Abstract
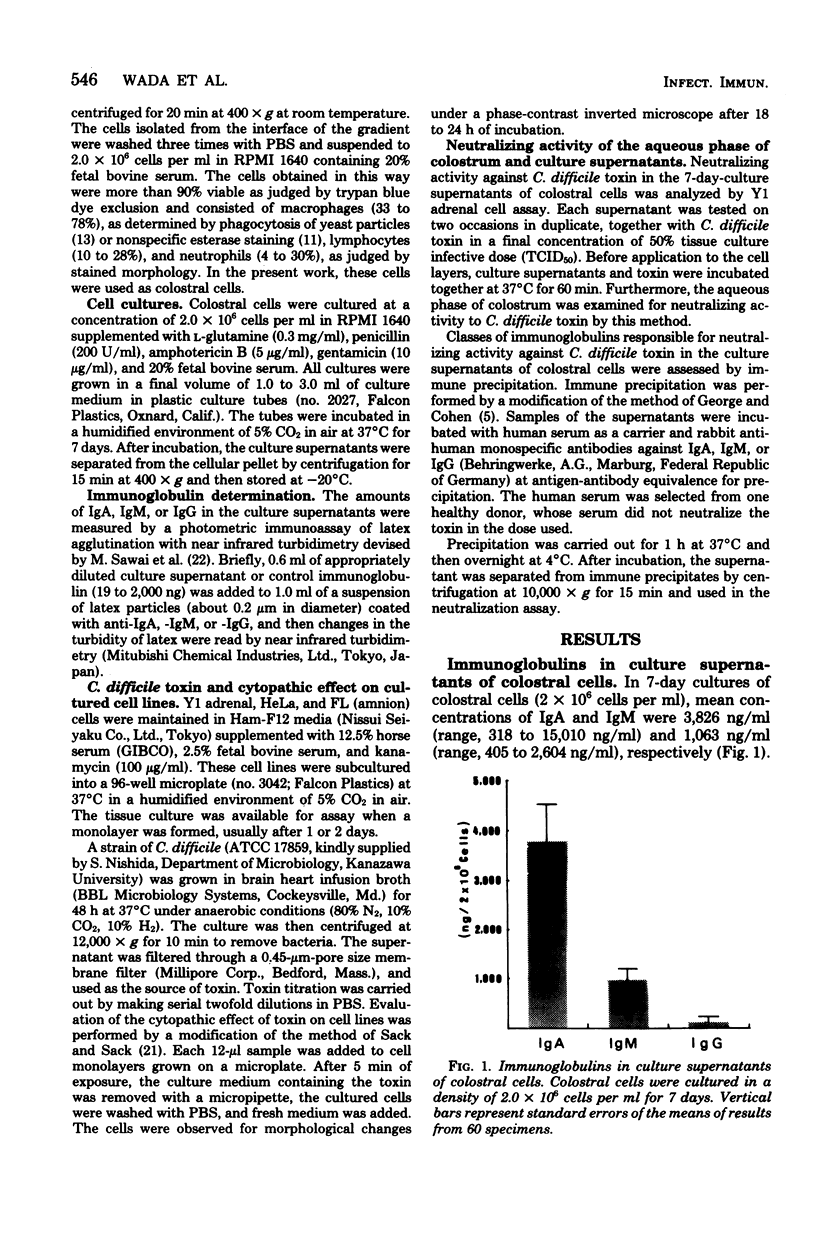
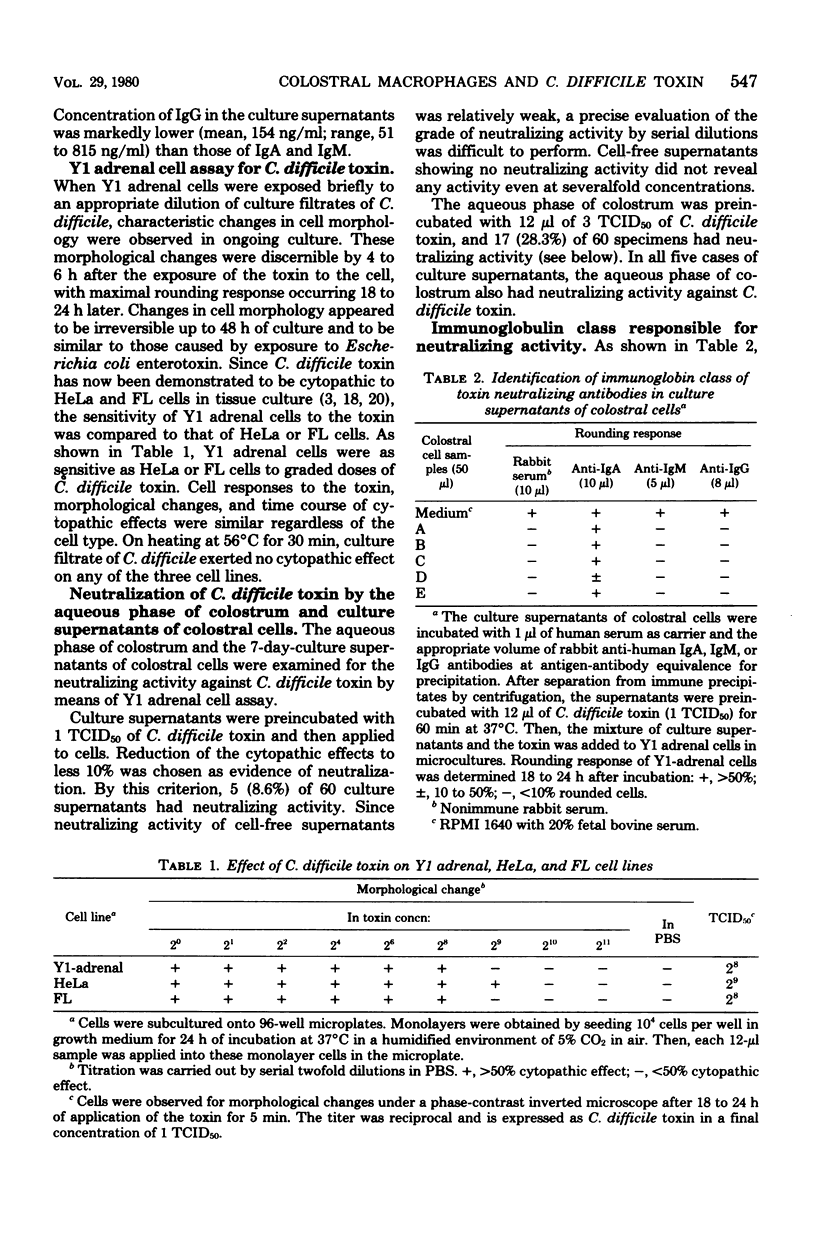
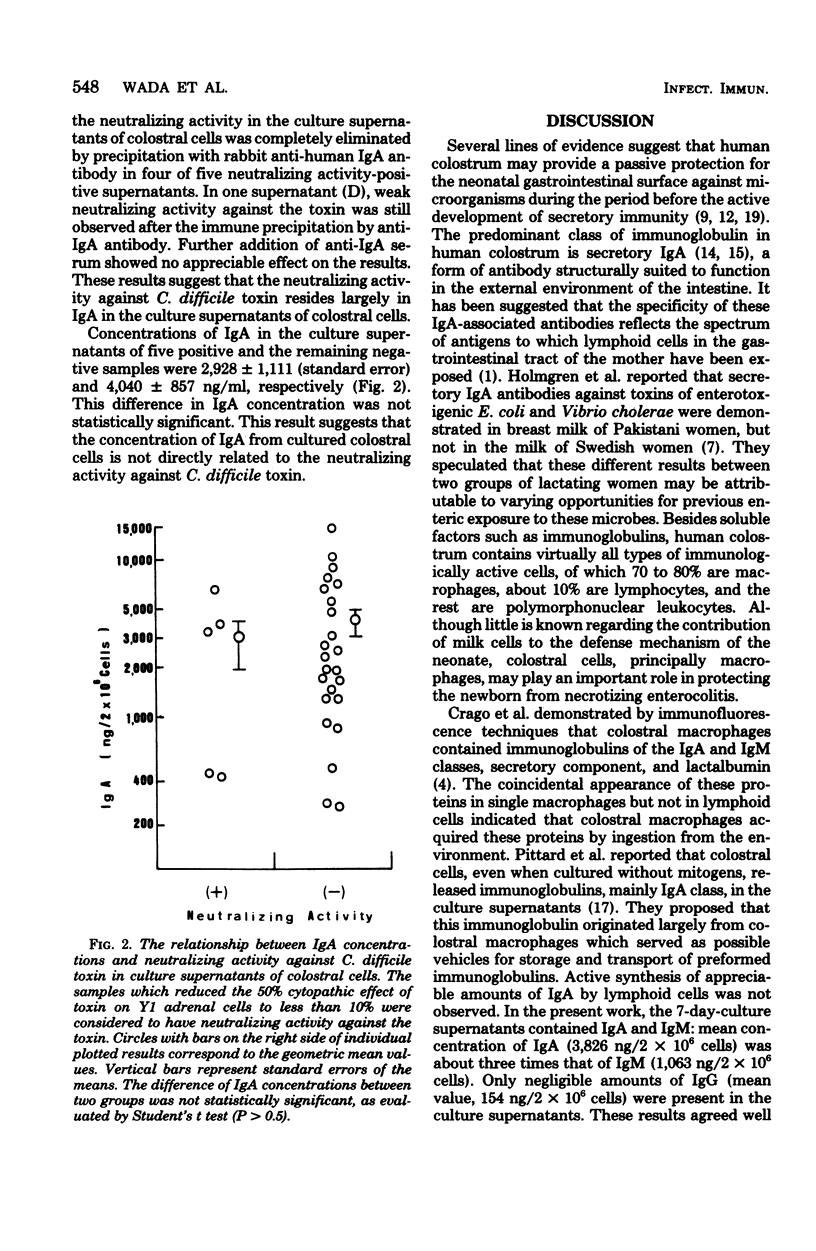
Human colostral specimens were obtained from 60 Japanese postpartum women within the first 3 days after delivery. Neutralizing activity against Clostridium difficile toxin was evaluated with Y1 adrenal cells in miniculture. When Y1 adrenal cells were exposed briefly to the toxin, they showed a rounding response in culture, resembling that effected by Escherichia coli enterotoxin; however, preincubation of the toxin with aqueous phase of colostrum significantly reduced its cytopathic effect on Y1 adrenal cells. Of 60 colostral specimens, 17 samples had neutralizing activity against the toxin. Cell-free supernatants of colostral cells cultured for 7 days without mitogens contained significant amounts of both immunoglobulin A (IgA) and IgM, but very small amounts of IgG. Neutralizing activity of cell-free supernatants of cultured colostral cells was evaluated as described above. Neutralizing activity against the toxin was identified in five samples of culture supernatants out of 60 colostral cell specimens. In all five cases, the aqueous phase of colostrum also had a neutralizing effect against C. difficile toxin. Neutralizing activity against the toxin found in five supernatants of cultured colostral cells was completely abolished only by anti-human IgA antibody as assessed by immune precipitation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlstedt S., Carlsson B., Hanson L. A., Goldblum R. M. Antibody production by human colostral cells. I. Immunoglobulin class, specificity, and quantity. Scand J Immunol. 1975 Sep;4(5-6):535–539. doi: 10.1111/j.1365-3083.1975.tb02659.x. [DOI] [PubMed] [Google Scholar]
- Barlow B., Santulli T. V., Heird W. C., Pitt J., Blanc W. A., Schullinger J. N. An experimental study of acute neonatal enterocolitis--the importance of breast milk. J Pediatr Surg. 1974 Oct;9(5):587–595. doi: 10.1016/0022-3468(74)90093-1. [DOI] [PubMed] [Google Scholar]
- Chang T. W., Lin P. S., Gorbach S. L., Bartlett J. G. Ultrastructural changes of cultured human amnion cells by Clostridiu difficile toxin. Infect Immun. 1979 Mar;23(3):795–798. doi: 10.1128/iai.23.3.795-798.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crago S. S., Prince S. J., Pretlow T. G., McGhee J. R., Mestecky J. Human colostral cells. I. Separation and characterization. Clin Exp Immunol. 1979 Dec;38(3):585–597. [PMC free article] [PubMed] [Google Scholar]
- George E. R., Cohen H. J. Kinetics of immunoglobulin synthesis and secretion by resting and pokeweed mitogen-transformed human lymphocytes. Clin Immunol Immunopathol. 1979 Jan;12(1):94–104. doi: 10.1016/0090-1229(79)90114-4. [DOI] [PubMed] [Google Scholar]
- George R. H., Symonds J. M., Dimock F., Brown J. D., Arabi Y., Shinagawa N., Keighley M. R., Alexander-Williams J., Burdon D. W. Identification of Clostridium difficile as a cause of pseudomembranous colitis. Br Med J. 1978 Mar 18;1(6114):695–695. doi: 10.1136/bmj.1.6114.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Hanson L. A., Carlson B., Lindblad B. S., Rahimtoola J. Neutralizing antibodies against Escherichia coli and Vibrio cholerae enterotoxins in human milk from a developing country. Scand J Immunol. 1976;5(6-7):867–871. doi: 10.1111/j.1365-3083.1976.tb03036.x. [DOI] [PubMed] [Google Scholar]
- Kliegman R. M. Neonatal necrotizing enterocolitis: implications for an infectious disease. Pediatr Clin North Am. 1979 May;26(2):327–344. doi: 10.1016/s0031-3955(16)33709-9. [DOI] [PubMed] [Google Scholar]
- Larsen S. A., Jr, Homer D. R. Relation of breast versus bottle feeding to hospitalization for gastroenteritis in a middle-class U.S. population. J Pediatr. 1978 Mar;92(3):417–418. doi: 10.1016/s0022-3476(78)80430-2. [DOI] [PubMed] [Google Scholar]
- Larson H. E., Price A. B., Honour P., Borriello S. P. Clostridium difficile and the aetiology of pseudomembranous colitis. Lancet. 1978 May 20;1(8073):1063–1066. doi: 10.1016/s0140-6736(78)90912-1. [DOI] [PubMed] [Google Scholar]
- Li C. Y., Lam K. W., Yam L. T. Esterases in human leukocytes. J Histochem Cytochem. 1973 Jan;21(1):1–12. doi: 10.1177/21.1.1. [DOI] [PubMed] [Google Scholar]
- Michael J. G., Ringenback R., Hottenstein S. The antimicrobial activity of human colostral antibody in the newborn. J Infect Dis. 1971 Nov;124(5):445–448. doi: 10.1093/infdis/124.5.445. [DOI] [PubMed] [Google Scholar]
- Miller M. E. Phagocytosis in the newborn infant: humoral and cellular factors. J Pediatr. 1969 Feb;74(2):255–259. doi: 10.1016/s0022-3476(69)80073-9. [DOI] [PubMed] [Google Scholar]
- Murillo G. J. Synthesis of secretory IgA by human colostral cells. South Med J. 1971 Nov;64(11):1333–1337. doi: 10.1097/00007611-197111000-00006. [DOI] [PubMed] [Google Scholar]
- Ogra S. S., Ogra P. L. Immunologic aspects of human colostrum and milk. I. Distribution characteristics and concentrations of immunoglobulins at different times after the onset of lactation. J Pediatr. 1978 Apr;92(4):546–549. doi: 10.1016/s0022-3476(78)80285-6. [DOI] [PubMed] [Google Scholar]
- Pitt J., Barlow B., Heird W. C. Protection against experimental necrotizing enterocolitis by maternal milk. I. Role of milk leukocytes. Pediatr Res. 1977 Aug;11(8):906–909. doi: 10.1203/00006450-197708000-00011. [DOI] [PubMed] [Google Scholar]
- Pittard W. B., 3rd, Polmar S. H., Fanaroff A. A. The breastmilk macrophage: a potential vehicle for immunoglobulin transport. J Reticuloendothel Soc. 1977 Dec;22(6):597–603. [PubMed] [Google Scholar]
- Rifkin G. D., Fekety F. R., Silva J., Jr Antibiotic-induced colitis implication of a toxin neutralised by Clostridium sordellii antitoxin. Lancet. 1977 Nov 26;2(8048):1103–1106. doi: 10.1016/s0140-6736(77)90547-5. [DOI] [PubMed] [Google Scholar]
- Robinson J. E., Harvey B. A., Soothill J. F. Phagocytosis and killing of bacteria and yeast by human milk cells after opsonisation in aqueous phase of milk. Br Med J. 1978 Jun 3;1(6125):1443–1445. doi: 10.1136/bmj.1.6125.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe R. D., Finegold S. M. Purification and characterization of Clostridium difficile toxin. Infect Immun. 1979 Jul;25(1):191–201. doi: 10.1128/iai.25.1.191-201.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack D. A., Sack R. B. Test for enterotoxigenic Escherichia coli using Y-1 adrenal cells in miniculture. Infect Immun. 1975 Feb;11(2):334–336. doi: 10.1128/iai.11.2.334-336.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll B. J., Nahmias A. J., Wickliffe C., Brann A. W., Jr, Dowell V. R., Jr, Whaley D. N. Bacterial toxin and neonatal necrotizing enterocolitis. J Pediatr. 1980 Jan;96(1):114–115. doi: 10.1016/s0022-3476(80)80345-3. [DOI] [PubMed] [Google Scholar]


