Abstract
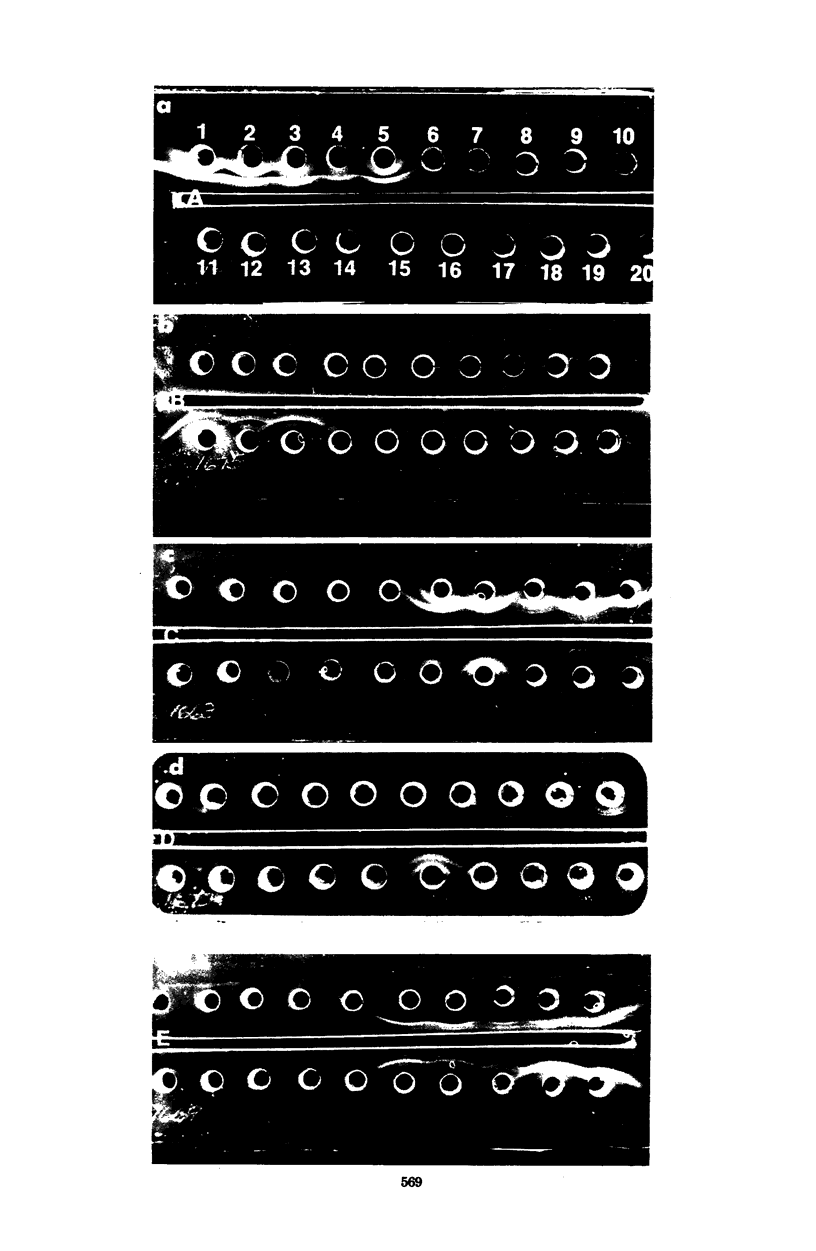
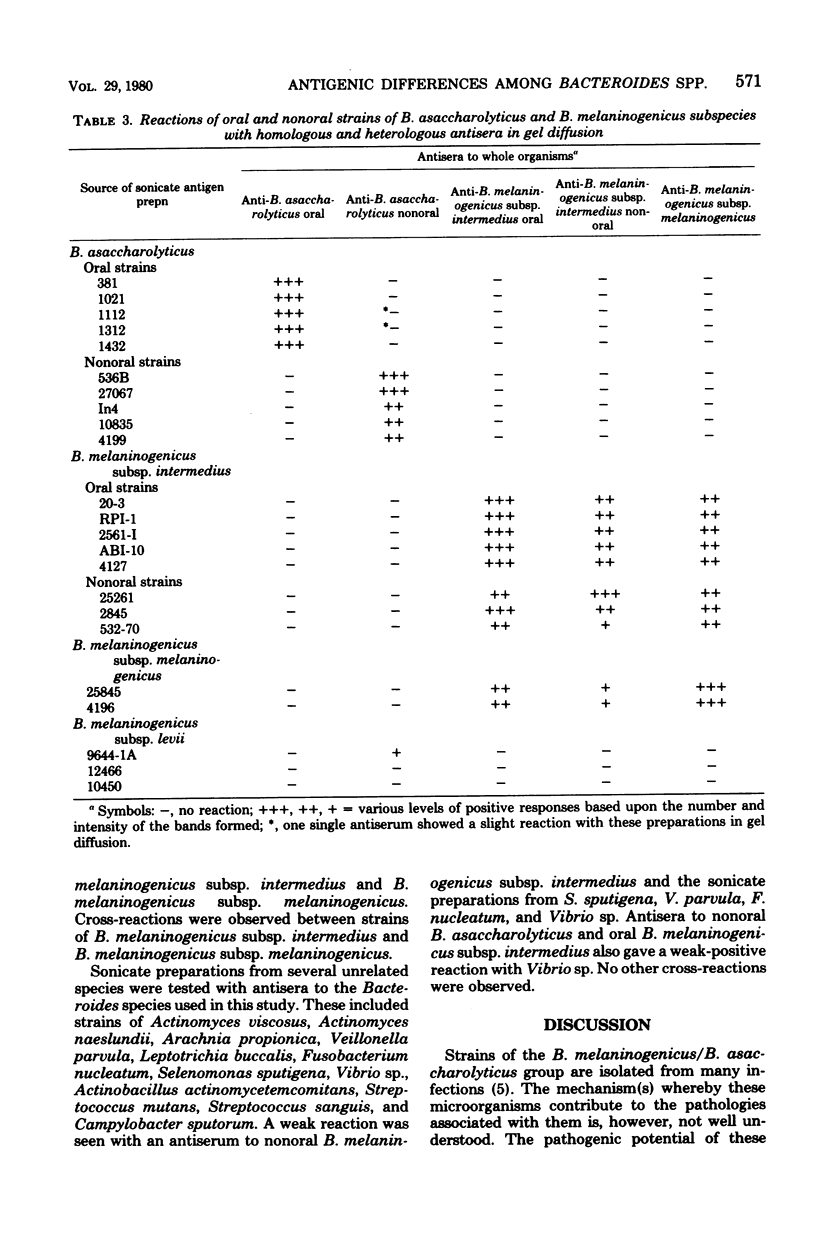
Antigens of several oral and nonoral strains of Bacteroides asaccharolyticus (proposed classification of oral B. asaccharolyticus, Bacteroides gingivalis), Bacteroides melaninogenicus subsp. intermedius, B. melaninogenicus subsp. melaninogenicus, and B. melaninogenicus subsp. levii were identified in soluble preparations obtained by sonication, autoclaving, and NaOH treatment of whole bacterial cells. The sonicate preparations contained the most complete representation of soluble antigens using antisera to the whole organism in gel precipitation tests. Among strains of B. melaninogenicus subsp. intermedius many common antigens were detected, and no consistent antigenic differences were seen between strains from oral and nonoral sites. None of the antigens of B. melaninogenicus subsp. intermedius reacted with sera raised to several strains of oral or nonoral B. asaccharolyticus, nor did antigens prepared from the latter strains react with antisera to B. melaninogenicus subsp. intermedius. At least one common antigen was shared by strains of B. melaninogenicus subsp. intermedius and strains of B. melaninogenicus subsp. melaninogenicus; however, subspecies-specific antigens were also found. Antigens from and antisera to oral and nonoral strains of B. asaccharolyticus did not react with sera to and antigens from B. melaninogenicus subsp. melaninogenicus. Strains of B. asaccharolyticus isolated from the oral cavity were antigenically distinct from strains of B. asaccharolyticus obtained from nonoral sites and lesions. This lack of cross-reactivity between the oral and nonoral strains of B. asaccharolyticus together with recent findings of marked genetic differences between oral and nonoral strains of B. asaccharolyticus suggest that these groups of organisms may represent different species.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett J. G., Onderdonk A. B., Drude E., Goldstein C., Anderka M., Alpert S., McCormack W. M. Quantitative bacteriology of the vaginal flora. J Infect Dis. 1977 Aug;136(2):271–277. doi: 10.1093/infdis/136.2.271. [DOI] [PubMed] [Google Scholar]
- Darwish S., Hyppa T., Socransky S. S. Studies of the predominant cultivable microbiota of early periodontitis. J Periodontal Res. 1978 Jan;13(1):1–16. doi: 10.1111/j.1600-0765.1978.tb00149.x. [DOI] [PubMed] [Google Scholar]
- GIBBONS R. J., MACDONALD J. B. Degradation of collagenous substrates by Bacteroides melaninogenicus. J Bacteriol. 1961 Apr;81:614–621. doi: 10.1128/jb.81.4.614-621.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbach S. L., Bartlett J. G. Anaerobic infections. 1. N Engl J Med. 1974 May 23;290(21):1177–1184. doi: 10.1056/NEJM197405232902106. [DOI] [PubMed] [Google Scholar]
- Hausmann E., Courant P. R., Arnold D. S. Conditions for the demonstration of collagenolytic activity in Bacteroides melaninogenicus. Arch Oral Biol. 1967 Feb;12(2):317–320. doi: 10.1016/0003-9969(67)90055-6. [DOI] [PubMed] [Google Scholar]
- Hausmann E., Raisz L. G., Miller W. A. Endotoxin: stimulation of bone resorption in tissue culture. Science. 1970 May 15;168(3933):862–864. doi: 10.1126/science.168.3933.862. [DOI] [PubMed] [Google Scholar]
- Hofstad T. Antibodies reacting with lipopolysaccharides from Bacteroides melaninogenicus, in serum from normal human subjects. J Infect Dis. 1974 Mar;129(3):349–352. doi: 10.1093/infdis/129.3.349. [DOI] [PubMed] [Google Scholar]
- Hofstad T. Serological responses to antigens of Bacteroidaceae. Microbiol Rev. 1979 Mar;43(1):103–115. doi: 10.1128/mr.43.1.103-115.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham H. R., Sisson P. R., Tharagonnet D., Selkon J. B., Codd A. A. Inhibition of phagocytosis in vitro by obligate anaerobes. Lancet. 1977 Dec 17;2(8051):1252–1254. doi: 10.1016/s0140-6736(77)92662-9. [DOI] [PubMed] [Google Scholar]
- Kaufman E. J., Mashimo P. A., Hausmann E., Hanks C. T., Ellison S. A. Fusobacterial infection: enhancement by cell free extracts of Bacteroides melaninogenicus possessing collagenolytic activity. Arch Oral Biol. 1972 Mar;17(3):577–580. doi: 10.1016/0003-9969(72)90073-8. [DOI] [PubMed] [Google Scholar]
- Kiger R. D., Wright W. H., Creamer H. R. The significance of lymphocyte transformation responses to various microbial stimulants. J Periodontol. 1974 Nov;45(11):780–785. doi: 10.1902/jop.1974.45.11.780. [DOI] [PubMed] [Google Scholar]
- LEV M. Apparent requirement for vitamin K of rumen strains of Fusiformis nigrescens. Nature. 1958 Jan 18;181(4603):203–204. doi: 10.1038/181203a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lambe D. W., Jr Determination of Bacteroides melaninogenicus serogroups by fluorescent antibody staining. Appl Microbiol. 1974 Oct;28(4):561–567. doi: 10.1128/am.28.4.561-567.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe D. W., Jr, Jerris R. C. Description of a polyvalent conjugate and a new serogroup of Bacteroides melaninogenicus by fluorescent antibody staining. J Clin Microbiol. 1976 May;3(5):506–512. doi: 10.1128/jcm.3.5.506-512.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansheim B. J., Coleman S. E. Immunochemical differences between oral and nonoral strains of Bacteroides asaccharolyticus. Infect Immun. 1980 Feb;27(2):589–596. doi: 10.1128/iai.27.2.589-596.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansheim B. J., Kasper D. L. Purification and immunochemical characterization of the outer membrane complex of Bacteroides melaninogenicus subspecies asaccharolyticus. J Infect Dis. 1977 May;135(5):787–799. doi: 10.1093/infdis/135.5.787. [DOI] [PubMed] [Google Scholar]
- Mashimo P. A., Ellison S. A., Slots J. Microbial composition of monkey dental plaque (Macaca arctoides and Macaca fascicularis). Scand J Dent Res. 1979 Feb;87(1):24–31. doi: 10.1111/j.1600-0722.1979.tb01936.x. [DOI] [PubMed] [Google Scholar]
- McCARTY M., LANCEFIELD R. C. Variation in the group-specific carbohydrate of group A streptococci. I. Immunochemical studies on the carbohydrates of variant strains. J Exp Med. 1955 Jul 1;102(1):11–28. doi: 10.1084/jem.102.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patters M. R., Genco R. J., Reed M. J., Mashimo P. A. Blastogenic response of human lymphocytes to oral bacterial antigens: comparison of individuals with periodontal disease to normal and edentulous subjects. Infect Immun. 1976 Nov;14(5):1213–1220. doi: 10.1128/iai.14.5.1213-1220.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANTZ L. A., RANDALL E. Use of autoclaved extracts of hemolytic streptococci for serological grouping. Stanford Med Bull. 1955 May;13(2):290–291. [PubMed] [Google Scholar]
- Reed M. J., Patters M. R., Mashimo P. A., Genco R. J., Levine R. J. Blastogenic response of human lymphocytes to oral bacterial antigens: characterization of bacterial sonicates. Infect Immun. 1976 Nov;14(5):1202–1212. doi: 10.1128/iai.14.5.1202-1212.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOCRANSKY S. S., GIBBONS R. J. REQUIRED ROLE OF BACTEROIDES MELANINOGENICUS IN MIXED ANAEROBIC INFECTIONS. J Infect Dis. 1965 Jun;115:247–253. doi: 10.1093/infdis/115.3.247. [DOI] [PubMed] [Google Scholar]
- Shah H. N., Williams R. A., Bowden G. H., Hardie J. M. Comparison of the biochemical properties of Bacteroides melaninogenicus from human dental plaque and other sites. J Appl Bacteriol. 1976 Dec;41(3):473–495. doi: 10.1111/j.1365-2672.1976.tb00660.x. [DOI] [PubMed] [Google Scholar]
- Slots J., Genco R. J. Direct hemagglutination technique for differentiating Bacteroides asaccharolyticus oral strains from nonoral strains. J Clin Microbiol. 1979 Sep;10(3):371–373. doi: 10.1128/jcm.10.3.371-373.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Gibbons R. J. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect Immun. 1978 Jan;19(1):254–264. doi: 10.1128/iai.19.1.254-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Hausmann E. Longitudinal study of experimentally induced periodontal disease in Macaca arctoides: relationship between microflora and alveolar bone loss. Infect Immun. 1979 Feb;23(2):260–269. doi: 10.1128/iai.23.2.260-269.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J. Microflora in the healthy gingival sulcus in man. Scand J Dent Res. 1977 May;85(4):247–254. doi: 10.1111/j.1600-0722.1977.tb00560.x. [DOI] [PubMed] [Google Scholar]
- Slots J., Möenbo D., Langebaek J., Frandsen A. Microbiota of gingivitis in man. Scand J Dent Res. 1978 May;86(3):174–181. doi: 10.1111/j.1600-0722.1978.tb01929.x. [DOI] [PubMed] [Google Scholar]
- Slots J. Subgingival microflora and periodontal disease. J Clin Periodontol. 1979 Oct;6(5):351–382. doi: 10.1111/j.1600-051x.1979.tb01935.x. [DOI] [PubMed] [Google Scholar]
- Slots J. The predominant cultivable microflora of advanced periodontitis. Scand J Dent Res. 1977 Jan-Feb;85(2):114–121. doi: 10.1111/j.1600-0722.1977.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Socransky S. S. Microbiology of periodontal disease -- present status and future considerations. J Periodontol. 1977 Sep;48(9):497–504. doi: 10.1902/jop.1977.48.9.497. [DOI] [PubMed] [Google Scholar]
- Takazoe I., Okuda K., Yammoto A. Distribution of a K-antigen among oral strains of Bacteroides melaninogenicus. Bull Tokyo Dent Coll. 1975 Feb;16(1):1–5. [PubMed] [Google Scholar]
- Tanner A. C., Haffer C., Bratthall G. T., Visconti R. A., Socransky S. S. A study of the bacteria associated with advancing periodontitis in man. J Clin Periodontol. 1979 Oct;6(5):278–307. doi: 10.1111/j.1600-051x.1979.tb01931.x. [DOI] [PubMed] [Google Scholar]






