Abstract
Objective
To determine if hepatocyte growth factor (HGF), a promising biomarker of coronary heart disease (CHD) given its release into circulation in response to endothelial damage, is associated with subclinical and clinical CHD in a racial/ethnic diverse population.
Methods
HGF was measured in 6738 participants of the Multi-Ethnic Study of Atherosclerosis. Highest mean HGF values (pg/mL) were observed in Hispanic, followed by African, non-Hispanic white, then Chinese Americans.
Results
In all races/ethnicities, HGF levels were associated with older age, higher systolic blood pressure and BMI, lower HDL, diabetes, and current smoking. In fully adjusted models, each standard deviation (SD) higher HGF was associated with an average increase in coronary artery calcium of 55 Agatston units for non-Hispanic white (p<0.001) and 51 for African (p=0.007) Americans, but was not in the other race/ethnic groups (interaction p=0.02). There were 529 incident CHD events and CHD risk was 41% higher in African (p<0.001), 17% in non-Hispanic white (p=0.026) and Chinese (p=0.36), and 6% in Hispanic (p=0.56) Americans per SD increase in HGF.
Conclusion
In a large and diverse population-based cohort, we report that HGF is associated with subclinical and incident CHD. We demonstrate evidence of racial/ethnic heterogeneity within these associations, as the results are most compelling in African and non-Hispanic white Americans. We provide evidence that HGF is a biomarker of atherosclerotic disease that is independent of traditional risk factors.
Keywords: atherosclerosis, coronary disease, epidemiology, risk factors
INTRODUCTION
Hepatocyte growth factor (HGF) was originally identified and studied due to its mitogenic role in liver regeneration.[1] Evidence is mounting that indicates HGF activities have cardioprotective effects in tissues through activation of anti-apoptotic, anti-inflammatory, anti-oxidant, and anti-fibrotic, pathways.[2] However, it is unknown if HGF contributes to the progression of atherosclerosis given that it is also a strong promotor of angiogenesis,[3, 4] an essential component of atherosclerotic plaque neovascularization. Subsequent research has shown that circulating HGF is elevated as a compensatory response to endothelial damage and accumulates in injured organs via its receptor c-Met.[5] Therefore, circulating HGF has been proposed as a potential clinical biomarker for assessing disease burden and predicting cardiovascular disease (CVD).
Studies of circulating HGF in humans are predominantly limited to clinical populations with CVD. Collectively, these studies found higher circulating levels of HGF were associated with intima medial thickness (IMT), aorto-iliac artery atherosclerosis, and presence of coronary atherosclerosis.[6–8] Similarly, higher concentrations were associated with myocardial infarction (MI), unstable angina, and heart failure.[9, 10] Likewise, HGF has been shown to be higher in those with cardiovascular risk factors such as hypertension, diabetes, and obesity.[11, 12]
Despite the evidence linking HGF and atherosclerotic disease, little information is known about the relationship of HGF with disease and related risk factors in the general population. Prior studies were limited in scope, sample size, and racial/ethnic diversity. Importantly, previous research has demonstrated race/ethnicity-specific genetic regulation of HGF levels, justifying the exploration of potential heterogeneity in phenotype associations.[13] Therefore, using the diverse cohort comprising the prospective Multi-Ethnic Study of Atherosclerosis (MESA), the objective of this study is to describe the shared and race/ethnicity-specific relationships of circulating HGF with cardiovascular risk factors and determine if levels of HGF are associated with subclinical atherosclerosis and incident coronary heart disease (CHD).
METHODS
Study participants
MESA enrolled 6814 participants from 2000–2002 without known clinical CVD who were aged 45–84 years of which 38% were non-Hispanic white, 28% African, 22% Hispanic, and 12% Chinese Americans. MESA participants were examined at 6 field centers located in Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; Northern Manhattan, NY; and Saint Paul, MN. The MESA study has been described in detail elsewhere.[14] As part of the MESA ancillary study titled Multi-Scale Biology of Atherosclerosis in the Cellular Adhesion Pathway (HL98077), 6738 participants had serum HGF measured at the first exam (2000–2002). The study was approved by the Institutional Review Boards at each research center and informed consent was obtained from all participants.
Measurements
Questionnaires were used to collect data such as environmental exposures (e.g., smoking history), and health status (e.g., menopause). Height was measured while participants were standing without shoes, heels together against a vertical mounted ruler. Body mass index (BMI) was calculated as weight (kg)/height2(m2). Resting seated blood pressure was measured 3 times using an automated oscillometric method (Dinamap), and the average of the second and third readings was used in analyses. Hypertension was defined as systolic blood pressure (SBP) of ≥140 mm Hg, diastolic blood pressure (DBP) of ≥90 mm Hg, or taking antihypertensive medication.
Serum glucose was assayed by a glucose oxidase method on the Vitros analyzer (Johnson and Johnson Clinical Diagnostics, Rochester, NY). Diabetes was defined as any participant who self-reported a physician diagnosis, used diabetes medication, or had a fasting glucose≥126 mg/dL. Total cholesterol was measured in ethylenediaminetetraacetic (EDTA) plasma using a cholesterol oxidase method (Roche Diagnostics, Indianapolis, IN) on a Roche COBAS FARA centrifugal analyzer. After precipitation of non-high density lipoprotein (HDL)-cholesterol with magnesium/dextran, HDL-cholesterol was also measured in EDTA plasma using the cholesterol oxidase cholesterol method (Roche Diagnostics). Triglyceride was measured in EDTA plasma using Triglyceride Glycerol Blanked reagent (Roche Diagnostics) on a Roche COBAS FARA centrifugal analyzer. Serum creatinine was measured by rate reflectance spectrophotometry using thin film adaptation of the creatine amidinohydrolase method on the Vitros analyzer (Johnson & Johnson Clinical Diagnostics, Inc., Rochester, NY). Glomerular filtration rate (GFR) was estimated using the simplified MDRD (Modification of Diet in Renal Disease study) equation.
Circulating levels of HGF protein were measured at Exam 1 in serum by a quantitative sandwich enzyme-linked immunosorbent assay (ELISA) using the Human soluble HGF/CD62P Immunoassay kit (R&D Systems, Minneapolis, MN), with a lower limit of detection of 40 pg/mL. The interassay laboratory coefficients of variation for the HGF method were 12.0%, 8.0%, and 7.4% at respective mean concentrations of 686.6, 2039.1, and 4079.5 pg/mL for lyophilized manufacturer’s controls; and 10.4% at a mean concentration of 687.7 pg/mL for an in-house pooled serum control.
Computed tomography (CT) of the coronary arteries was performed at exam 1 and methods have been previously described.[15] In brief, at 3 of 6 centers, electron beam scanners (Imatron C-150; Imatron, Inc., San Francisco, CA) were used with cardiac-gating at 80% of the R-R interval. At the other 3 centers, a prospective electrocardiogram-triggered multi-detector scan was acquired at 50% of the R-R interval. All scanners were comparable in their ability to measure calcium.[15] Scans were read centrally at Harbor-University of California Medical Center (Los Angeles, CA), and Agatston coronary artery calcium (CAC) scores were quantified by blinded CT image analysts. Using high-resolution B-mode ultrasonography, images of the near and far walls of the bilateral common and internal carotid arteries were obtained using a Logiq 700 ultrasound machine (GE Medical Systems, Waukesha, Wisconsin). Central reading of intima-media thickness (IMT) was done at the Tufts Medical Center (Boston, Massachusetts). A semi-quantitative scale was used to report the presence of atherosclerotic plaque; those with 0% were considered to be absent a plaque, and those with >0% were positive for the presence of a plaque.
Coronary heart disease events
The MESA exam and follow-up forms for ascertaining events are available on the MESA website (http://www.mesa-nhlbi.org). In brief, the cohort was followed for 12.3 years via telephone interviews with participants at 9–12 month intervals, and with next of kin for out-of-hospital deaths. Hospital records were obtained on an estimated 99% of hospitalized cardiovascular events and some information on 97% of outpatient encounters. Trained personnel abstracted any hospital records suggesting possible cardiovascular events that included MI, angina, resuscitated cardiac arrest, stroke (not transient ischemic attack), CHD, or other CVD death. MI was defined by integrating cardiac pain, biomarker level, and ECG changes using the Minnesota code. CHD events included all MI, resuscitated cardiac arrest, definite angina, probable angina (if followed by revascularization), and CHD death.
Statistical analyses
Exam 1 characteristics were compared across racial/ethnic strata using linear regression models for continuous variables and the chi-square test for categorical variables. In race/ethnicity-stratified analyses, regression models were used to assess the association of HGF levels and CVD risk factors (i.e., age, sex, BMI, SBP, hypertension treatment, total and HDL cholesterol, and smoking and diabetes status). To investigate the association of HGF levels and CAC, IMT, and presence of plaque at Exam 1, regression models were fit with HGF as the independent variable with adjustment for risk factors. Assumptions of linearity for HGF were evaluated using generalized additive models with cubic B-splines. There were no indications of major departures from linearity. Linear regression was used for IMT and logistic regression was used for plaque. Because the distribution of CAC has a large percentage of zero measurements, standard normalization transformations are not adequate; therefore, the Tobit model (type II) was used to investigate the relationship between CAC and protein concentration levels.[16] An additional two-stage modeling analysis was performed to assess the association of HGF with CAC. Stage 1 assessed the relationship of HGF with CAC limited to subjects with CAC>0 using linear regression models as described previously; Stage 2 assessed the relationship of HGF with any CAC using logistic regression. The association of HGF with time to CHD was assessed using Cox proportional hazards regression, adjusting for CHD risk factors. Associations are reported per race-pooled standard deviation increase in HGF (259 pg/mL). Proportional hazards were verified by examination of the Schoenfeld residuals. Race/ethnicity-stratified Kaplan Meier curves, adjusted for traditional risk factors, were created to illustrate cumulative incidence of CHD by tertile of HGF. We used a Bonferroni correction to account for multiple comparisons (0.05/4 strata × 5 outcomes= 0.0025). All testing for interactions between HGF and strata was conducted by pooling subjects across strata and testing the significance of HGF-by-strata interaction terms.
RESULTS
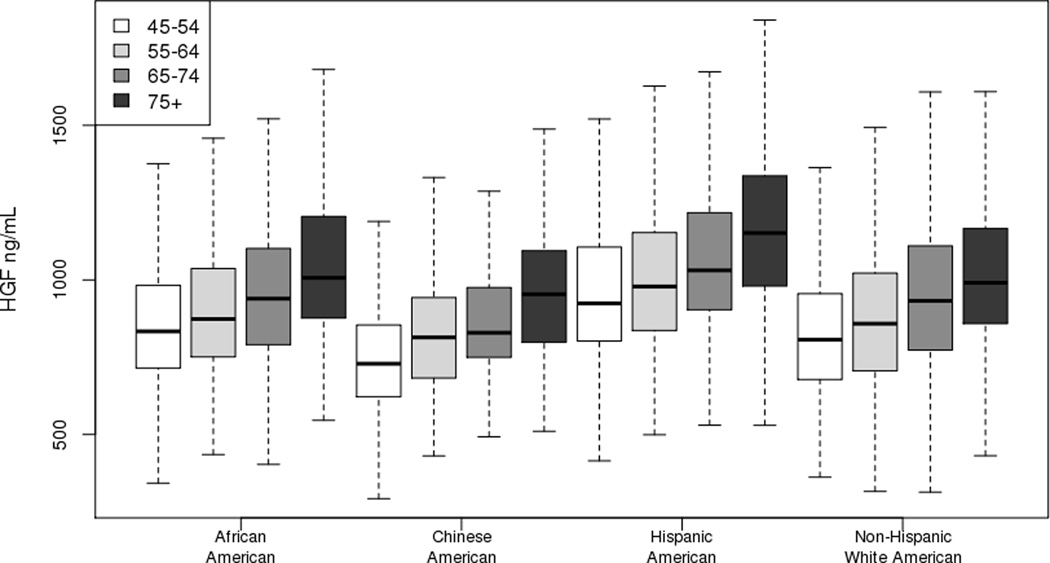
Baseline characteristics stratified by race/ethnicity are provided in Table 1. Significant differences in HGF (pg/mL) by race/ethnicity were observed, with highest mean values in Hispanic (1036±269), followed by African (934±249), non-Hispanic white (916±255), then Chinese (839±216) Americans (race/ethnicity interaction p<0.001). Furthermore, levels of HGF were positively associated with age (Figure 1). Exploring the relationship of HGF and traditional CVD risk factors, HGF levels were associated with higher BMI and SBP and lower HDL in all race/ethnicities after adjustment for age and sex (Table 2). Likewise, hypertensives, current smokers, and diabetics had higher HGF levels. In females, lower levels of HGF were associated with use of hormone replacement therapy, albeit the association was strongest in non-Hispanic white and African American women. Exclusively in non-Hispanic whites, HGF levels were higher in females compared to males independent of age and inversely associated with current alcohol consumption.
Table 1.
Baseline characteristics by race/ethnicity for those with hepatocyte growth factor measured at exam 1 (mean ± standard deviation or percentage)
| Characteristics | African American |
Chinese American |
Hispanic American |
Non-Hispanic white American |
p Value |
|---|---|---|---|---|---|
| N | 1861 | 799 | 1477 | 2604 | |
| HGF, pg/mL | 934.4 (249.8) | 839.4 (216.1) | 1035.9 (268.7) | 915.8 (255.1) | <0.001 |
| Age, years | 62.1 (10.0) | 62.4 (10.3) | 61.2 (10.3) | 62.6 (10.2) | <0.001 |
| Sex, % female | 56 | 51 | 52 | 52 | 0.058 |
| Body mass index, kg/m2 | 30.2 (5.8) | 24.0 (3.3) | 29.4 (5.1) | 27.7 (5.1) | <0.001 |
| Education | <0.001 | ||||
| ≤11th grade | 12 | 25 | 45 | 5 | |
| High school graduate | 19 | 16 | 20 | 17 | |
| Some college | 35 | 20 | 25 | 28 | |
| Bachelor’s degree | 17 | 23 | 6 | 22 | |
| Graduate/professional school | 17 | 16 | 4 | 28 | |
| Income | <0.001 | ||||
| < $25,000 | 30 | 50 | 49 | 16 | |
| $25,000 – $50,000 | 32 | 22 | 33 | 27 | |
| $50,000 – $100,000 | 29 | 18 | 15 | 33 | |
| ≥ $100,000 | 8 | 10 | 2 | 25 | |
| Systolic blood pressure, mmHg | 131.7 (21.6) | 124.6 (21.6) | 126.5 (21.8) | 123.5 (20.4) | <0.001 |
| Diastolic blood pressure, mmHg | 74.5 (10.2) | 72.0 (10.4) | 71.5 (10.1) | 70.2 (10.0) | <0.001 |
| Hypertension, % yes | 59 | 38 | 41 | 38 | <0.001 |
| Blood pressure status | <0.001 | ||||
| Normotensive < 120 mmHg, % yes | 41 | 63 | 59 | 62 | |
| Hypertensive (controlled), % yes | 48 | 26 | 30 | 28 | |
| Hypertensive (uncontrolled), % yes | 12 | 12 | 12 | 11 | |
| Diabetes mellitus, % yes | 17.5 | 12.9 | 17.3 | 6 | <0.001 |
| Laboratory Values | |||||
| Total cholesterol, mg/dL | 189.6 (36.1) | 192.7 (31.8) | 198.3 (37.5) | 195.8 (35.1) | <0.001 |
| HDL cholesterol, mg/dL | 52.4 (15.2) | 49.5 (12.7) | 47.7 (13.1) | 52.3 (15.7) | <0.001 |
| LDL cholesterol, mg/dL | 116.5 (32.9) | 115.2 (29.0) | 119.8 (32.9) | 117.1 (30.1) | 0.003 |
| Triglycerides, mg/dL | 104.8 (68.8) | 142.9 (84.9) | 157.5 (101.5) | 132.9 (90.4) | <0.001 |
| Creatinine, mg/dL | 1.0 (0.3) | 0.9 (0.2) | 0.9 (0.3) | 1.0 (0.2) | <0.001 |
| Glomerular filtration rate (GFR) | 86.4 (19.2) | 82.3 (16.6) | 83.4 (18.2) | 75.9 (17.0) | <0.001 |
| Lifestyle factors | |||||
| Smoking status | <0.001 | ||||
| Never, % yes | 45 | 75 | 54 | 44 | |
| Former, % yes | 37 | 19 | 32 | 44 | |
| Current, % yes | 18 | 6 | 14 | 11 | |
| Current use of alcohol, % yes | 50 | 31 | 47 | 72 | <0.001 |
| Current medication use | |||||
| Antilipidemic therapy, % yes | 16 | 14 | 13 | 18 | <0.001 |
| Statin use, % yes | 15 | 13 | 12 | 17 | <0.001 |
| Hypertension medication use, % yes | 50 | 29 | 32 | 33 | <0.001 |
| Calcium channel blockers, % yes | 21 | 10 | 11 | 8 | <0.001 |
| Inhibitors of ADP-induced platelet aggregation, % yes | 0.2 | 0.1 | 0.3 | 0.3 | 0.6 |
| Angiotensin type 2 antagonists | 4 | 5 | 3 | 3 | 0.003 |
| Aspirin use, % yes | 31 | 18 | 26 | 40 | <0.001 |
| Diabetes medication use, % yes (diabetics only) | 79 | 74 | 80 | 70 | 0.073 |
| Diuretic use, % yes | 22 | 4 | 8 | 13 | <0.001 |
| Hormone replacement therapy % yes (females only) | 46 | 34 | 40 | 64 | <0.001 |
| Subclinical and Clinical Disease | |||||
| CAC > 0, % yes | 43 | 50 | 45 | 57 | <0.001 |
| CAC categories | <0.001 | ||||
| < 50, % | 76 | 71 | 74 | 63 | |
| 50–149, % | 10 | 14 | 11 | 11 | |
| 150–399, % | 7 | 9 | 7 | 12 | |
| > 400, % | 8 | 6 | 8 | 13 | |
| Common carotid IMT, mm | 0.9 (0.2) | 0.8 (0.2) | 0.9 (0.2) | 0.9 (0.2) | <0.001 |
| Internal carotid IMT, mm | 1.1 (0.6) | 0.9 (0.5) | 1.0 (0.6) | 1.1 (0.6) | <0.001 |
| Presence of plaque, % Yes | 44 | 26 | 39 | 47 | <0.001 |
CAC, coronary artery calcium; GFR, glomerular filtration rate; HDL, high-density lipoproteins; IMT, intima medial thickness; HGF, hepatocyte growth factor; LDL, low-density lipoprotein.
p Value from linear regression model for continuous variables and chi-square test for categorical variables comparing variables across ethnic groups.
Figure 1.
Boxplot of hepatocyte growth factor by race/ethnicity and age strata. The lower and upper edges of the rectangle are the 25th and 75th percentiles and the horizontal line within the rectangle is the median. The lines extend from each rectangle to the lowest and highest values within 1.5 × interquartile range.
Table 2.
Race-ethnicity specific association of tertiles of hepatocyte growth factor and cardiovascular disease risk factors
| African American | Chinese American | Hispanic American | Non-Hispanic white American | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Tertile 1 | Tertile 2 | Tertile 3 | p Value | Tertile 1 | Tertile 2 | Tertile 3 | p Value | Tertile 1 | Tertile 2 | Tertile 3 | p Value | Tertile 1 | Tertile 2 | Tertile 3 | p Value |
| HGF Range (pg/mL) | 342.6–813.9 | 814.3–999.6 | 1000.0–2151.8 | 292.4–738.3 | 738.3–892.1 | 892.4–2135.9 | 317.7–903.2 | 903.7–1112.9 | 1113.4–2094.1 | 313.6–783.5 | 783.6–993.1 | 993.2–2117.0 | ||||
| Age, years | 59.1 (9.3) | 62.3 (9.9) | 65.0 (10.0) | <0.001 | 57.9 (9.5) | 62.6 (9.3) | 66.6 (10.3) | <0.001 | 58.0 (9.4) | 61.3 (9.9) | 64.3 (10.7) | <0.001 | 59.3 (9.5) | 62.6 (10.1) | 65.9 (10.1) | <0.001 |
| Sex, % female | 50 | 61 | 55 | 0.39 | 52 | 48 | 54 | 0.64 | 49 | 52 | 55 | 0.08 | 47 | 52 | 57 | <0.001 |
| Body Mass Index, kg/m2 | 29.3 (5.3) | 30.4 (5.9) | 30.7 (6.2) | <0.001 | 23.2 (2.9) | 24.2 (3.3) | 24.5 (3.6) | <0.001 | 28.2 (4.5) | 29.2 (4.6) | 30.9 (5.7) | <0.001 | 26.4 (4.3) | 27.6 (4.9) | 29.2 (5.5) | <0.001 |
| Education | <0.001 | <0.001 | 0.33 | <0.001 | ||||||||||||
| ≤11th grade | 9 | 11 | 15 | 16 | 26 | 33 | 39 | 50 | 46 | 3 | 5 | 7 | ||||
| High school graduate | 16 | 19 | 23 | 12 | 18 | 19 | 21 | 18 | 23 | 14 | 15 | 22 | ||||
| Some college | 35 | 34 | 36 | 25 | 16 | 19 | 30 | 22 | 24 | 23 | 29 | 33 | ||||
| Bachelor’s degree | 21 | 19 | 13 | 28 | 22 | 18 | 5 | 7 | 5 | 27 | 23 | 17 | ||||
| Graduate/professional school | 20 | 17 | 13 | 20 | 18 | 11 | 6 | 4 | 3 | 33 | 29 | 20 | ||||
| Income | 0.002 | 0.08 | 0.10 | <0.001 | ||||||||||||
| < $25,000 | 22 | 32 | 37 | 41 | 49 | 60 | 44 | 50 | 55 | 11 | 14 | 23 | ||||
| $25,000 – $50,000 | 31 | 32 | 34 | 22 | 21 | 23 | 35 | 33 | 31 | 23 | 26 | 32 | ||||
| $50,000 – $100,000 | 37 | 27 | 23 | 24 | 19 | 12 | 19 | 15 | 12 | 33 | 35 | 30 | ||||
| ≥ $100,000 | 10 | 9 | 6 | 13 | 11 | 6 | 3 | 3 | 2 | 33 | 25 | 15 | ||||
| Systolic Blood Pressure, mmHg | 128.4 (20.0) | 131.6 (22.2) | 135.2 (22.1) | 0.01 | 118.6 (18.8) | 125.6 (21.7) | 129.8 (22.7) | 0.02 | 121.0 (19.4) | 126.9 (21.2) | 131.8 (23.2) | <0.001 | 119.3 (18.7) | 122.7 (19.9) | 128.4 (21.7) | <0.001 |
| Hypertension, % Yes | 49 | 59 | 70 | <0.001 | 25 | 38 | 49 | 0.001 | 30 | 41 | 53 | <0.001 | 29 | 36 | 50 | <0.001 |
| Diabetes Mellitus, % Yes | 11 | 17 | 24 | <0.001 | 6 | 13 | 20 | <0.001 | 8 | 19 | 25 | <0.001 | 2 | 5 | 11 | <0.001 |
| Total Cholesterol, mg/dL | 190.0 (35.6) | 192.8 (36.8) | 186.0 (35.6) | <0.001 | 194.0 (32.8) | 190.1 (32.0) | 194.1 (30.5) | 0.47 | 202.4 (38.0) | 200.9 (37.9) | 191.6 (35.5) | <0.001 | 195.3 (33.3) | 197.3 (36.1) | 194.9 (35.8) | 0.31 |
| HDL Cholesterol, mg/dL | 54.1 (15.4) | 52.3 (15.4) | 50.8 (14.7) | <0.001 | 51.3 (13.7) | 49.1 (11.8) | 48.1 (12.3) | <0.001 | 49.2 (13.9) | 47.4 (12.9) | 46.4 (12.2) | <0.001 | 54.2 (16.7) | 52.2 (15.4) | 50.4 (14.7) | <0.001 |
| Triglycerides, mg/dL | 97.2 (75.2) | 107.3 (70.3) | 109.9 (59.5) | <0.001 | 133.0 (83.7) | 135.3 (72.2) | 160.5 (94.9) | <0.001 | 155.6 (111.6) | 162.4 (109.2) | 154.5 (80.9) | 0.82 | 117.8 (69.4) | 133.6 (105.1) | 147.3 (90.6) | <0.001 |
| Creatinine, mg/dL | 1.0 (0.2) | 1.0 (0.2) | 1.0 (0.3) | <0.001 | 0.9 (0.2) | 0.9 (0.2) | 0.9 (0.3) | 0.03 | 0.9 (0.2) | 0.9 (0.2) | 0.9 (0.4) | 0.68 | 0.9 (0.2) | 1.0 (0.2) | 1.0 (0.2) | <0.001 |
| Glomerular Filtration Rate (GFR) | 88.4 (17.0) | 85.9 (18.5) | 84.8 (21.7) | 0.43 | 85.2 (14.1) | 83.0 (16.4) | 78.7 (18.5) | 0.3 | 83.6 (15.8) | 83.9 (17.4) | 82.7 (21.0) | 0.005 | 78.3 (20.4) | 75.6 (13.8) | 73.8 (15.9) | 0.15 |
| Current Smoker, % Yes | 13 | 17 | 23 | <0.001 | 3 | 6 | 8 | <0.001 | 9 | 14 | 18 | <0.001 | 8 | 11 | 15 | <0.001 |
| Current Use of Alcohol, % Yes | 52 | 51 | 46 | 0.58 | 34 | 32 | 28 | 0.45 | 51 | 49 | 43 | 0.56 | 76 | 73 | 66 | <0.001 |
| Antilipidemic Therapy, % Yes | 15 | 16 | 19 | 0.91 | 9 | 14 | 20 | 0.02 | 12 | 13 | 14 | 0.22 | 15 | 18 | 22 | 0.008 |
| Statin Use, % Yes | 14 | 15 | 18 | 0.89 | 9 | 12 | 17 | 0.4 | 11 | 12 | 13 | 0.25 | 14 | 16 | 20 | 0.05 |
| Hypertension Medication Use, % Yes | 40 | 49 | 62 | <0.001 | 18 | 28 | 40 | <0.001 | 24 | 31 | 42 | <0.001 | 24 | 33 | 42 | <0.001 |
| Calcium Channel Blockers, % Yes | 18 | 17 | 28 | 0.005 | 5 | 10 | 14 | 0.03 | 9 | 9 | 14 | 0.03 | 6 | 6 | 11 | 0.01 |
| Angiotensin Type 2 Antagonists | 4 | 3 | 5 | 0.36 | 3 | 4 | 9 | 0.07 | 1 | 3 | 4 | 0.02 | 2 | 2 | 4 | 0.1 |
| Aspirin Use, % Yes | 29 | 30 | 33 | 0.51 | 10 | 21 | 24 | 0.03 | 23 | 26 | 30 | 0.61 | 35 | 41 | 42 | 0.55 |
| Diabetes Medication Use, % Yes | 80 | 74 | 83 | 0.31 | 67 | 79 | 72 | 0.85 | 66 | 84 | 82 | 0.12 | 47 | 70 | 75 | 0.26 |
| Diuretic Use, % Yes | 18 | 21 | 26 | 0.17 | 2 | 5 | 6 | 0.004 | 5 | 8 | 12 | 0.004 | 7 | 12 | 21 | <0.001 |
| Females Only | ||||||||||||||||
| Hormone Replacement Therapy, % Yes | 55 | 45 | 40 | 0.004 | 44 | 31 | 28 | 0.09 | 45 | 41 | 34 | 0.76 | 68 | 69 | 57 | <0.001 |
| Post-menopause, % Yes | 82 | 85 | 87 | 0.65 | 78 | 85 | 94 | 0.55 | 82 | 90 | 90 | 0.95 | 79 | 85 | 90 | 0.33 |
GFR, glomerular filtration rate; HDL, high-density lipoproteins; HGF, hepatocyte growth factor.
p Values from a linear regression model adjusted for age and sex.
Education and income level did not impact the effect, therefore, Table 3 summarizes the association of HGF with atherosclerotic disease after adjustment for traditional risk factors. In fully adjusted models, one standard deviation (SD) increase in HGF was associated with an average increase in CAC of 55 Agatston units for non-Hispanic white Americans (p<0.001) and 51 for African (p=0.007) Americans, with no significant association in either Chinese or Hispanic Americans. A formal test of the race/ethnicity interaction term was significant (p=0.02). Similar results were observed for models limited to CAC>0 and when modeling any CAC. For those participants with measurable CAC, a one SD increase in HGF associated with an average increase in CAC of 33 Agatston units for non-Hispanic white Americans (p=0.025) and 53 for African (p=0.01) Americans. However, when modeling any CAC, we only see a significant increase in odds in Non-Hispanic whites (OR=1.23, 95% CI 1.10–1.36).
Table 3.
Association of hepatocyte growth factor and subclinical and clinical atherosclerotic disease
| Pooled Sample | Race/Ethnicity Interaction p Value |
African American | Chinese American | Hispanic American |
Non-Hispanic
white American |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| *Beta (S.E.) | p Value | Beta (S.E.) | p Value | Beta (S.E.) | p Value | Beta (S.E.) | p Value | Beta (S.E.) | p Value | ||
| CAC, Agatston Score | |||||||||||
| Model 1 | 65 (8) | <0.001 | 0.031 | 71 (18) | <0.001 | 31 (19) | 0.097 | 38 (19) | 0.044 | 86 (13) | <0.001 |
| Model 2 | 37 (8.9) | <0.001 | 0.022 | 51 (19) | 0.007 | −2.5 (19) | 0.90 | 17 (20) | 0.39 | 55 (14) | <0.001 |
| CAC>0, Agatston Score | |||||||||||
| Model 1 | 37 (9) | <0.001 | 0.041 | 62 (20) | 0.002 | −3 (19) | 0.87 | 14 (21) | 0.50 | 46 (13) | <0.001 |
| Model 2 | 23 (10) | 0.016 | 0.028 | 53 (21) | 0.011 | −24 (19) | 0.22 | 11 (22) | 0.62 | 33 (15) | 0.025 |
| Common Carotid IMT, mm | |||||||||||
| Model 1 | 0.009 (0.002) | <0.001 | 0.53 | 0.007 (0.004) | 0.13 | 0.015 (0.007) | 0.042 | 0.008 (0.004) | 0.069 | 0.01 (0.004) | 0.005 |
| Model 2 | −0.001 (0.002) | 0.81 | 0.74 | 0.002 (0.005) | 0.62 | 0.001 (0.007) | 0.88 | −0.009 (0.005) | 0.84 | −0.004 (0.004) | 0.28 |
| Internal Carotid IMT, mm | |||||||||||
| Model 1 | 0.06 (0.007) | <0.001 | <0.001 | 0.036 (0.015) | 0.015 | 0.05 (0.02) | 0.014 | 0.03 (0.014) | 0.035 | 0.098 (0.012) | <0.001 |
| Model 2 | 0.036 (0.008) | <0.001 | <0.001 | 0.014 (0.015) | 0.35 | 0.029 (0.021) | 0.18 | 0.008 (0.015) | 0.60 | 0.071 (0.013) | <0.001 |
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | ||
| Presence of Plaque | |||||||||||
| Model 1 | 1.19 (1.13–1.26) | <0.001 | 0.35 | 1.11 (1.0 – 1.24) | 0.045 | 1.21 (0.99–1.48) | 0.070 | 1.18 (1.06–1.32) | 0.004 | 1.26 (1.15–1.38) | <0.001 |
| Model 2 | 1.10 (1.04–1.17) | 0.002 | 0.37 | 1.04 (0.93–1.16) | 0.52 | 1.10 (0.89–1.37) | 0.38 | 1.12 (0.99–1.27) | 0.064 | 1.14 (1.04–1.26) | 0.007 |
| Any CAC, Agatston Score | |||||||||||
| Model 1 | 1.24 (1.16–1.31) | <0.001 | 0.052 | 1.15 (1.03–1.28) | 0.012 | 1.31 (1.07–1.60) | 0.009 | 1.14 (1.01–1.28) | 0.029 | 1.39 (1.26–1.53) | <0.001 |
| Model 2 | 1.12 (1.05–1.19) | <0.001 | 0.11 | 1.07 (0.96–1.20) | 0.22 | 1.13 (0.92–1.39) | 0.25 | 1.05 (0.93–1.20) | 0.43 | 1.23 (1.10–1.36) | <0.001 |
| Number of CHD Events | 529 | 134 | 48 | 109 | 238 | ||||||
| Total Person-Years | 72833 | 19552 | 8805 | 15709 | 28767 | ||||||
| Crude CHD Rate, per 1,000 person-years | 7.3 | 6.9 | 5.5 | 6.9 | 8.3 | ||||||
| HR (95% CI) | pValue | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | ||
| Time to Coronary Heart Disease | |||||||||||
| Model 1 | 1.30 (1.20–1.41) | <0.001 | 0.24 | 1.47 (1.27–1.71) | <0.001 | 1.32 (0.98–1.77) | 0.067 | 1.13 (0.95–1.35) | 0.17 | 1.30 (1.15–1.47) | <0.001 |
| Model 2 | 1.20 (1.10–1.31) | <0.001 | 0.18 | 1.41 (1.20–1.66) | <0.001 | 1.17 (0.84–1.62) | 0.36 | 1.06 (0.87–1.28) | 0.56 | 1.17 (1.02–1.33) | 0.026 |
CAC, coronary artery calcium; CHD, coronary heart disease; IMT, intima medial thickness.
Results are reported per standard deviation increase in hepatocyte growth factor (SD=259 pg/mL).
Model 1 = age and sex (+ race/ethnicity in pooled analyses).
Model 2 = age, sex, BMI, systolic blood pressure, hypertension treatment, total cholesterol, HDL cholesterol, smoking and diabetes status (+ race/ethnicity in pooled analyses).
There was race/ethnicity differences in the relation between HGF and internal carotid IMT with a 1 SD increase in HGF associated with 0.07 mm higher internal carotid IMT in non-Hispanic whites (p<0.001). In contrast, levels of HGF were not associated with common carotid IMT in any of the four race/ethnicities. Furthermore, we observed an increase in the odds of the presence of plaque (OR=1.10 per SD of HGF; p=0.002); similar effect sizes were observed for Chinese (OR=1.10; p=0.38), Hispanic (OR=1.12; p=0.064) and non-Hispanic White Americans (OR=1.14; p<0.001).
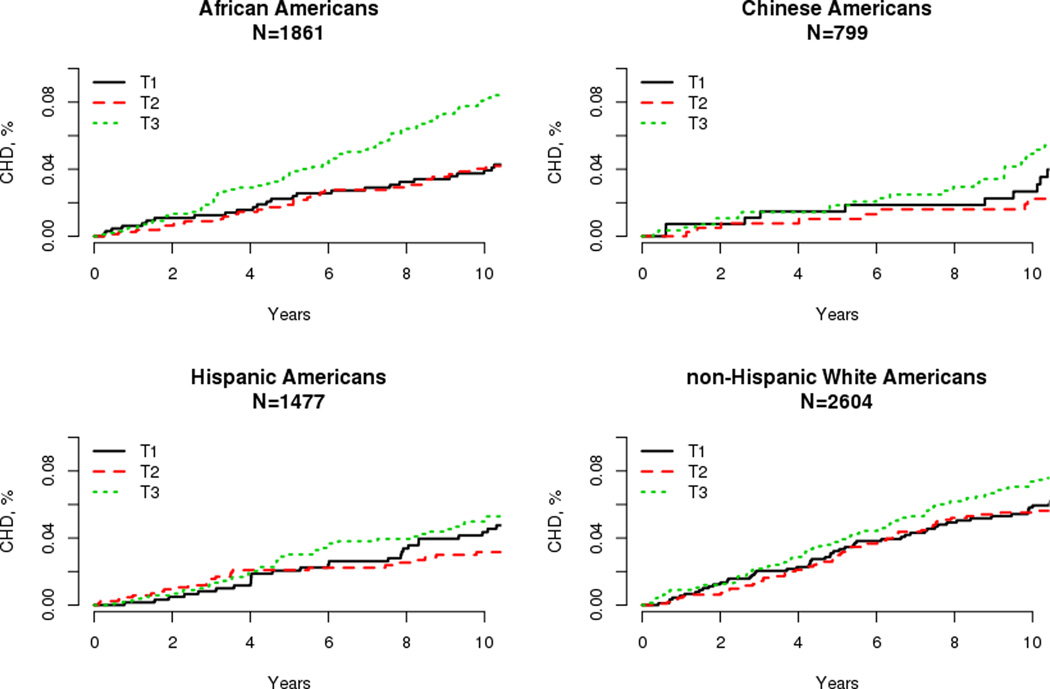
There were 529 incident CHD events during the follow-up period. We observed racial/ethnic differences in crude CHD rates, with non-Hispanic whites having the highest rates at 8.3 per 1000 person years, followed by Hispanic and African (6.9), and Chinese (5.5) Americans. In models adjusted for traditional risk factors, CHD risk was 41% higher per SD increase in HGF in African (p<0.001) and 17% in non-Hispanic whites (p=0.026). Although not statistically significant, we observed a 17% increase in CHD risk in Chinese (p=0.36), and 6% in Hispanic (p=0.56) Americans. Similarly, adjusted Kaplan Meier curves of the cumulative incidence of CHD by tertile of HGF displayed racial/ethnic differences (Figure 2). However, the formal test of interaction between the races was not significant (p=0.18). In race/ethnicity pooled analyses, each 1 SD increase in HGF was associated with a 20% higher risk of CHD (p<0.001).
Figure 2.
Kaplan-Meier curves for incident coronary heart disease by tertile of hepatocyte growth factor by Race/Ethnicity.
To assess the impact of healthy participant bias in MESA, we conducted sensitivity analyses stratifying by age. Stratifying the MESA cohort into two age groups (i.e., 45–64 and 65–84), we observed similar associations with subclinical disease (Table 4). In contrast, a significant difference by age group was observed for incident CHD with a 1 SD increase in HGF associated with a 32% increased risk of CHD in the younger group (p<0.001), while a 12% increased risk was observed in the older group (p=0.04, age by HGF interaction p=0.03).
Table 4.
Association of hepatocyte growth factor and subclinical and clinical atherosclerotic disease by baseline age grouping
| Exam 1 Age 45–64 (n=3790) | Exam 1 Age 65–84 (n=2951) | |||
|---|---|---|---|---|
| Beta (S.E.) | p Value | Beta (S.E.) | p Value | |
| CAC, Agatston Score | ||||
| Model 1 | 56 (8.3) | <0.001 | 61 (13) | <0.001 |
| Model 2 | 27 (8.8) | 0.002 | 37 (14) | 0.008 |
| Common Carotid IMT, mm | ||||
| Model 1 | 0.015 (0.003) | <0.001 | 0.001 (0.004) | 0.75 |
| Model 2 | 0.003 (0.003) | 0.27 | −0.005 (0.004) | 0.17 |
| Internal Carotid IMT, mm | ||||
| Model 1 | 0.054 (0.008) | <0.001 | 0.065 (0.014) | <0.001 |
| Model 2 | 0.025 (0.008) | 0.002 | 0.044 (0.014) | 0.002 |
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Presence of Plaque | ||||
| Model 1 | 1.23 (1.14–1.33) | <0.001 | 1.16 (1.07–1.26) | <0.001 |
| Model 2 | 1.10 (1.01–1.19) | 0.028 | 1.11 (1.02–1.21) | 0.02 |
| Number of CHD Events | 193 | 336 | ||
| Total Person-Years | 43,379 | 29,454 | ||
| Crude CHD Rate, per 1,000 person-years | 4.4 | 11.4 | ||
| HR (95% CI) | p Value | HR (95% CI) | pValue | |
| Time to Coronary Heart Disease | ||||
| Model 1 | 1.52 (1.35–1.72) | <0.001 | 1.18 (1.06–1.31) | 0.002 |
| Model 2 | 1.32 (1.15–1.51) | <0.001 | 1.12 (1.01–1.26) | 0.04 |
CAC, coronary artery calcium; CHD, coronary heart disease; IMT, intima medial thickness.
Model 1 = age, sex and race/ethnicity.
Model 2 = age, sex, BMI, systolic blood pressure, hypertension treatment, total cholesterol, HDL cholesterol, smoking and diabetes status and race/ethnicity.
DISCUSSION
HGF was positively associated with subclinical and clinical CHD in this large and diverse population. Increased circulating levels of HGF were associated with greater CAC and incident CHD in African and non-Hispanic white Americans independent of other cardiovascular risk factors. In contrast, levels of HGF were not associated with subclinical disease in Chinese or Hispanic Americans nor were levels of HGF significantly associated with increased risk of CHD in these groups. These findings provide initial evidence that HGF may be to be a valuable clinical marker and further demonstrate the utility of HGF may be limited to specific populations.
HGF is thought to be primarily produced by mesenchymal cells and acts on cells expressing MET.[2] HGF and MET expression are stimulated in response to tissue injury resulting in the activation of anti-apoptotic pathways, increased angiogenesis, and upregulation of IL-10, a cytokine that limits inflammation.[17] Additionally, activation of HGF/Met enhances critical protective pathways that act against hypoxia-induced autophagy.[18] A substantial body of evidence exists regarding the cardioprotective effects of these tissue activities in atherosclerotic heart disease, which have been summarized previously.[2] In contrast, the angiogenic properties of HGF and associations of circulating HGF and cardiovascular risk factors and disease implicate HGF in atherosclerotic disease. Despite the uncertainty regarding the role of HGF in atherosclerosis, HGF has been hypothesized as a potential biomarker for disease prediction as well as a biomarker of disease burden. Herein, we demonstrate racial/ethnic heterogeneity in the association of HGF with atherosclerotic heart disease.
The mechanisms underlying the racial/ethnic heterogeneity in the association of HGF with CAC and internal carotid IMT remain unclear. However, these results support the notion that subclinical measures of atherosclerosis may not reflect the extent of disease similarly across race/ethnicity groups. Numerous studies, including MESA, have found that non-Hispanic whites have higher prevalence and density of CAC compared other race/ethnicities and that African Americans have the lowest prevalence.[19, 20] Our novel finding that higher HGF is associated with CAC in both non-Hispanic whites and African Americans suggest that despite the distributional differences in CAC between the two groups, HGF is a potential biomarker of underlying disease.
Prior investigations of HGF and IMT have focused predominantly in small Japanese clinical populations.[7, 21] The largest study investigated 317 Japanese residents and reported that those in the upper 50th percentile of HGF had increased common carotid IMT compared to those with lower levels.[6] Of note, a previous MESA study demonstrated that common carotid IMT predicted CHD, albeit not as strongly as CAC.[22] In contrast and despite the large sample size in MESA, HGF was associated specifically with internal carotid IMT in non-Hispanic whites. These seemingly mixed results need to be viewed in the context of MESA IMT measurements. The common carotid artery IMT measurements were made below the bulb but did not consider presence or absence of early plaque and thus there was no exclusion of plaque. Therefore, this IMT measure is less of a surrogate for atherosclerosis and more likely related to hypertrophy of the medial layer. In contrast, the internal carotid artery IMT focused on capturing any plaque present in either the bulb or proximal internal carotid artery and thus there are site-specific differences in the association of risk factors and these two IMT measurements.[23]
Similar to the results for CAC, the increased risk of CHD in those with higher levels of HGF was most compelling for African and non-Hispanic white Americans. However, in contrast to the results for subclinical disease, low CHD event numbers in Chinese and Hispanic Americans may be impacting our ability to detect meaningful associations. The increased risk of CHD observed with higher levels of HGF in MESA, extends our knowledge of this relationship beyond highly selected clinical populations that have dominated the literature to date. For example, higher HGF has been associated with increased risk of death in heart failure patients,[24] and in patients undergoing percutaneous coronary revascularization.[25] Likewise, higher levels of HGF are associated with acute MI. [9] Herein we show that levels of HGF predict the development of clinical disease.
HGF is released in response to tissue injury and thus we expect circulating levels to be associated with adverse risk factors. In relatively small clinical populations, circulating HGF has been associated with advanced age, current smoking and diabetes,[10, 25–27] and systolic blood pressure.[7, 28, 29] Likewise, obesity is associated with higher levels of HGF[12, 30] with concomitant decreases following weight loss.[31] In MESA, we more fully elucidated the shared and race/ethnicity specific relationships with traditional cardiovascular risk factors. Collectively, these results support the hypothesis that HGF levels are associated with a more adverse risk profile suggestive of systemic inflammation and endothelial injury. We further demonstrate that HGF levels add additional information as to underlying disease risk.
The major limitation of the study is the relatively small number of CHD events in Chinese and Hispanic Americans that may have hindered our ability to detect an association with HGF. Likewise, our ability to formally evaluate the prognostic value of HGF is limited by the number of events to date and thus will be the focus of future research as the cohort ages. Furthermore, MESA participants were required to be free of known CVD at baseline and thus are likely more representative of a primary prevention population as opposed to the general population. Healthy participant bias may be a factor and one that is likely stronger in the older ages. To attempt to understand if bias could affect the association of HGF and incident CHD, we stratified the cohort by age and observed a stronger association in the 45–64 year olds then in the older group. These results suggest that our pooled point estimate could be biased toward the null, and the increased risk of CHD per SD of HGF could be higher in a general population sample. Strengths of the study include the large sample sizes in four race/ethnicity groups as prior investigations were limited in both size and diversity. Given the high prevalence of CAC in all four racial/ethnic groups (>40%), there was adequate power to detect an association in race/ethnicity stratified analyses.
Conclusion
In a large and diverse population-based cohort, we report that HGF is associated with subclinical and incident CHD. We demonstrate evidence of racial/ethnic heterogeneity within these associations, as the results are most compelling in African and non-Hispanic white Americans. We provide evidence that HGF is a biomarker of atherosclerotic disease that is independent of traditional risk factors. However, further research is needed to assess HGF as a clinically useful risk marker of atherosclerotic disease.
Key questions.
What is already known about this subject?
HGF has previously been associated with cardiovascular risk factors and atherosclerotic disease.
What does this this study add?
Previous studies have predominantly focused on clinical populations that were limited in scope, sample size, and racial/ethnic diversity. By using the Multi-Ethnic Study of Atherosclerosis (MESA), we observed that each standard deviation higher HGF was associated with an average increase in coronary artery calcium of 55 Agatston units for non-Hispanic white and 51 for African Americans, but was not in the other race/ethnic groups (interaction p=0.02). Likewise, coronary heart disease risk was 41% higher in African (p<0.001), 17% in non-Hispanic white (p=0.026) and Chinese (p=0.36), and 6% in Hispanic (p=0.56) Americans per standard deviation increase in HGF.
How might this impact on clinical practice?
These findings provide initial evidence that HGF may be to be a valuable clinical marker and further demonstrate the utility of HGF may be limited to specific populations.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. MESA is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with MESA investigators.
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive license (or non-exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in HEART editions and any other BMJPGL products to exploit all subsidiary rights.
Funding This research was supported by the NIH contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute (NHLBI) at NIH and by grants UL1-TR-000040 and UL1-TR-001079 from National Center for Research Resources at NIH. Funding for adhesion protein levels was provided by the NHLBI by grant R01 HL98077. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Contributors
Study design: SJB, CB, PAD, NBL, EJB, CLW
Data collection: SJB, NQH, MYT
Data analysis: PAD, NBL, MA
Outcome adjudication: MB, JFP
Manuscript draft: All authors contributed, read and approved the final manuscript.
Completing interests None declared.
Ethics approval MESA and its ancillary studies were approved by the Institutional Review Board at participating centers and all participants gave written informed consent.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement MESA Coordinating Center distributes only deidentified Datasets (with HIPAA defined identifiers removed) to Recipient Investigators with approved manuscripts proposals in compliance with MESA and NHLBI/NIH data privacy and sharing standard practices and policy. Additionally, access to MESA datasets require completion of a Data Distribution Agreement (MESA Data and Materials Distribution Agreement if the Investigator is not at a MESA affiliated Institution) and review and approval of a detailed proposal by MESA Publications and Steering Committees for soundness in science, methodology, and adherence to MESA policy. Manuscript pen drafts (and presentation abstracts) are also reviewed by these same committees.
REFERENCES
- 1.Nakamura T, Nawa K, Ichihara A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun. 1984;122:1450–1459. doi: 10.1016/0006-291x(84)91253-1. [DOI] [PubMed] [Google Scholar]
- 2.Gallo S, Sala V, Gatti S, et al. Cellular and molecular mechanisms of HGF/Met in the cardiovascular system. Clin Sci. 2015;129:1173–1193. doi: 10.1042/CS20150502. [DOI] [PubMed] [Google Scholar]
- 3.Bussolino F, Di Renzo MF, Ziche M, et al. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992;119:629–641. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant DS, Kleinman HK, Goldberg ID, et al. Scatter factor induces blood vessel formation in vivo. Proc Natl Acad Sci U S A. 1993;90:1937–1941. doi: 10.1073/pnas.90.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tajima H, Higuchi O, Mizuno K, et al. Tissue distribution of hepatocyte growth factor receptor and its exclusive down-regulation in a regenerating organ after injury. J Biochem (Tokyo) 1992;111:401–406. doi: 10.1093/oxfordjournals.jbchem.a123769. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto Y, Kohara K, Tabara Y, et al. Plasma hepatocyte growth factor and the relationship between risk factors and carotid atherosclerosis. Hypertens Res. 2002;25:661–667. doi: 10.1291/hypres.25.661. [DOI] [PubMed] [Google Scholar]
- 7.Kawamoto R, Oka Y, Yoshida O, et al. Significance of serum circulating hepatocyte growth factor in the development of carotid atherosclerosis. J Atheroscler Thromb. 2003;10:154–159. doi: 10.5551/jat.10.154. [DOI] [PubMed] [Google Scholar]
- 8.Tateishi J, Waku S, Masutani M, et al. Hepatocyte growth factor as a potential predictor of the presence of atherosclerotic aorto-iliac artery disease. Am Heart J. 2002;143:272–276. doi: 10.1067/mhj.2002.120151. [DOI] [PubMed] [Google Scholar]
- 9.Sato T, Yoshinouchi T, Sakamoto T, et al. Hepatocyte growth factor(HGF): a new biochemical marker for acute myocardial infarction. Heart Vessels. 1997;12:241–246. doi: 10.1007/BF02766790. [DOI] [PubMed] [Google Scholar]
- 10.Lamblin N, Susen S, Dagorn J, et al. Prognostic significance of circulating levels of angiogenic cytokines in patients with congestive heart failure. Am Heart J. 2005;150:137–143. doi: 10.1016/j.ahj.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 11.Bancks MP, Bielinski SJ, Decker PA, et al. Circulating level of hepatocyte growth factor predicts incidence of type 2 diabetes mellitus: the Multi-Ethnic Study of Atherosclerosis (MESA) Metabolism. 2016;65:64–72. doi: 10.1016/j.metabol.2015.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lieb W, Safa R, Benjamin EJ, et al. Vascular endothelial growth factor, its soluble receptor, and hepatocyte growth factor: clinical and genetic correlates and association with vascular function. Eur Heart J. 2009;30:1121–1127. doi: 10.1093/eurheartj/ehp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larson NB, Berardi C, Decker PA, et al. Trans-ethnic meta-analysis identifies common and rare variants associated with hepatocyte growth factor levels in the Multi-Ethnic Study of Atherosclerosis (MESA) Ann Hum Genet. 2015;79:264–274. doi: 10.1111/ahg.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 15.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 16.Tobin J. Estimation for relationships with limited dependent variables. Econometrica. 1958;26:24–36. [Google Scholar]
- 17.Rutella S, Bonanno G, Procoli A, et al. Hepatocyte growth factor favors monocyte differentiation into regulatory interleukin (IL)-10 + + IL-12low/neg accessory cells with dendritic-cell features. Blood. 2006;108:218–227. doi: 10.1182/blood-2005-08-3141. [DOI] [PubMed] [Google Scholar]
- 18.Gallo S, Gatti S, Sala V, et al. Agonist antibodies activating the Met receptor protect cardiomyoblasts from cobalt chloride-induced apoptosis and autophagy. Cell Death Dis. 2014;5:e1185. doi: 10.1038/cddis.2014.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bild DE, Detrano R, Peterson D, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111:1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 20.Lee TC, O’Malley PG, Feuerstein I, et al. The prevalence and severity of coronary artery calcification on coronary artery computed tomography in black and white subjects. J Am Coll Cardiol. 2003;41:39–44. doi: 10.1016/s0735-1097(02)02618-9. [DOI] [PubMed] [Google Scholar]
- 21.Satani K, Konya H, Hamaguchi T, et al. Clinical significance of circulating hepatocyte growth factor, a new risk marker of carotid atherosclerosis in patients with Type 2 diabetes. Diabet Med. 2006;23:617–622. doi: 10.1111/j.1464-5491.2006.01849.x. [DOI] [PubMed] [Google Scholar]
- 22.Gepner AD, Young R, Delaney JA, et al. Comparison of coronary artery calcium presence, carotid plaque presence, and carotid intima-media thickness for cardiovascular disease prediction in the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2015:8. doi: 10.1161/CIRCIMAGING.114.002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polak JF, Post WS, Carr JJ, et al. Associations of common carotid intima-media thickness with coronary heart disease risk factors and events vary with distance from the carotid bulb. J Am Soc Echocardiogr. 2014;27:991–997. doi: 10.1016/j.echo.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rychli K, Richter B, Hohensinner PJ, et al. Hepatocyte growth factor is a strong predictor of mortality in patients with advanced heart failure. Heart. 2011;97:1158–1163. doi: 10.1136/hrt.2010.220228. [DOI] [PubMed] [Google Scholar]
- 25.Susen S, Sautiere K, Mouquet F, et al. Serum hepatocyte growth factor levels predict long-term clinical outcome after percutaneous coronary revascularization. Eur Heart J. 2005;26:2387–2395. doi: 10.1093/eurheartj/ehi436. [DOI] [PubMed] [Google Scholar]
- 26.Rajpathak SN, Wassertheil-Smoller S, Crandall J, et al. Hepatocyte growth factor and clinical diabetes in postmenopausal women. Diabetes Care. 2010;33:2013–2015. doi: 10.2337/dc10-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto Y, Kohara K, Tabara Y, et al. Association between carotid arterial remodeling and plasma concentration of circulating hepatocyte growth factor. J Hypertens. 2001;19:1975–1979. doi: 10.1097/00004872-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura Y, Morishita R, Nakamura S, et al. A vascular modulator, hepatocyte growth factor, is associated with systolic pressure. Hypertension. 1996;28:409–413. doi: 10.1161/01.hyp.28.3.409. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura S, Moriguchi A, Morishita R, et al. A novel vascular modulator, hepatocyte growth factor (HGF), as a potential index of the severity of hypertension. Biochem Biophys Res Commun. 1998;242:238–243. doi: 10.1006/bbrc.1997.7800. [DOI] [PubMed] [Google Scholar]
- 30.Rehman J, Considine RV, Bovenkerk JE, et al. Obesity is associated with increased levels of circulating hepatocyte growth factor. J Am Coll Cardiol. 2003;41:1408–1413. doi: 10.1016/s0735-1097(03)00231-6. [DOI] [PubMed] [Google Scholar]
- 31.Swierczynski J, Korczynska J, Goyke E, et al. Serum hepatocyte growth factor concentration in obese women decreases after vertical banded gastroplasty. Obes Surg. 2005;15:803–808. doi: 10.1381/0960892054222678. [DOI] [PubMed] [Google Scholar]