Abstract
Pralatrexate is a folic acid analog metabolic inhibitor similar to methotrexate, that has shown tolerability and efficacy with an overall response rate of 45% in a phase 1 dose de-escalation study of patients with relapsed/refractory cutaneous T-cell lymphoma (CTCL). The object of this phase I/II open-label, multi-center clinical trial was to determine the maximum tolerated dose (MTD) and recommended dose of pralatrexate plus oral bexarotene in 34 patients with relapsed/refractory CTCL who had failed prior systemic therapies. Pralatrexate was administered by IV push at 15 mg/m2 given weekly 3 weeks out of 4 weeks with daily oral bexarotene (150 or 300 mg/m2), levothyroxine, atorvastatin, folate, and with B12 every two months. At the maximum tolerated dose (MTD), the response rate was 60% (4 CR, 14 PR), the maximum observed response duration was 28.9+ months, and duration of response for 4 CRs ranged from 9.0-28.3 months. The median progression free survival was 12.8 months (0.5-29.9). Mucositis was the most common adverse event. The combination of pralatrexate (15 mg/m2) and oral bexarotene (150 mg/m2) is active with high response rates and minimal toxicity for cutaneous T-cell lymphomas.
Keywords: Cutaneous T-cell lymphoma, Mycosis Fungoides, Sézary Syndrome, Folic Acid Inhibitor, rexinoid
Introduction
Cutaneous T-cell lymphomas (CTCLs) are extra-nodal, non-Hodgkin's lymphomas, distinguished by their clinical features and clonal T-cell surface receptors.1 The most common subtype of CTCL is mycosis fungoides (MF) accounting for 60% of new diagnoses, with Sézary syndrome (SS) or erythrodermic, leukemic variant representing 10%, and CD30+ primary cutaneous anaplastic T cell lymphoma (pc-ALCL) 20%. Although MF patients often have an indolent course limited to skin patches and/or plaques, MF patients with tumors, extra-cutaneous disease, and large cell transformation experience a more aggressive disease behavior and have a worse overall survival.2-4 Skin directed therapies are preferred for early stage MF (T1, T2), but patients with transformed MF and advanced stages need additional systemic therapy. Currently available systemic therapies have demonstrated response rates of 25-50%.5-8
Pralatrexate is an anti-folate 9, 10 with demonstrated tolerability and activity in subjects with relapsed/refractory peripheral T cell lymphoma (PTCL) and CTCL However, the approved dose of pralatrexate for PTCL is 30 mg/m2 weekly for 6 of 7 weeks. 10, 11 In a CTCL specific study, the dose of 15 mg/m2 pralatrexate given weekly 3 of 4 weeks was identified as active and was better tolerated than the full standard dosing with an overall response rate of 45% among patients with relapsed or refractory disease.12 The dose limiting toxicity for pralatrexate is mucositis;12 bexarotene induces central hypothyroidism and hypertriglyceridemia.13 The overall response rate in advanced MF patients treated with an initial dose of 300 mg/m2/day of oral bexarotene was 45%.13 Heterogeneous patient populations and evolving staging criteria over time do not allow head to head comparisons of response rates. Pre-clinical studies in MF/SS cell lines, patients' Sézary cells, and a mouse xenograph model support the hypothesis that pralatrexate combined with bexarotene may be synergistic in human subjects. (Xiang, unpublished data)
The object of this phase I/II open-label, multi-center clinical trial was to determine the maximum tolerated dose (MTD) and recommended dose of pralatrexate plus oral bexarotene in patients with relapsed/refractory CTCL who had failed prior systemic therapies.
Study Design
This was a multi-center, open-label, non-randomized dose finding study (PDX-018; NCT01134341) conducted between July 15, 2010 and March 9, 2015. It followed a standard 3+3 dose escalation design to determine the maximum tolerated dose using the dose levels in Table 1. The study was conducted in accordance with the Good Clinical Practices in accordance with the US Department of Health and Human Services. All patients signed informed consent approved by each center's IRB. As shown in Figure 1, pralatrexate was administered by IV infusion of 30 seconds to 5 minutes once weekly for 3 weeks of a 4-week treatment cycle. Subjects could be treated until progression or intolerance. Bexarotene was self-administered orally once daily with food. Vitamin B12 (1 mg IM every 8-10 weeks) and folic acid (1-1.25 mg PO once daily) were supplements administered at least 7 days prior to initiation of the study treatment and continued until 30 days after last dose of study drug.
Table 1. Patient cohorts and Drug Escalation Criteria.
| Cohorts | Pralatrexate Dose (IV) | Bexarotene Dosea (PO) |
|---|---|---|
| Cohort 1 | 15 mg/m2 | 150 mg/m2 |
| Cohort 2a | 15 mg/m2 | 300 mg/m2 |
| Cohort 2b | 10 mg/m2 | 150 mg/m2 |
| Cohort 3 | 10 mg/m2 | 300 mg/m2 |
Dose of bexarotene determined by guidelines per package insert.
Figure 1.
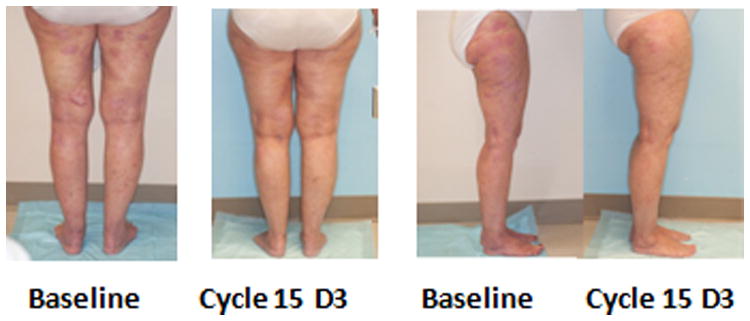
Clinical partial response to pralatrexate and bexarotene comparing baseline to course 15 day 3. The patient was on drug for over two years with almost complete CR.
Inclusion/Exclusion Criteria
CTCL patients with MF stage ≥IB, Sézary Syndrome, or primary cutaneous anaplastic large cell lymphoma were eligible if they had relapsed or were refractory to at least one prior systemic therapy or were intolerant to their last prior therapy. The required ECOG status was 0-2. Exclusion criteria included having a history of prior malignancies with disease-free duration <5 years unless treatment resulted in complete resolution with no current clinical, radiologic or laboratory evidence of active or recurrent disease. Patients with active central nervous system disease, uncontrolled hypercholesterolemia or hypertriglyceridemia, and previous exposure to pralatrexate were excluded. Oral retinoids, except bexarotene, within 4 weeks of study treatment or high-dose vitamin A were not allowed.
Response Criteria
Global response criteria were used to assess response to the therapy (Table 2). The modified skin weighted assessment tool (mSWAT) was used to assess the extent of skin disease, flow cytometry was used to measure blood involvement, and CT scans were used for assessing nodal involvement.14 Complete response was defined as 100% clearance of disease in all areas (skin, blood, viscera, and lymph nodes) and partial response was 50% improvement in all of the above. Stable disease was <25% increase to <50% reduction in mSWAT from baseline or failure to meet criteria for complete response (CR), partial response (PR), or progressive disease (PD). Progressive disease was defined as ≥ 25% in mSWAT score from baseline or, in patients who achieved CR or PR, as a ≥ 25% increase of mSWAT score from the sum of nadir and 50% baseline mSWAT scores.
Table 2. Global Response: Criteria for Integrated Response Evaluation.
| Response Designation | Criteria |
|---|---|
| Complete response |
|
| Partial response |
|
| Stable disease |
|
| Progressive disease |
|
Results
Demographics and DLT
The disposition of patients in each cohort is shown in Table 3. For all patients the median prior therapies was 3.5 (1-14). Eighteen patients (53%) had prior bexarotene exposure including two of four with complete responses and ten of sixteen with partial responders. The demographics and CTCL sub-types at baseline are shown in Table 4. The patient exposure to each drug is in Table 5. Patients who had a baseline evaluation and received all three doses of pralatrexate in the first cycle or had a DLT were evaluable for efficacy. No dose limiting toxicities (DLTs) were observed at dose level 1 (pralatrexate 15 mg/m2 and bexarotene 150 mg/m2). Of three patients in the second cohort who received pralatrexate 15 mg/m2 with bexarotene 300 mg/m2 at dose level 2, two had DLTs during cycle 1. One patient had grade 2 hypotension and grade 3 neutropenia and the other had grade 4 thrombocytopenia and neutropenia. An additional 3 subjects were enrolled on dose level one and 0 of three experienced a dose limiting toxicity. Level 1 was then determined to be the maximum tolerated dose (MTD). Overall, 33 of 34 evaluable patients with relapsed or refractory CTCL were treated with up to 33 cycles of combination therapy.
Table 3. Patient Disposition: Safety Populationa.
| Parameter | Cohort 1/Expansion (n=31) | Cohort 2a (n=3) | All Patients (N = 34) |
|---|---|---|---|
|
| |||
| Discontinuation of treatment, n (%) | 31 (100) | 3 (100) | 34 (100) |
|
| |||
| Primary reasons for treatment discontinuation, n (%) | |||
| AE | 11 (35) | 0 (0) | 11 (32) |
| Disease progression | 7 (23) | 2 (67) | 9 (26) |
| Patient decision | 6 (19) | 1 (33) | 7 (21) |
| Investigator decision | 4 (13) | 0 (0) | 4 (12) |
| Initiation of subsequent therapy | 2 (6) | 0 (0) | 2 (6) |
| Sponsor decision | 1 (3) | 0 (0) | 1 (3) |
Included all patients who received at least 1 dose of pralatrexate or bexarotene.
Table 4. Baseline Characteristics.
| Characteristic | Cohort 1/Expansion (n=31) | Cohort 2a (n=3) | All Patients (N = 34) |
|---|---|---|---|
|
| |||
| Median age, y (range) | 66 (41-85) | 55 (39-77) | 66 (39-85) |
|
| |||
| Age ≥65 y, n (%) | 18 (58) | 1 (33) | 19 (56) |
|
| |||
| Male, n (%) | 16 (52) | 2 (67) | 18 (53) |
|
| |||
| Median BSA, m2 (range) | 1.82 (1.49-2.45) | 2.00 (1.95-2.35) | 1.86 (1.49-2.45) |
|
| |||
| Histology at study entry, n (%) | |||
| MF | 17 (55) | 2 (67) | 19 (56) |
| Transformed MF | 10 (32) | 1 (33) | 11 (32) |
| Sézary syndrome | 3 (10) | 0 (0) | 3 (9) |
| Primary cutaneous ALCL, ALK– | 1 (3) | 0 (0) | 1 (3) |
|
| |||
| ECOG performance status 0/1/2, % | 68/16/16 | 67/33/0 | 68/18/15 |
|
| |||
| Median number of prior therapies (range) | 3 (1-12) | 4 (3-14) | 3.5 (1-14) |
Table 5. Exposure to Study Treatments: Safety Populationa.
| Parameter | Cohort 1/Expansion (n=31) | Cohort 2a (n=3) | All Patients (N = 34) |
|---|---|---|---|
| Exposure to pralatrexate | |||
| Median number of cycles (range) | 6 (1-33) | 5 (2-9) | 5.5 (1-33) |
| Median number of doses (range) | 15 (2-84) | 10 (2-24) | 14 (2-84) |
| Median total dose, mg/m2 (range) | 227.8 (30-1238) | 113.4 (30-360) | 205.5 (30-1238) |
| Dose reductions, n (%) | 8 (26) | 1 (33) | 9 (26) |
| Exposure to bexarotene | |||
| Median number of cycles (range) | 6 (1-33) | 5 (2-9) | 6 (1-33) |
| Median duration, days (range) | 168.5 (15-916) | 127 (30-240) | 168 (15-916) |
| Dose reductions, n (%) | 9 (29) | 1 (33) | 10 (29) |
Included all patients who received at least 1 dose of pralatrexate or bexarotene.
Efficacy
The response rate (CR+PR) in 33 evaluable patients who had a baseline exam and received at least one dose of medication was 61%. One patient was not evaluable because they only received two doses of pralatrexate. Response by each CTCL subtype is shown in Table 7. Of note, the response rate was 65% in Sézary Syndrome and 50% in transformed MF. Complete responses were seen in one ALCL, one IIB, and two IVA2 MF patients. Partial responses were seen in one transformed IA, three IV, three IIA, seven IIB, and one each: III, IVA1, and IVB. Stable disease was seen in one IV, I t-IIB, one III, One iVA1, four IVA2 and two unknown. Two IB patients had stable disease. Figure 1 shows an example of a patient with transformed MF who achieved a long lasting partial response for over two years. Study treatment also provided a potential survival benefit to patients, with a median PFS of 12.8 months (range 0.5 to 29.9+ months) at the MTD. The probability of remaining in response, estimated using the Kaplan-Meier method, was 89.1% at 3 months, 79.1% at 6 months, 72.5% at 9 months, and 57.7% at 12 months.
Table 7. Response by CTCL Subtype.
| CTCL Subtype | ORR | CR | PR |
|---|---|---|---|
| All evaluable patients (N=33)* | 20 (61%) | 4 (12%) | 16 (48%) |
| MF (n=19) | 12 (63%) | 2 (11%) | 10 (53%) |
| Transformed MF (n=10) | 5 (50%) | 1 (10%) | 4 (40%) |
| Sézary syndrome (n=3) | 2 (67%) | 0 (0) | 2 (67%) |
| ALCL (n=1) | 1 (100%) | 1 (100%) | 0 (0) |
| Stage of MF Responders | IIB, IVA2(2) | t-IA;IB(3);IIa (3); IIb(7); & III;IVA1;IVB (1) |
Included all patients who received at least 1 dose of pralatrexate or bexarotene and had a baseline response evaluation t = transformed
Safety
The most common reasons for discontinuation on the protocol were adverse events (32%), progressive disease (26%), or decision of investigator or patient (32%) (Table 3). In the last group who did complete one cycle, six discontinued for lack of efficacy, one had worsening Parkinson disease, one had difficult venous access, and one was concerned about recurrent venous access. Adverse events reported in ≥20% patients are shown in Table 6. The most common events at any grade were stomatitis/mucositis, fatigue, hypertriglyceridemia, nausea, and neutropenia. The most common AEs of any grade considered attributed to pralatrexate by the investigator included stomatitis (65%), fatigue (44%), nausea (32%), neutropenia (32%), and anemia (24%). The most common AEs considered attributed to bexarotene by the investigator included hypertriglyceridemia (56%), fatigue (44%), neutropenia (32%), nausea (26%), and central hypothyroidism (24%). Since both drugs can cause anemia, fatigue, and neutropenia, it was not possible to determine whether bexarotene or pralatrexate or both were responsible.
Table 6. Treatment-Emergent AEs Experienced by ≥20% of Patients (N=34).
| Adverse Event | Any Graden (%) | Grade 3 or 4 n (%) |
|---|---|---|
| Stomatitis | 23 (68) | 7 (21) |
| Fatigue | 19 (56) | 0 |
| Hypertriglyceridemia | 19 (56) | 10 (29) |
| Nausea | 16 (47) | 0 |
| Neutropenia | 12 (35) | 12 (35) |
| Peripheral edema | 11 (32) | 2 (6) |
| Anemia | 10 (29) | 1 (3) |
| Skin infection | 9 (26) | 2 (6) |
| Cough | 8 (24) | 0 |
| Diarrhea | 8 (24) | 2 (6) |
| Dizziness | 8 (24) | 1 (3) |
| Dyspnea | 8 (24) | 2 (6) |
| Hypothyroidism | 8 (24) | 0 |
| Pain in extremity | 7 (21) | 0 |
Grade 3 and 4 adverse events are shown in Table 6 and included stomatitis, hypertriglyceridemia, and neutropenia. Grade 3/4 hematology values were reported for a total of 19 patients (56%) on study, including 17 patients (50%) with Grade 3 values and 4 patients (12%) with Grade 4 values. Hematology parameters that were observed at a severity of Grade 3/4 included lymphocyte and neutrophil counts (each 32%), WBC counts (12%), platelet counts (9%), and hemoglobin (3%). Grade 3 serum chemistry values were reported for 14 patients (41%), most of which (13/14 patients) involved elevated triglycerides.
Dose reductions were made in 21% of the patients. Dose omissions due to adverse events occurred in 6% of patients. Dose reductions due to bexarotene related AEs occurred in 24% of patients. Dose omissions due to bexarotene AEs occurred in 59% of patients. Fifteen percent of patients experienced both pralatrexate and bexarotene reductions and 50% of patients experienced both pralatrexate and bexarotene omission.
Conclusions
The combination of pralatrexate and bexarotene was well-tolerated with a safety profile consistent with that observed previously with the individual study drugs. No patient deaths were attributed to either study treatment. The MTD identified in this study (pralatrexate 15 mg/m2 given weekly 3 out of 4 weeks and bexarotene 150 mg/m2 daily) is the recommended dose for future clinical trials using this combination. The combination of low pralatrexate and bexarotene doses was associated with a higher overall response rate (61%) than response rate of 45% reported for pralatrexate or bexarotene alone.12, 13 The combination showed activity even among heavily pretreated patients and across the various CTCL subtypes, including two of three subjects with Sézary syndrome (67%) and five of ten with transformed MF (50%).12 The overall response was 61% with 4 CRs and 14 PRs treated at the MTD. Remarkably, sixty-seven percent of the patients treated at the MTD responded and maintained their response for > 6 months. The median progression free survival at the MTD was 12.8 months (range 0.5-29.9). Thus, pralatrexate with bexarotene is capable of producing durable responses, even in patients with advanced disease such as blood involvement and with large cell transformation and further investigation is warranted.
Statement of Translational Relevance.
The two major variants of cutaneous T-cell lymphoma, mycosis fungoides and Sézary syndrome, are currently incurable and approved agents have response rates of around 30-35%. In this Phase I/II dose escalation trial we tested the combination of two active agents, bexarotene, the first rexinoid, and pralatrexate, a powerful folic acid inhibitor. Preliminary in vitro and ex vivo studies conducted in T-cell lymphoma cell lines, Sézary cells, and immunodeficient mice supported the synergism of the two agents and formed the basis for this Phase II clinical trial. The combination of pralatrexate (15 mg/m2 three weeks out of four) and bexarotene (150 mg daily) gave a superior response rate of 60% compared to 45% and 35% respectively and long term responses were seen.
Acknowledgments
“The authors declare no potential conflicts of interest with the exception of Grant Support for conducting the Clinical Trial” – by Spectrum. The authors thank Pankaj Sharma for help providing the data.
References Cited
- 1.Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R, et al. ISCL/EORTC. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC) Blood. 2007;110:1713–1722. doi: 10.1182/blood-2007-03-055749. [DOI] [PubMed] [Google Scholar]
- 2.Talpur R, Singh L, Daulat S, Liu P, Seyfer S, Trynosky, et al. Long term outcomes of 1263 patients with Mycosis fungoides and Sezary syndrome from 1982 to 2009. Clinical cancer research. 2012;18(4):5051–5060. doi: 10.1158/1078-0432.CCR-12-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agar NS, W W, Crichton S, Mitchell TJ, Cox M, Ferreira S, Robson A, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730–4739. doi: 10.1200/JCO.2009.27.7665. [DOI] [PubMed] [Google Scholar]
- 4.Kim YH, Chow S, Varghese A, Hoppe RT. Clinical characteristics and long-term outcome of patients with generalized patch and/or plaque (T2) mycosis fungoides. Arch Dermatol. 1999;135:26–32. doi: 10.1001/archderm.135.1.26. [DOI] [PubMed] [Google Scholar]
- 5.Duvic M, Foss FM. Mycosis fungoides: pathophysiology and emerging therapies. Semin Oncol. 2007;34:S21–28. doi: 10.1053/j.seminoncol.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Talpur R, Sui D, Gangar P, Dabaja BS, Duvic M. Retrospective Analysis of Prognostic Factors in 187 Cases of Transformed Mycosis Fungoides. Clin Lymphoma Myeloma Leuk. 2016;16:49–56. doi: 10.1016/j.clml.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Scarisbrick JJ, Prince HM, Vermeer MH, Quaglino P, Horwitz S, Porcu P, et al. Cutaneous Lymphoma International Consortium Study of Outcome in Advanced Stages of Mycosis Fungoides and Sézary Syndrome: Effect of Specific Prognostic Markers on Survival and Development of a Prognostic Model. J Clin Oncol. 2015;33:3766–3773. doi: 10.1200/JCO.2015.61.7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alberti-Violetti S, Talpur R, Schlichte M, Sui D, Duvic M. Advanced-stage mycosis fungoides and Sézary syndrome: survival and response to treatment. Clin Lymphoma Myeloma Leukemia. 2015;15:105–112. doi: 10.1016/j.clml.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 9.Wood GS, Wu J. Methotrexate and Pralatrexate. Dermatol Clin. 2015;33:747–755. doi: 10.1016/j.det.2015.05.009. 33(4):747-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dondi A, Bari A, Pozzi S, Ferri P, Sacchi S. The potential of pralatrexate as a treatment of peripheral T-cell lymphoma. Expert Opin Investig Drugs. 2014;23:711–718. doi: 10.1517/13543784.2014.902050. [DOI] [PubMed] [Google Scholar]
- 11.Marci E, O'Connor Safety and Efficacy of parlatrexate in the treatment ofof patients with relapsed or refractory peripheral T-cell lymphoma. Ther Adv Hematol. 2012;3:227–235. doi: 10.1177/2040620712445330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horwitz SM, Kim YH, Foss F, Zain JM, Myskowski PL, Lechowicz MJ. Identification of an active, well-tolerated dose of pralatrexate in patients with relapsed or refractory cutaneous T-cell lymphoma. Blood. 2012;119:4115–4122. doi: 10.1182/blood-2011-11-390211. [DOI] [PubMed] [Google Scholar]
- 13.Duvic M, Martin AG, Kim Y, Olsen E, Wood G, Crowley CA, Yocum RC the Worldwide Bexarotene Study Group. Phase 2 and 3 clinical trial of oral bexarotene (Targretin capsules) for the treatment of refractory or persistent early stage cutaneous T-cell lymphoma. Arch Dermatol. 2001;137:581–593. [PubMed] [Google Scholar]
- 14.Olsen EA, Whittaker S, Kim YH, Duvic M, Prince HM, Lessin SR, Wood GS, et al. International Society for Cutaneous Lymphomas; United States Cutaneous Lymphoma Consortium; Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598–2607. doi: 10.1200/JCO.2010.32.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunn TJ, Dinner S, Price E, Coutré SE, Gotlib J, Hao Y, et al. A phase 1, open-label, dose-escalation study of pralatrexate in combination with bortezomib in patients with relapsed/refractory multiple myeloma. Br J Haematol. 2016;173:253–259. doi: 10.1111/bjh.13946. [DOI] [PubMed] [Google Scholar]


