Abstract
Objectives:
The aim was to examine efficacy and safety after Implantable Collamer Lens (ICL) implantation for correction of myopia et myopic astigmatism.
Methods:
This prospective clinical study included 28 eyes of 16 patients which underwent implantation of ICL for correction of myopia up to -18,00 diopters (D) and myopic astigmatism up to -6,00 D in the Eye Clinic Svjetlost Sarajevo, from January 2013 to January 2016. Uncorrected distance visual acuity (UDVA), spherical equivalent (SE), corrected distance visual acuity (CDVA), intraocular pressure (IOP), endothelial cell (EC) density were evaluated at one, six and twelve months. For statistical analysis SPSS for Windows and Microsoft Excel were used.
Results:
Out of 16 patients, with mean age of 28,21 ± 4,06 years, 12 of them had binocular and 4 of them had monocular procedure. After 12 months mean UDVA was 0,76 ± 0,16 compared to UDVA 0,04 ± 0,03 preoperatively. Mean SE preoperatively was -0,21 ± 0,27 D compared to -9,52 ± 3,69 D preoperatively. At 12 months one eye (3,57%) lost 2 Snellen lines. In this study 8 eyes (28,57%) gained 1 line, 5 eyes gained (17,56%) 2 lines, and 3 eyes (10,72%) gained 3 lines. EC loss was 5,50±4.71% after 12 months. There was no significant change of IOP by the end of 12 months follow up period. One haptic crack was reported as the only intraoperative complication. Three postoperative complications were: two lens rotations and one retinal detachment.
Conclusion:
Implantation of ICL is an effective and safe method for reducing or correcting myopia and myopic astigmatism.
Keywords: implantable collamer lens, myopia, myopic astigmatism
1. INTRODUCTION
Different surgical procedures for correction of myopia have been developed over the last decades. Corneal refractive surgery is predictable and safe for treatment of low and moderate myopia (1). For treatment of high myopia it has poor predictability and can cause increased aberrations (2). Implantation of phakic intraocular lenses (pIOL) can be beneficial for patients who are not suitable candidates for Laser In Situ Keratomileusis (LASIK) and Photorefractive Keratectomy (PRK) since it does not involve corneal tissue removal (3). The other advantages are reversibility of the procedure and preservation of accommodation.
The Visian Implantable Collamer Lens (ICL) and The Visian Toric ICL (ICL™, STAAR Surgical, Nidau, Switzerland) are made from collamer, a 100% biocompatible material. The lens is designed to treat a broad range of refractive errors generally between +10.0 and -18.0 D with astigmatism between 1.0 to 6.0 D (4). The lens is foldable, which allows insertion in posterior chamber (PC) through a sutureless 2,8 mm incision.
The main disadvantage of PC pIOL is potential cataract formation due to close contact to the lens. Other complications include IOP elevation, rotation of pIOL, retinal detachment, pigment dispersion glaucoma, endothelial cell loss (5)
Posterior chamber pIOL has been reported to be effective for the correction of moderate to high ametropia (6).
2. MATERIAL AND METHODS
This was a prospective clinical study conducted at Eye Clinic Svjetlost Sarajevo. It included 28 eyes from 16 patients who underwent implantation of PC pIOL for the correction of myopia and myopic astigmatism. All procedures were performed by one surgeon and all patients signed informed consent.
Each patient included in this study was not suitable for corneal refractive surgery, had stable myopia for two years, anterior chamber depth (ACD) ≥ 2,8 mm (measured form endothelium), endothelial cell (CE) count ≥ 2300 cells/mm2, mesopic pupil ≤ 6,5 mm.
Exclusion criteria were: patients younger than 21 years, active pathology of the eye, cataract, glaucoma, chronic or recurrent uveitis, prior ocular surgery, autoimmune systemic diseases, diabetes, pregnancy and breastfeeding.
Preoperative examination included: uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA) measured in decimal Snellen values, manifest and cycloplegic refraction (autorefractometer- Rexxam CO, LTD, Kagava, Japan), intraocular pressure (IOP), (Auto Non-Contact Tonometer, Reichert Inc., Buffalo, NY, USA), biomicroscopic examination (Slit Lamp SL980, CSO Costruzione Strumenti Oftalmici, Florence, Italy), fundus examination, corneal topography (Wavelight, Allegretto Oculyzer, Erlangen, Germany), endothelial cell density (ECD) count (CSO Specular Microscope, CSO Costruzione Strumenti Oftalmici, Florence, Italy), pupil size (Sirius Pupilographer, CSO Costruzione Strumenti Oftalmici, Florence, Italy), horizontal corneal diameter (white to white–WTW) was measured by apparatus (IOL Master, Carl Zeiss, Germany) and also manually by caliper.
Calculation of the ICL power was performed using software provided by manufacturer (7), with target of emmetropia. Toric ICLs were manufactured to minimize rotation, and required the surgeon to rotate the ICL no more than 22.5 degrees. The size of ICL was chosen based on WTW measures. In the study only ICL V4c model was used, which has a central hole, therefore there was no need for preoperative Yag laser iridotomy.
Prior to the surgery patients dilating pupil agents Phenylephrinehydrochloride (Neosynephrin-POS 10%, Ursapharm, Saarbrücken, Germany) were administered to the patients. Patients were operated under peribulbar anaesthesia of 2mm3 lidocaine (Lidokain-hlorid 2%, Galenika, Beograd, Republika Srbija). Two paracentesis incisions were made with 15° blade at 10 and 2 o’clock positions. Sodium hyaluronate (Provisc, Alcon, Rijksweg, Belgium) was instilled in anterior chamber (AC) followed by 2,8 mm corneal incision at 12 o’clock. ICL was inserted in the PC with the use of an injector cartridge (STAAR Surgical, Nidau, Switzerland). All viscoelastic material was carefully removed by bimanual irrigation and aspiration probes and using Balanced Salt Solution (AMO Endosol, AMO, Groningen, The Netherlands). Main incision was hydrated after Cefuroxime Natrium (Cefuroxime, Astro-pharma, Wien, Österrreich) installation in AC.
The following postoperative medication regimen was recommended: one drop of Tobramicin/Dexamethason (Tobradex, Alcon, Rijksweg, Begium) 4 times daily/4 weeks.
Biomicroscopic examination, UDVA and IOP were obtained and recorded at day 1 and day 7 after surgery. At other testing intervals (1, 6 and 12 months), a complete examination was performed, which included refraction, spherical equivalent (SE), corneal topography and ECD count. Index of safety and efficacy and CDVA were evaluated after 12 months.
Statistical calculation was performed with SPSS for Windows (19.0, SPSS Inc, Chicago, Illinois, SAD) and Microsoft Excel (11.0, Microsoft Corporation, Redmond, WA, SAD). The comparison between the preoperative and postoperative periods was performed with the Wilcoxon signed rank test. Value of p<0.05 was considered statistically significant.
3. RESULTS
Visual and refractive outcomes of 28 eyes from 16 patients, who underwent implantation of ICL for correction of myopia or myopic astigmatism were described in this study. Nine (9/16) patients were male (56,25%) and seven (7/16) were female (43,75%). Out of 16 patients 4 of them underwent monocular and 12 binocular ICL implantation. All preoperative values are presented in Table 1. Graph 1 shows significant improvement of mean UDVA preoperatively and measurements in the first month (p<0,001) while after first month until 12 months there was no change (p=0.705).
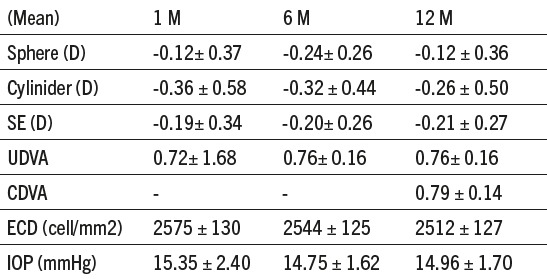
Table 1.
Preopertive values (SE – spheric equivalent, UDVA – uncorrected distance visual acuity, CDVA – corrected distance visual acuity, IOP – intraocular pressure, ECD – endothelial cell density, ACD – anterior chamber depth).
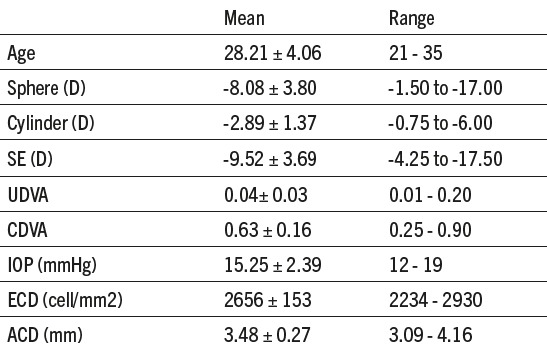
Graph 1.
UDVA in follow up period of 12 months (UDVA – uncorrected distance visual acuity)
From 0% preoperatively UDVA of 20/40 or better increased to 92.86% postoperatively, and UDVA of 20/20 increased to 67.86%.
In our study efficacy index (defined as UDVA at 12 month postoperatively/CDVA preoperatively) was 1.2. Safety index (defined as CDVA at 12 months/CDVA preoperatively) was 1.25.
Values of SE decreased significantly at one month postoperatively to -0.19 ± 0.34 D from preoperative -9.52 ± 3.69 D (p<0.001) and remained stable over the follow up period (p=0.621). The results are shown in Graph 2.
Graph 2.
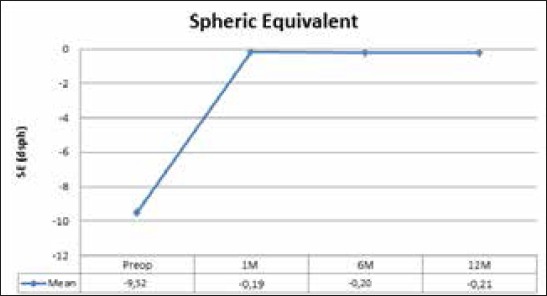
Spheric equivalent (SE) in follow up period of 12 months
At 12 months one eye (3.57%) lost 2 lines, 8 eyes (28.57%) gained 1 line, 5 eyes gained (17.56%) 2 lines, and 3 eyes (10.72%) gained 3 lines.
The loss of EC after 12 months was 5,50% and was the highest in the first postoperative month 3,06% and continued to fall in follow up period for 2,44% more, as shown in Graph 3. ECD loss after 1 month was statistically significant (p>0.001), and after this period shows no statically significant change (p=0.361).
Graph 3.
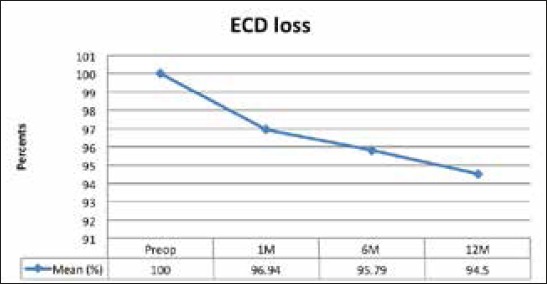
ECD loss in follow up period of 12 months (ECD – endothelial cell density)
There was no statistically significant difference in IOP postoperatively (p=0.469). All other postoperative results are presented in Table 2.
Table 2.
Postoperative results in follow up period of 12 months (SE – spheric equivalent, UDVA – uncorrected distance visual acuity, CDVA – corrected distance visual acuity, IOP – intraocular pressure, ECD – endothelial cell density)
4. DISCUSSION
The primary variable for this kind of study, UDVA, showed significant improvement in first month (0.72±1.68) compared to preoperatively (0.04±0.03) and stayed stable throughout the 12 months period (0.76±0.16). UDVA of 20/40 or better increased from 0% preoperatively to 92.86%, and UDVA of 20/20 increased to 67.86%. Food and Drug Administration (FDA) (8) reported UDVA 20/40 or better in 94.70% and UDVA 20/20 in 59.23% of eyes after 3 years. These results show efficacy of this procedure and in our study index of efficacy is 1.20.
In our study, SE decreased from -9.5 ± 3.69 D preoperatively to -0.19 ± 0.34 D at first month and stayed stable to one year -0.21 ± 0.27 D. In fact, 82.86% eyes had an SE within ±1.00 D and 78.57% eyes had an SE ±0,50 D. Uusitalo (9) reported 81.6% eyes within ±0,50 D and 71.10% within ±1.00 D. We had no eye underwent bioptics for residual refractive error.
At one year, only one eye had (3.57%) lost two Snellen lines. Eighth eyes gained one line (28.57%), 5 eyes gained two lines (17.56%) and 3 eyes (10.72%) gained 3 lines. Other also reported changes in lines after implantation of ICL phakic lenses. For example, Al Sabani (10) found 1.5% eyes that lost two lines, 10.7% gained two lines and 9.2% gained more than two lines. This gain could be the result of magnification of the retinal image after ICL implantation and avoiding of minimization of picture by spectacles in high myopia. The reason that one eye lost two Snellen lines in this study is rotation of ICL probably because of small size of diameter, but the patient was satisfied and refused explaining and changing of ICL.
There was no significant change (p= 0.469) in the IOP during postoperative period. Shimicu et al (11) presented the same results in their investigation (p=0.53). We explained this result with using V4c model of ICL with central hole which prevents pupillary block and does not require preoperative iridotomy or intraoperative iridectomy.
Only one intraoperative complication occurred. ICL haptic was cracked during the surgery in one eye (3.57%). There were no postoperative complications after event in this case, and no loss of CDVA. Al Sabaani et al reports 3 haptic tears and 1 haptic crack, and it is argued to be due to learning curve (10).
Postoperative complications included two rotations of the lens. In one case the patient underwent ICL replacement with the bigger lens after which the lens remained in correct position. Second surgery was performed one month after the first surgery. Second patient decided he does not want to reposition or replace existing ICL, which was rotated approximately 15°, and the patient lost two Snellen lines of CDVA.
Cataract formation was one of the expected complications of pIOL implantation. Al Sabaani (10) reports anterior subcapsular cataract in 4.34% eyes one year after surgery. In our study during 12 months postoperative period cataract was not reported. We explain this difference with very experienced surgeon. Gonvers (12) reports increase of cataract formation with longer follow up period. This is the reason why studies with shorter follow up period mostly report none or very small number of cataracts postoperatively. Further and longer observation is needed to make any conclusions
One eye (3.57%) had retinal detachment 3 months after the ICL implantation. The patient had high degenerative myopia and preoperatively underwent prophylactic laser photocoagulation on both eyes. Pars plana vitrectomy was successfully preformed, with no further complications on this eye. No other retinal complications were observed. In a large study Bamashmu (13) reports 9.9% (61 of 617) eyes required prophylactic laser photocoagulation, with development of one spontaneous rhegmatogenous detachment and one traumatic, which is 0,32% in follow-up up to 40 months. Percentage of posterior segment complications in our study can hardly be compared to Bamashmu (13) due to much higher number of patients. Patients with high degenerative myopia must undertake prophylactic treatment and even then, there is still a risk of retinal detachment.
Loss EDC at one year in our study was 5.5%, likewise Muslubas (14) reports 6.5% and Al Sabaani 5.6% after 12 months. In our study the main loss occurred during first month (3.06%) and was statistically significant, compared to other studies where the biggest loss was in first few months. After this period in all studies the was no significantly significant decrease during the follow up period (3, 15). ECD loss in first few months can be explained by surgical trauma. There are few studies with follow up period of 2 to 4 years, and they found no statistically significant change in ECD loss during their follow up after the first major ECD drop (3, 16).
Implantation of ICL is an effective treatment for correction of myopia and myopic astigmatism with advantages such as immediate correction, stability, relative simplicity and reversibility.
Footnotes
• Conflict of interest: none declared.
REFERENCES
- 1.Tanzer DJ, Brunstetter T, Zeber R, Hofmeister E, Kaupp S, Kelly N, et al. Laser in situ keratomileusis in United States Naval aviators. J Cataract Refract Surg. 2013;39(7):1047–58. doi: 10.1016/j.jcrs.2013.01.046. doi:10.1016/j.jcrs.2013.01.046. [DOI] [PubMed] [Google Scholar]
- 2.Igarashi A, Kamiya K, Shimizu K, Komatsu M. Visual performance after implantable collamer lens implantation and wavefront-guided laser in situ keratomileusis for high myopia. Am J Ophthalmol. 2009;148(1):164–70. doi: 10.1016/j.ajo.2009.02.001. doi:10.1016/j.ajo.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Pineda-Fernández A, Jaramillo J, Vargas J, Jaramillo M, Jaramillo J, Galindez A. Phakic posterior chamber intraocular lens for high myopia. J Cataract Refract Surg. 2004;30:2277–83. doi: 10.1016/j.jcrs.2004.03.035. doi:10.1016/j.ajo.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Guell JL, Morrel M, Kook D, Kohnen T. Phakic intraocular lenses. Part 1: Historical overview, current models, selection criteria and surgical techniques. J Cataract Refract Surg. 2010;36:1976–93. doi: 10.1016/j.jcrs.2010.08.014. doi:10.1016/j.jcrs.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 5.Packer M. Meta-analysis and review: effectiveness, safety, and central port design of the intraocular collamer lens. Clin Ophthalmol. 2016;9(10):1059–77. doi: 10.2147/OPTH.S111620. doi:10.2147/OPTH.S111620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alfonso JF, Baamonde B, Fernández-Vega L, Fernandes P, González-Méijome JM, et al. Posterior chamber collagen copolymer phakic intraocular lenses to correct myopia: five-year follow-up. J Cataract Refract Surg. 2011;37:873–80. doi: 10.1016/j.jcrs.2010.11.040. doi:10.1007/s00417-012-1957-0. [DOI] [PubMed] [Google Scholar]
- 7.Sarver EJ, Sanders DR. Astigmatic power calculations for intraocular lenses in the phakic and aphakic eye. J Refract Surg. 2004;20:472–7. doi: 10.3928/1081-597X-20040901-10. doi:10.1371/journal.pone.0056453. [DOI] [PubMed] [Google Scholar]
- 8.Sanders DR, Doney K, Poco M ICL in Treatment of Myopia Study Group. United States Food and Drug Administration clinical trial of the Implantable Collamer Lens (ICL) for moderate to high myopia: three-year follow-up. Ophthalmology. 2004;111(9):1683–92. doi: 10.1016/j.ophtha.2004.03.026. doi:10.1016/j.ophtha.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 9.Uusitalo RJ, Aine E, Sen NH, Laatikainen L. Implantable contact lens for high myopia. J Cataract Refract Surg. 2002;28:29–36. doi: 10.1016/s0886-3350(01)01218-4. doi:10.1016/S0886-3350(01)01218-4. [DOI] [PubMed] [Google Scholar]
- 10.Al Sabaani N, Al Assiri A, Al Torbak A, Al Motawa S. Outcome of posterior chamber phakic intraocular lens procedure to correct myopia. Saudi J Ophthalmol. 2013;27(4):259–66. doi: 10.1016/j.sjopt.2013.06.009. doi:10.1016/j.sjopt.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimizu K, Kamiya K, Igarashi A, Kobashi H. Long-Term Comparison of Posterior Chamber Phakic Intraocular Lens With and Without a Central Hole (Hole ICL and Conventional ICL) Implantation for Moderate to High Myopia and Myopic Astigmatism: Consort-Compliant Article. Abdulrahman. K, ed. Medicine. 2016;95(14):e3270. doi: 10.1097/MD.0000000000003270. doi:10.1097/MD.0000000000003270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonvers M, Bornet C, Othenin-Girard P. Implantable contact lens for moderate to high myopia-relationship of vaulting to cataract formation. J Cataract Refract Surg. 2003;29:918–24. doi: 10.1016/s0886-3350(03)00065-8. doi:10.1016/S0886-350(03)00065-8. [DOI] [PubMed] [Google Scholar]
- 13.Bamashmus MA, Al-Salahim SA, Tarish NA, Saleh MF, Mahmoud HA, Elanwar MF, Awadalla MA. Posterior Vitreous Detachment and Retinal Detachment After Implantation of the Visian Phakic Implantable Collamer Lens. Middle East African Journal of Ophthalmology. 2013;20(4):327–31. doi: 10.4103/0974-9233.120019. doi.org/10.4103/0974-9233.120019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muslubas IBS, Cabi C, Oral AYA, Akcay L, Kaplan AT, Ozgur OR, et al. Comparison of Outcomes of Posterior Chamber and Iris-Claw Anterior Chamber Phakic Intraocular Lens Implantation for Moderate to High Myopia. Arsan J Clin Exp Ophthalmol. 2013;4:263. doi:10.4172/2155-9570.1000263. [Google Scholar]
- 15.Jimenez-Alfaro I, Benitez del Castillo JM, Garcia-Feijoo J, et al. Safety of posterior chamber phakic intraocular lenses for the correction of high myopia: anterior segment changes after posterior chamber phakic intraocular lens implantation. Ophthalmology. 2001;108:90–9. doi: 10.1016/s0161-6420(00)00403-6. doi:10.1016/S0161-6420(00)00403-6. [DOI] [PubMed] [Google Scholar]
- 16.Dejaco-Ruhswurm I, Scholz U, Pieh S, et al. Long-term endothelial changes in phakic eyes with posterior chamber intraocular lenses. J Cataract Refract Surg. 2002;28:1589–93. doi: 10.1016/s0886-3350(02)01210-5. doi:10.1016/S0886-3350(02)01210-5. [DOI] [PubMed] [Google Scholar]


