnI- cardiac troponin I; SUA- serum uric acid; ACS- acute coronary syndrome; AMI- acute myocardial infarction; UAP- unstable angina pectoris; Group A-hyperuricemic acute coronary syndrome patients; Group B-normouricemic acute coronary syndrome patients; *difference between AMI and UAP subjects in group of hyperuricemia; P-probability; †difference between AMI and UAP subjects in group of normouricemia; n- number of subjects; ‡difference between hyperuricemic and normouricemic subjects in acute myocardial infarction; ¦difference between hyperuricemic and normouricemic subjects in unstable angina pectoris.
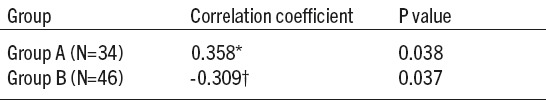
Values of SUA correlated with cTnI (Table 4), moderate positively in the group A and moderate negatively in the group B of ACS patients (p=0.038 and p=0.037, respectively).
Table 4.
Correlation between cardiac troponin I (cTnI) and serum uric acid (SUA) levels in hyperuricemic and normouricemic group of patients with acute coronary syndrome (ACS). *Spearman’s rho correlation coefficient; p value<0.05 was considered to be statistically significant. †-Pearson’s r correlation coefficient; P- probability;
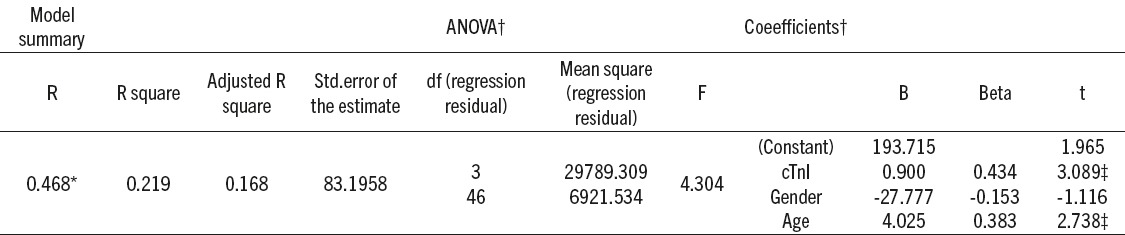
Presented in Table 5, multiple logistic regression analysis revealed that cTnI and age were independently associated with the SUA levels in the group A of ACS patients (p<0.05).
Table 5.
Summary of multiple linear regression analysis for the variables predicting the serum uric acid (SUA) levels in the hyperuricemic group of patients with acute coronary syndrome (ACS). *Predictors: (constant), cardiac troponin I (cTnI), gender, age; †Dependent variable: uric acid; ‡Significant at p value<0,05
5. DISCUSSION
Our study demonstrated median SUA levels to be in reference range in ACS patients, but some of those exceeded cut-off values for hyperuricemic status, which pointed on greater intensity of oxidative stress in those patients independently on ACS type. In addition, hyperuricemic and normouricemic status noticed in ACS patients was not associated with ACS type. The results of our study showed that median values of SUA in all ACS patients, as well as in patients with AMI and UAP separately, are within reference range, in the upper third. Considering cTnI, as gold standard for myocardial necrosis we tried to find out if it can be connected to SUA levels in ACS patient. Results of our study showed that cTnI was insignificantly higher in hyperuricemic versus normouricemic patients which points at cTnI failure to detect the higher oxidative stress and possible poor outcome associated with higher SUA level. In addition, we found that serum uric acid can differentiate ACS type in hyperuricemic group contrary to values in normouricemic group of ACS patients. Negative correlation of SUA with cTnI levels in the group B demonstrates lower levels of SUA in response to higher extent of myocardial lesion what indicates that normouricemia could be the result of the antioxidative SUA role and higher consumption of UA in the struggle with reactive oxygen species (ROS), in order to reduce oxidative stress, in those ACS patients. We didn’t find any association of SUA with type of acute coronary syndrome, but independently of type of ACS we found the different response in SUA synthesis/excretion recognized due to hyperuricemia and normouricemia independently on type ACS. Previous studies did not find any association between SUA levels and type and site of AMI but there was correlation of SUA and C-reactive protein as a marker of the systemic inflammation in ACS (3, 12, 13). Uric acid has antioxidant properties in early phases of atherosclerosis and later, due to its increase, paradoxically becomes prooxidant (antioxidant – prooxidant urate redox shuttle) (14). Anyway, with regard to oxygen scavenging, UA shows an opposite, dual oxidant effects, extracellular, antioxidant protection and intracellular, prooxidant damage. When UA penetrates into the endothelium and the myocyte, or produced in them, its effects change from an antioxidant to a strong pro-oxidant, due to its degradation and generation of ROS, as by products, which increase vascular oxidative stress and have a major role in cardiac dysfunction (15-17). The association of SUA with CVDs is noted not only in patients with hyperuricemia, but also with normal to high-normal range of UA levels (18, 19). Since activity of xanthine oxidase is increased under ischaemic conditions, which leads to increased synthesis of UA, SUA may also act as a marker of tissue ischemia (20). In the study of Lippi et al. (21), a potential association between UA and Troponin T (TnT) has been investigated in patients with an ACS. They revealed significantly higher SUA levels in patients with TnT values above decisional threshold and confirmed association between SUA and ACS. Nevertheless, SUA contributes about 60% of free radical scavenging activity in humans and this property could be expected to protect against ischaemic stress (22).
Multiple logistic regression analysis revealed that cTnI was independently associated with the SUA levels in the group A of ACS patients. Positive correlation of SUA with cTnI levels in the group A points that higher SUA levels are associated with higher extent of myocardial lesion in those ACS patients, that could be explained by pronounced prooxidative SUA effects in these ACS patients. Some authors agree that UA is one of the most important antioxidants in plasma, but at high concentrations, its protective antioxidant effect is overwhelmed by negative effects that may promote oxidative stress (23). Madole et al (24) suggest that elevated SUA levels indicate increased oxidative stress directly or it may indicate a defense against acute oxidative stress. All of the mentioned above could cause individual differences in SUA alteration in ACS patients.
Our findings support the link of the SUA and type of ACS only in hyperuricemic patients, but because of the cross-sectional design, we could not establish a causal relationship between them. Since the present study has its limitations through its retrospective nature, large prospective studies should be considered.
6. CONCLUSIONS
Power of serum uric acid to differentiate AMI and UAP in hyperuricemic ACS patients indicates on its possible role as an indicator of myocardial lesion extent in those patients. Obtained results showing correlation between cTnI and SUA in hyperuricemic ACS patients as well as the role of cTnI and age in prediction of uric acid serum level implicate that the intensity of uric acid production might depend on other factors such as oxidative stress in addition to age and myocardial lesion severity evaluated by cTnI.
Authors’ contribution: Sabaheta Hasic is responsible for study design, data analysis, data interpretation, the article writing and critical revision of the article. Damira Kadic participated in drafting, critical revision of the article and conducting of the final review before publishing. Emina Kiseljakovic conducted the final review by giving the critical revision of the article intellectual content. Radivoj Jadric revised the design and intellectual content of the article. Emina Spahic collected the data and critically evaluated the article.
Footnotes
• Conflict of interest: none declared.
REFERENCES
- 1.Kumar A, Cannon CP. Acute coronary syndromes: diagnosis and management, part I. Mayo Clinic Proceedings. 2009;84(10):917–38. doi: 10.4065/84.10.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third Universal Definition of Myocardial Infarction. Circulation. 2012;126(16):2020–35. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 3.Timóteo AT, Lousinha A, Labandeiro J, et al. Serum uric acid: a forgotten prognostic marker in acute coronary syndromes? Eur Heart J Acute Cardiovasc Care. 2013;2(1):44–52. doi: 10.1177/2048872612474921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duran M, Murat SN, Ornek E. Does serum uric acid level affect coronary collaterals in patients with acute coronary syndrome? Angiology. 2013;64(4):325–6. doi: 10.1177/0003319712463421. [DOI] [PubMed] [Google Scholar]
- 5.Hsu PC, Su HM, Lin TH. Association between coronary collaterals and serum uric acid level in Chinese population with acute coronary syndrome. Angiology. 2013;64(4):323–4. doi: 10.1177/0003319712463263. [DOI] [PubMed] [Google Scholar]
- 6.Kivity S, Kopel E, Maor E, Abu-Bachar F, Segev S, Sidi Y, et al. Association of serum uric acid and cardiovascular disease in healthy adults. Am J Cardiol. 2013;111(8):1146–51. doi: 10.1016/j.amjcard.2012.12.034. [DOI] [PubMed] [Google Scholar]
- 7.Ndrepepa G, Braun S, King L, Fusaro M, Tada T, Cassese S, et al. Uric acid and prognosis in angiography-proven coronary artery disease. Eur J Clin Invest. 2013;43(3):256–66. doi: 10.1111/eci.12039. [DOI] [PubMed] [Google Scholar]
- 8.Lin GM, Li YH, Zheng NC, Lai CP, Lin CL, Wang JH, et al. Serum uric acid as an independent predictor of mortality in high-risk patients with obstructive coronary artery disease: a prospective observational cohort study from the ET-CHD registry 1997-2003. J Cardiol. 2013;61(2):122–7. doi: 10.1016/j.jjcc.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Gagliardi AC, Miname MH, Santos RD. Uric acid: a marker of increased cardiovascular risk. Atherosclerosis. 2009;202(1):11–7. doi: 10.1016/j.atherosclerosis.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 10.Karim B, Nasution SA, Wijaya IP, Harimurti K. Hyperuricemia as a Risk Factors of Major Adverse Cardiac Events in Patients with Acute Coronary Syndrome: a Retrospective Cohort Study. Acta Med Indones. 2015;47(4):320–5. [PubMed] [Google Scholar]
- 11.Yu J, Han J, Mao J, Guo L, Gao W. Association between serum uric acid level and the severity of coronary artery disease in patients with obstructive coronary artery disease. Chin Med J. 2014;127(6):1039–45. [PubMed] [Google Scholar]
- 12.Hayden MR, Tyagi SC. Uric acid: a new look at an old risk marker for cardiovascular disease, metabolic syndrome, and type 2 diabetes mellitus: the urate redox shuttle. Nutr Metab. 2004;19(1):10. doi: 10.1186/1743-7075-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spahić E, Hasić S, Kiseljaković E, Resić H, Kulić M. Positive correlation between uric acid and C-reactive protein serum level in healthy individuals and patients with acute coronary syndromes. Med Glas (Zenica) 2015;12(2):128–32. doi: 10.17392/821-15. [DOI] [PubMed] [Google Scholar]
- 14.Biscaglia S, Ceconi C, Malagù M, Pavasini R, Ferrari R. Uric acid and coronary artery disease: An elusive link deserving further attention. Int J Cardiol. 2016;213:28–32. doi: 10.1016/j.ijcard.2015.08.086. [DOI] [PubMed] [Google Scholar]
- 15.Nabipour I, Sambrook PN, Blyth FM, Janu MR, Waite LM, Naganathan V, et al. Serum uric acid is associated with bone health in older men: a cross-sectional population-based study. J Bone Miner Res. 2011;26(5):955–64. doi: 10.1002/jbmr.286. [DOI] [PubMed] [Google Scholar]
- 16.Schulz E, Gori T, Münzel T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens Res. 2011;34(6):665–73. doi: 10.1038/hr.2011.39. [DOI] [PubMed] [Google Scholar]
- 17.Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension. 2003;42(3):247–52. doi: 10.1161/01.HYP.0000085858.66548.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakagawa T, Tuttle KR, Short RA, Johnson RJ. Hypothesis: fructose-induced hyperuricemia as a casual mechanism for the epidemic of the metabolic syndrome. Nat Clin Pract Nephrol. 2005;1(2):80–6. doi: 10.1038/ncpneph0019. [DOI] [PubMed] [Google Scholar]
- 19.Nadkar MY, Jain VI. Serum uric acid in acute myocardial infarction. J Assoc Physicians India. 2008;56:759–62. [PubMed] [Google Scholar]
- 20.Krishnan E, Baker JF, Furst DE, Schumacher HR. Gout and the risk of acute myocardial infarction. Arthritis Rheum. 2006;54(8):2688–96. doi: 10.1002/art.22014. [DOI] [PubMed] [Google Scholar]
- 21.Lippi G, Montagnana M, Franchini M, Guidi GC, Targher G. Uric acid concentration in patient with acute coronary syndrome. Intern Emerg Med. 2008;3(4):409–11. doi: 10.1007/s11739-008-0165-8. [DOI] [PubMed] [Google Scholar]
- 22.Johnson RJ, Kang DH, Feig D, Kivlighn S, Kanellis J, Watanabe S, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003;41(6):1183–90. doi: 10.1161/01.HYP.0000069700.62727.C5. [DOI] [PubMed] [Google Scholar]
- 23.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359(17):1811–21. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madole MB, Bachewar NP, Aiyar CM. Study of oxidants and antioxidants in patients of acute myocardial infarction. Adv Biomed Res. 2015;29(4):241. doi: 10.4103/2277-9175.168608. [DOI] [PMC free article] [PubMed] [Google Scholar]


