Abstract
Purpose
Standard therapy for newly diagnosed glioblastoma (GBM) is surgical resection, followed by concurrent radiotherapy and temozolomide chemotherapy. In this phase 2 clinical trial, the addition of an autologous heat shock protein vaccine to standard therapy was evaluated. Tumor-induced immunosuppression, mediated by expression of PD-L1 on tumor and circulating immune cells, may impact the efficacy of vaccination. Expression of PD-L1 on peripheral myeloid cells was evaluated for the first time as a predictor of survival.
Experimental Design
In this single arm, phase 2 study, adult patients with GBM underwent surgical resection followed by standard radiation and chemotherapy. Autologous vaccine (Prophage) was generated from resected tumors and delivered in weekly vaccinations after completion of radiotherapy. The primary endpoint was overall survival.
Results
Forty-six patients received the vaccine with a median overall survival of 23.8 months (95% Confidence Interval [CI], 19.8 – 30.2). Median overall survival for patients with high PD-L1 expression on myeloid cells was 18.0 months (95% CI, 10.0 – 23.3) as compared to 44.7 months (95% CI, incalculable) for patients with low PD-L1 expression (hazard ratio 3.3; 95% CI, 1.4 – 8.6; p=0.007). A multivariate proportional hazards model revealed MGMT methylation, Karnofsky performance status, and PD-L1 expression as the primary independent predictors of survival.
Conclusions
Vaccination with autologous tumor-derived heat shock proteins may improve survival for GBM patients when combined with standard therapy, and warrants further study. Systemic immunosuppression mediated by peripheral myeloid expression of PD-L1 is a recently identified factor that may significantly impact vaccine efficacy.
Keywords: glioblastoma, immunotherapy, heat shock protein, vaccine, PD-L1
INTRODUCTION
Glioblastoma (GBM), the most common primary brain malignancy, is associated with a generally poor prognosis and a median overall survival of approximately 16 months. (1, 2) The current standard of care for GBM at initial diagnosis is surgical resection followed by radiation therapy and temozolomide chemotherapy. (3) Recent studies investigating the addition of bevacizumab to radiation and temozolomide have been disappointing, with no improvement in overall survival compared to placebo. (2, 4) The addition of immunotherapy to standard treatment has been explored in a variety of studies through vaccination with tumor specific antigens. (5-8) The efficacy of immunotherapy has been limited, in part, by glioma-induced immunosuppression. (9) Although multifactorial, immunoresistance of tumors is largely mediated by expression of immunosuppressive proteins such as the programmed death ligand- 1 (PD-L1). (10) Like many solid-organ tumors, gliomas express cell-surface PD-L1 (11, 12), but expression has not been associated with clinical outcome. (13, 14) We have shown that gliomas also induce PD-L1 expression on circulating myeloid cells and tumor-infiltrating macrophages, which may promote significant systemic immunosuppression and resistance to vaccination. (15) The clinical implications of this PD-L1 expression have not previously been demonstrated.
Heat-shock proteins (HSP), which function as intracellular chaperones, can be used to deliver tumor antigens for immune stimulation. (16) Tumor proteins bound to the gp96 HSP can be internalized by antigen presenting cells through the CD91 receptor, resulting in cleavage and cross-presentation of antigenic peptides on MHC class I and II. (17) By purifying HSP complexes from a patient’s tumor, an autologous, polyvalent vaccine can be developed and administered for treatment.
The safety and efficacy of a heat shock protein peptide complex-96 vaccine (HSPPC-96, Prophage) has been previously been studied in phase 1 and phase 2 single-arm trials for the treatment of recurrent GBM. (18, 19) These studies demonstrated robust peripheral immune stimulation in response to vaccination and modest improvements in survival compared to historical standards. Here we report the results of a phase 2, single-arm, multi-centered trial of HSPPC-96 vaccination in combination with standard radiation and chemotherapy for the treatment of newly diagnosed GBM. We also evaluate the impact of PD-L1 expression in circulating myeloid cells on clinical response to vaccination.
METHODS
Study Design
Patients 18 years of age or older with newly diagnosed, histopathologically confirmed GBM were eligible for participation in the study. Patients were initially screened for inclusion at the time of radiographic diagnosis and underwent surgical resection with collection of tissue for vaccine generation. Additional eligibility criteria included a post-operative Karnofsky performance status (KPS) of at least 70 (on a scale of 0 to 100), an extent of surgical resection in excess of 90% of the contrast-enhancing tumor on post-operative MRI within 30 days of surgery, and sufficient tumor tissue collected to generate a minimum of four 25 μg doses of vaccine. Patients were excluded from the study for known systemic autoimmune diseases, primary or secondary immunodeficiency, concurrent malignancy within the past 5 years, a bleeding diathesis, uncontrolled active infection, or other serious unstable medical condition. Following surgical resection, patients received conformal radiotherapy with concurrent temozolomide chemotherapy according to the standard of care. Patients were excluded from receiving vaccine if repeat MRI at the completion of radiotherapy demonstrated evidence of tumor progression. Patients were required to begin vaccine therapy within 2 to 5 weeks following completion of radiotherapy. All patients were required to provide written informed consent. The study was approved by the institutional review board at each center prior to patient enrollment. The study was registered on ClinicalTrials.gov (NCT00905060).
Study Treatment
Patients were registered for participation before surgical resection. All patients underwent aggressive resection with intra-operative collection of tissue to generate autologous vaccine. Fresh frozen tumor tissue was shipped to the vaccine manufacturing facility (Agenus, Inc., Lexington, MA) to generate vaccine after confirmation of the diagnosis. Approximately 7 grams of tissue were necessary to produce a minimum of four 25 μg vaccine doses. Vaccine quality was confirmed by post-production testing according to good manufacturing practice guidelines. Extent of surgical resection and participation eligibility was determined by the principle investigator at each site based on the post-operative MRI.
After surgical resection, patients received radiotherapy (60 Gy administered in 2 Gy fractions 5 days a week for 6 weeks) and oral temozolomide (75 mg per square meter of body-surface area daily for a maximum of 49 days). At completion of radiotherapy, patients underwent clinical and radiographic evaluation to demonstrate disease stability. Patients with stable disease received the HSPPC-96 vaccine beginning 2 to 5 weeks post radiotherapy. Vaccine was administered in 25 μg doses through intradermal injection every week for 4 weeks.
Maintenance temozolomide treatment began 2 weeks after administration of the 4th vaccine at an initial dose of 150 mg per square meter for 5 consecutive days in a 28-day cycle. The dose was increased to 200 mg per square meter for 5 days in subsequent cycles if no treatment-related adverse events greater than grade 2 were noted. The 5th vaccine dose (if available) was given on the same day as the start of maintenance temozolomide. Subsequent vaccine doses were given beginning 3 weeks after the 5th vaccine and administered on a monthly basis. Vaccinations continued until depletion of the vaccine or tumor progression. Maintenance temozolomide was planned for 6 cycles with the option of extension to a total of 12 cycles if there were no significant adverse events. The extent of temozolomide therapy and subsequent treatment at tumor progression was at the discretion of the patient’s primary neuro-oncologist.
Patient Evaluation
Baseline evaluation including neurologic assessment, complete blood analysis, and tumor imaging with contrast-enhanced MRI was performed pre-operatively and immediately post-operatively, usually within 48 hours. KPS was graded by the treating provider. Repeat assessment by physical examination and imaging was performed at the completion of initial chemoradiotherapy, just prior to the first vaccine administration. Final eligibility to receive vaccine was determined at this time point. After initiation of vaccine therapy, patients were evaluated every 4 weeks by clinical examination, and every 8 weeks by radiographic imaging with contrast-enhanced MRI. Tumor progression was assessed according to the modified RANO criteria (Supplementary Table S1). (20) Adverse events were assessed and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Event (CTCAE), version 4.0.
Immunologic Evaluation
Patients undergoing surgical resection at sites capable of processing of blood for immunologic assays had 30 mL of peripheral blood obtained for laboratory analysis at the time of craniotomy. Peripheral blood leukocytes (PBL) were extracted from whole blood by centrifugation on a Ficoll-Paque Plus (GE Healthcare) gradient. Extracted leukocytes were washed and suspended in phosphate-buffered saline (PBS) with 2% bovine serum albumin (BSA) in 96-well round bottom plates (Costar), plating 5 × 104 cells per well. Cells were stained extracellularly with CD45 FITC (clone HI30, eBioscience), CD11b PE-Cy7 (clone ICRF44, eBioscience), and PD-L1 PE (clone MIH1, eBioscience) or IgG1k isotype control (eBioscience) diluted in PBS with 2% BSA on ice for 30 minutes. After washing, cells were fixed with 2% paraformaldehyde (Sigma) prior to analysis. Cell counts and relative fluorescence were measured by flow cytometry on a FACSCalibur cytometer with CellQuest Software (BD Biosciences) and analyzed using FlowJo Software (TreeStar).
Study End Points
The primary end point for the study was duration of overall survival, defined as the time from surgical resection to death of any cause. The secondary endpoint was duration of progression-free survival, defined as the time from resection until either documented disease progression or death. All patients were followed on study protocol with regular clinical and radiographic assessment for evidence of progression for 24 months, then peripherally until death. Investigator assessment of medical records beyond the 24 month period was utilized to define further dates of progression.
Statistical Analysis
The trial was designed to test the alternative hypothesis that median survival for patients treated with the HSPPC-96 vaccine would be 23 months or greater against the null hypothesis that median survival would be 15 months or less. An exponential distribution with a 1 year accrual period and 2 years of follow-up was assumed. To achieve a power of 80% using a log-rank test with a two-sided alpha of 10%, a target accrual of 55 patients was planned.
The Kaplan-Meier method was used to estimate the survival distributions of the primary and secondary endpoints. To determine the relative impact of vaccine therapy on progression-free and overall survival within molecularly defined subgroups, survival analysis was performed on patient subsets separated by MGMT methylation status and peripheral myeloid cell PD-L1 expression. Differences between groups were assessed using the log-rank test, and a univariate proportional hazards model was fit to calculate hazard ratios. A multivariate proportional hazards model was developed to incorporate other known predictors of survival and vaccine efficacy, including age, KPS, time to vaccination, and number of vaccine doses administered. Differences between groups were accepted as statistically significant for p values less than 0.05.
RESULTS
Patients
From July 2009 to April 2012, 109 patients were screened for enrollment at 8 centers in the United States. Forty-six patients met all pre and post-operative criteria for enrollment and constitute the intention-to-treat population. (Supplementary Figure S1) The baseline characteristics of the 46 treated patients are shown in Table 1. Patients received a median of 9 vaccine doses (range 3 – 26). Vaccine administration was discontinued in 28 (61%) patients due to depletion of vaccine, 15 (33%) patients due to disease progression, and 3 (6%) patients due to withdrawal from the study. All patients were followed until death or closure of data analysis on January 16, 2015. No patients were lost to follow-up.
Table 1.
Baseline Characteristics of Patients
| Characteristic | Enrolled Patients (N=46) |
|---|---|
| Age, yr | |
| median | 58 |
| range | 30 - 75 |
| Gender, no. (%) | |
| male | 29 (63%) |
| female | 17 (37%) |
| KPS, no. (%) | |
| 100 | 7 (15%) |
| 90 | 26 (57%) |
| 80 | 11 (24%) |
| 70 | 2 (4%) |
| MGMT status, no. (%) | |
| methylated | 23 (50%) |
| unmethylated | 19 (41%) |
| data unavailable | 4 (9%) |
| Vaccinations Received, no. | |
| median | 9 |
| range | 3 – 26 |
| Time from surgery to first vaccine, weeks | |
| median | 13.4 |
| range | 10.0 – 17.1 |
| Reason for discontinuing vaccine, no. (%) | |
| vaccine depleted | 28 (61%) |
| tumor progression | 15 (33%) |
| patient non-compliance | 3 (6%) |
Treatment Outcomes
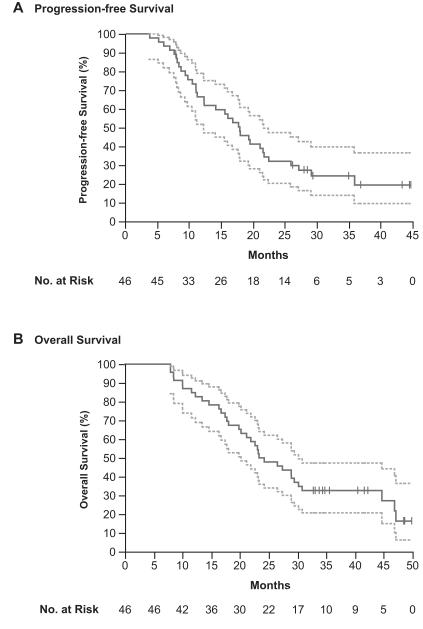
At the time of data analysis, 12 of 46 patients (26%) were alive and 12 of 46 (26%) had no evidence of progression. Median progression-free survival was 18.0 months (95% Confidence Interval [CI], 12.4 – 21.8). (Figure 1A) Median overall survival was 23.8 months (95% CI, 19.8 – 30.2) with actuarial 2-year survival of 50.0% (95% CI, 35.1% to 64.9%) and 3-year survival of 32.6% (95% CI, 20.0% to 48.1%). (Figure 1B)
Figure 1. Progression-free and Overall Survival.
Kaplan-Meier estimates of progression-free (A) and overall survival (B) in the intention-to-treat population. Vertical lines indicate time points at which patients were censored. Dotted-line curves indicate the 95% confidence interval.
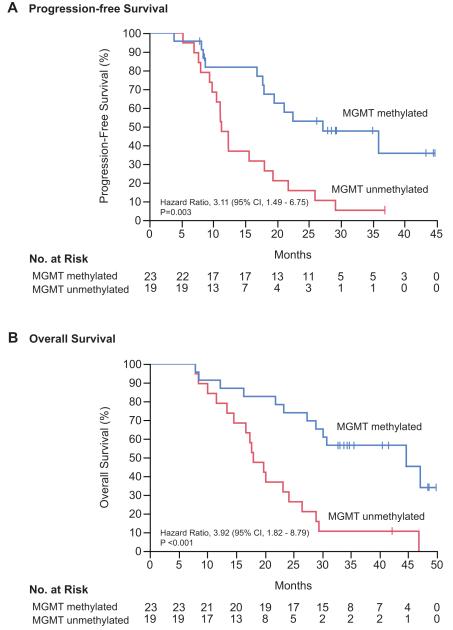
MGMT methylation status was evaluated as a prognostic factor for overall survival. The MGMT promoter was methylated in 23 of 46 patients (50%), with methylation status unavailable for 4 patients (9%). The median overall survival for MGMT unmethylated tumors was 18.0 months (95% CI, 13.4 – 24.2) as compared to 44.7 months (95% CI, 27.4 – incalculable) for MGMT methylated tumors (hazard ratio for death in unmethylated tumors 3.9; 95% CI, 1.8 – 8.8; p<0.001). (Figure 2)
Figure 2. Progression-free and Overall Survival by MGMT Methylation Status.
Kaplan-Meier estimates of progression-free (panel A) and overall survival (panel B) in patients with available MGMT methylation status (n=42). Vertical lines indicate time points at which patients were censored.
Impact of Myeloid Cell PD-L1 Expression on Survival
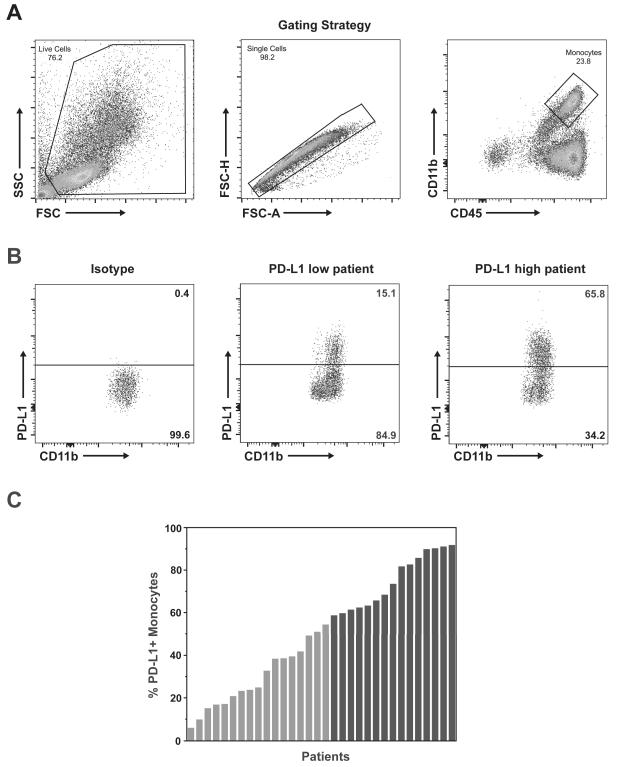
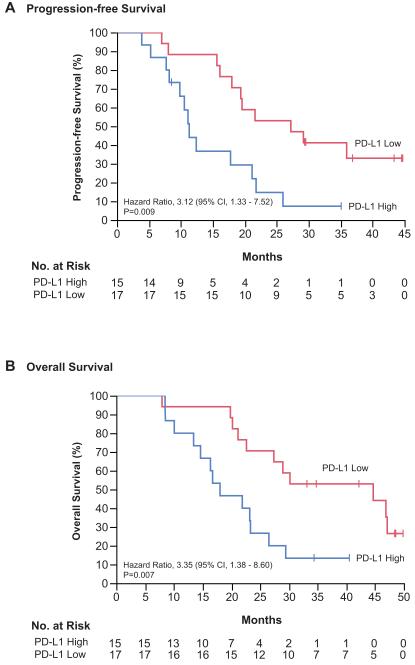
Expression of PD-L1 on circulating myeloid cells has previously been suggested to be an important contributor to tumor-induced immunosuppression. (15) Therefore, PD-L1 expression on peripheral myeloid cells was evaluated as a prognostic factor for overall survival. Circulating myeloid cells (CD45+/CD11b+) obtained from patients at the time of surgery were analyzed for PD-L1 expression to determine the percent of myeloid cells positive for PD-L1 (cutoff for positivity determined relative to FMO and isotype control). Peripheral blood was available for analysis from 32 vaccine-treated patients. Median PD-L1 positivity in circulating myeloid cells was 54.5% (range 5.9% to 91.8%). (Figure 3) Patients were categorized as high PD-L1 expressors (% PD-L1 greater than the median) or low PD-L1 expressors (% PD-L1 less than or equal to the median). The baseline patient and tumor characteristics did not differ significantly between PD-L1 expression groups. (Supplementary Table S2) Notably, MGMT methylation was balanced between high and low PD-L1 expressors (40% vs. 53%, p=0.27) and IDH1 mutation was evaluated by immunohistochemistry demonstrating no IDH1 mutant tumors in the entire cohort. The median progression-free survival for high PD-L1 expressors was 11.3 months (95% CI, 7.7 – 21.1) as compared to 27.2 months (95% CI, 16.1 – incalculable) for low PD-L1 expressors (hazard ratio for progression in high expressors 3.1; 95% CI, 1.3 – 7.5; p=0.009). (Figure 4A) The median overall survival for high PD-L1 expressors was 18.0 months (95% CI, 10.0 – 23.3) as compared to 44.7 months (95% CI, 21.1 – incalculable) for low PD-L1 expressors (hazard ratio for death in high expressors 3.3; 95% CI, 1.4 – 8.6; p=0.007). (Figure 4B)
Figure 3. Analysis of PD-L1 Expression on Myeloid Cells in Trial Patients.
(A) Representative gating scheme for identification of myeloid cells from peripheral blood leukocytes by flow cytometry. Live cells were gated from the total population (left panel), followed by identification of single cells (center panel), and gating for total monocyte/myeloid population of CD45+/CD11b+ cells (right panel). (B) Representative analysis of PD-L1+ cells within the gated monocyte population. The cutoff for positive expression was defined by PD-L1 FMO (not shown) and confirmed by staining with PD-L1 isotype control (left panel). Positive cell populations were identified with PD-L1 staining in 32 patients, ranging from low expression (center panel) to high expression (right panel). (C) Summary of PD-L1 expression in monocytes from each of the 32 evaluated patients ordered by percent positive expression. Patients colored in gray (n=17) had expression equal to or less than the median (54.5% PD-L1+ monocytes) and were defined as low expressors. Patients colored in black (n=15) had expression above the median and were defined as high expressors.
Figure 4. Progression-free and Overall Survival by PD-L1 Expression.
Kaplan-Meier estimates of progression-free (A) and overall survival (B) in the subgroup of patients with peripheral blood analysis divided by expression of PD-L1 on circulating monocytes (n=32). Vertical lines indicate time points at which patients were censored.
A multivariate proportional hazards model was developed for overall survival incorporating MGMT methylation status, PD-L1 expression in monocytes, and previously reported predictors of outcome (age, KPS) as well as vaccine administration factors (number of vaccines administered, time to first vaccination). (Supplementary Table S3) Only MGMT methylation (hazard ratio for unmethylated tumors 6.3; 95% CI, 2.1 – 22.0; p<0.001), PD-L1 expression (hazard ratio for high expression 4.0; 95% CI, 1.4 to 12.7; p=0.008), and KPS (hazard ratio for KPS < 90 5.1; 95% CI, 1.5 – 18.0; p=0.009) were found to be independent predictors of overall survival.
Adverse Events
All patients were followed for 24 months for safety. Adverse events of any type and severity were identified in 44 of 46 patients (96%). (Table 2) Adverse events attributable to the vaccine were identified in 34 patients (74%) with no serious (grade 3 or 4) events related to vaccination. The most common vaccine related events were minor injection site reactions or constitutional symptoms.
Table 2.
Adverse Events
| Adverse Events | Any Adverse Event no. of patients (%) |
Grade 3 – 4 Event no. of patients (%) |
|---|---|---|
| Any event | 44 (95.7%) | 31 (67.4%) |
| TMZ-related event | 42 (91.3%) | 21 (45.6%) |
| Vaccine-related event | 34 (73.9%) | 0 (0%) |
| Vaccine-related events | ||
| Constitutional | ||
| Fatigue | 4 (8.7%) | 0 (0%) |
| Flu-like illness | 2 (4.3%) | 0 (0%) |
| Injection Site Reaction | ||
| Induration | 1 (2.2%) | 0 (0%) |
| Pruritus | 1 (2.2%) | 0 (0%) |
| Erythema | 16 (34.8%) | 0 (0%) |
| Other reaction | 8 (17.4%) | 0 (0%) |
| Gastrointestinal | ||
| Diarrhea | 1 (2.2%) | 0 (0%) |
| Metabolic | ||
| Anorexia | 1 (2.2%) | 0 (0%) |
| Musculoskeletal | ||
| Myalgia | 1 (2.2%) | 0 (0%) |
| Nervous | ||
| Dizziness | 1 (2.2%) | 0 (0%) |
| Headache | 1 (2.2%) | 0 (0%) |
| Skin | ||
| Erythema | 3 (6.5%) | 0 (0%) |
| Pruritus | 2 (4.3%) | 0 (0%) |
| Rash | 1 (2.2%) | 0 (0%) |
| Vascular | ||
| Flushing | 1 (2.2%) | 0 (0%) |
DISCUSSION
Vaccination with autologous tumor-derived heat shock protein peptide complexes has previously been shown to stimulate a specific anti-tumor response in the majority of treated patients. (19) These results prompted a phase 2 trial of the vaccine in recurrent GBM with modest improvement in survival over historical controls. (18) The current study was designed to evaluate the benefit of autologous HSPPC-96 vaccination in combination with standard radiation and chemotherapy for patients with newly diagnosed GBM. Overall survival was selected as the primary endpoint since determination of progression by radiographic criteria can be misleading, particularly in patients with an immune-mediated inflammatory response.
The current standard of care for newly diagnosed GBM was established by Stupp and colleagues in a phase 3 trial of radiation and temozolomide in which they reported a median overall survival of 14.6 months. (3) More recently, two randomized phase 3 clinical trials of bevacizumab combined with standard chemoradiotherapy for newly diagnosed GBM (RTOG 0825 and AVAGlio) have reported no significant benefit of the addition of bevacizumab. (2, 4) Median overall survival in the placebo groups from both studies ranged from 16.1 to 16.7 months. Compared to these results, patients treated with the HSPPC-96 vaccine had a median overall survival of 23.8 months with nearly 33% of patients surviving greater than 3 years. It must be noted that patients enrolled in the HSPPC-96 vaccine trial were highly selected for positive predictors of outcome by requiring a near-total surgical resection and excluding early progressors after radiation. The extent of surgical resection has previously been shown to be a positive predictor of outcome for GBM patients (21, 22), and all patients in the current study had at least 90% extent of resection. However, in the RTOG 0825 study, greater than 60% of patients had a complete resection and the remainder had at least a partial resection, with biopsy-only patients excluded from the analysis. (2) Nonetheless, given the strict inclusion criteria of the current study, the best comparison cohort for expected survival is most appropriately found in a concurrent vaccine trial with similar inclusion criteria. ACT IV, the phase 3 randomized trial of rindopepimut, an EGFRvIII targeted peptide vaccine for newly diagnosed GBM, required similar criteria for enrollment, including a near-total surgical resection and disease stability after completion of radiation therapy. (NCT01480479) This study was recently halted due to an interim analysis demonstrating futility, with an inability to reach statistical efficacy in the treatment arm. Reported outcomes from 405 patients on the study with minimal residual disease after surgery demonstrated a median OS of 20.1 months in vaccine-treated patients and 20.0 months in controls. (23) Unexpectedly, the control arm had substantially improved survival compared to the median survival reported in the RTOG 0825 and AVAGlio trials. The difference may highlight the importance of aggressive surgical resection and the bias of excluding early progressors. Despite these biases, the median overall survival of 23.8 months in the HSPPC-96 vaccine study remains promising when compared to ACT IV with equivalent selection bias.
In the current study, the greatest improvement in survival was observed during the initial treatment period from surgical resection to first progression. PFS from initial treatment for the entire cohort was 18 months, compared to 7.3 months and 6.2 months in the placebo arms of RTOG 0825 and AVAGlio, respectively. (2, 4) However, after initial progression the remaining median survival with further therapy was only approximately 6 months, similar to outcomes observed in numerous studies for recurrent GBM. (24, 25) The impact of immune stimulation from vaccination may be best appreciated in the initial period of tumor control because once the tumor has progressed, escape from immune surveillance has occurred. Immune escape may be due to selection for tumor cells without the neo-antigens that are presented by the vaccine, and/or by development of secondary mechanisms of immunoresistance. As these tumors have become refractory to the initial immune response, they may be more aggressive than recurrent tumors in patients treated without immunotherapy, accounting for the relatively short survival from progression. Despite an apparently effective immune response at initial treatment, inflammatory pseudoprogression was not observed any more often than with standard therapies.
Another concern in comparing outcomes of this study with other trials is the number of patients with MGMT promoter methylation among vaccine-treated subjects. MGMT methylation was found in 50% of vaccine-treated patients compared to 30% in the bevacizumab trials. (2, 4) To ensure that MGMT methylation status alone did not account for the better than expected survival in vaccine-treated patients, overall survival was analyzed by MGMT methylation status. As expected, methylated patients had significantly improved survival compared to unmethylated patients, but both groups had better than expected survival compared to historical data. In the bevacizumab trials, median overall survival was 25.0 months in methylated patients and 14.6 months in unmethylated patients. (2) In the current study, median overall survival was 44.7 months for methylated patients and 18.0 for unmethylated patients, suggesting that vaccinated patients may have received some clinical benefit beyond what was expected from chemotherapy alone, based on molecular phenotype.
Ultimately, the efficacy of any immunotherapeutic approach is predicated on the ability to induce a systemic antitumor response. This response is dependent on stimulation with a sufficiently immunogenic tumor-specific antigen, and the ability to overcome tumor-induced immunosuppression. Immunogenicity arises from development of neo-epitopes as a result of tumor mutations. Although GBM has a relatively low somatic mutation rate compared to other malignancies, recent studies show that many patients harbor numerous potentially immunogenic mutations. (26, 27) Prior studies have demonstrated the potential of heat shock proteins, such as gp96, to act as antigen presenters for intracellular peptides, including mutationally derived neo-epitopes. (28, 29) The improved survival of patients treated with vaccine in this study suggests that most tumors harbor neo-antigenic mutations capable of stimulating a systemic immune response, when carried by heat shock proteins. The degree of the response is, in part, dependent on overcoming tumor-associated immunosuppression.
GBM is known to be highly immunosuppressive, inhibiting peripheral T cell activation and cytotoxic function. (30, 31) The mechanisms of glioma-induced immunosuppression are multifactorial, but often involve modulation of key immune checkpoints now recognized as important regulators of immunity in many cancers. (15, 32-34) PD-L1 is a surface protein expressed by leukocytes that binds to the programmed death 1 (PD-1) receptor on activated T cells, inducing T cell anergy and/or apoptosis. (10) It has been recognized that most solid organ cancers, including GBM, can express PD-L1 on the tumor cell surface, inducing local T cell immunoresistance within the tumor microenvironment. (11, 12) Inhibitors targeting PD-L1 and PD-1 have shown clinical efficacy in multiple cancers and are now approved for use in melanoma and lung carcinoma. (35-37) The majority of literature on the role of PD-L1 in tumor-induced immunosuppression has focused on tumor expression of the protein, which acts in the tumor microenvironment, but should not impact peripheral immunity. A study of 135 GBM specimens, including 117 newly diagnosed patients, demonstrated variable expression of PD-L1 within the tumors but no association with clinical outcomes. (13) We recently demonstrated that circulating myeloid cells in most GBM patients have upregulated PD-L1 expression, mediated by a tumor-derived cytokine. (15) Although similar findings have been documented in a small number of studies from other cancers, direct evidence of the immunologic impact of this expression on anti-tumor immunity has not been demonstrated. In this study, patients with the most elevated expression of PD-L1 on circulating myeloid cells were predicted to have more systemic immunosuppression and less response to vaccination. We demonstrated a decrease in median overall survival of over 24 months for patients with high peripheral PD-L1 expression compared to low expressors. The impact of PD-L1 expression was independent of MGMT methylation status, and was found to be highly predictive of outcome in multivariate analysis. This novel finding suggests that PD-L1 expression in circulating myeloid cells may be more important than tumor surface expression. Myeloid cell PD-L1 levels may represent a crucial predictive biomarker for response to vaccine therapy, and as such should be considered as a stratification factor in future immunotherapy trials for GBM. Additionally, combining a PD-L1/PD-1 inhibitor with vaccination may improve overall survival, particularly in high PD-L1 expressors. It is not known if PD-L1 expression on myeloid cells has an impact on survival for patients receiving standard of care chemotherapy and radiation without a concurrent immunotherapy, such as anti-tumor vaccination. We are currently investigating this question by prospective immune profiling and clinical outcomes assessment in patients receiving standard of care therapy only. The results of this investigation, not yet available, may impact how immune checkpoint inhibitors are utilized in GBM. Since prior studies demonstrate no association of tissue PD-L1 expression with clinical outcome, a lack of correlation between circulating myeloid cell PD-L1 expression and survival would suggest that the use of PD-L1/PD-1 inhibitors alone may not be an effective treatment strategy. The results of the current vaccine study suggest targeting PD-L1/PD-1 with a concurrent immune education strategy, such as vaccination, is a preferable approach.
In conclusion, vaccination with an autologous heat shock protein peptide complex vaccine in combination with standard therapy may improve survival for patients with GBM and warrants further investigation in a randomized, phase 3 trial. Expression of PD-L1 on circulating myeloid cells in GBM patients appears to impact systemic immunity and the efficacy of vaccination, suggesting a role for anti-PD-L1 therapy in combination with anti-tumor vaccines.
Supplementary Material
TRANSLATIONAL RELEVANCE.
Vaccination with tumor-derived heat shock protein peptide complexes has been shown to elicit a significant tumor-specific immune response. The current study confirms the potential efficacy of this approach for the treatment of glioblastoma. Tumor-induced immunosuppression, including upregulation of inhibitory immune checkpoints, is a major impediment of immunotherapy for numerous cancers. Significant attention has been given to PD-L1 expression on tumors and the dramatic response to therapy with PD-L1/PD-1 inhibitors. Although expression of PD-L1 on circulating myeloid cells has been described in glioblastoma and other malignancies, the impact of this finding on clinical outcomes has not been clearly documented. We now show that patients with low PD-L1 expression on myeloid cells have improved survival when treated with an anti-tumor vaccine, suggesting that PD-L1 expression on myeloid cells may be an important predictive biomarker in future clinical trials. Additionally, the combination of PD-1/PD-L1 inhibition and vaccination may increase the efficacy of this immunotherapeutic approach.
Acknowledgments
The authors would like to thank all of the patients and families who participated in this study, as well as the members of the neurosurgical and neuro-oncology teams at each participating institution. The authors particularly acknowledge the contributions of the neurosurgical and neuro-oncology team at the University of California, San Francisco (UCSF), headed by Dr. Jennifer Clarke, for continued support of the trial and analysis after the departure of Dr. Andrew Parsa. The authors also thank the vaccine manufacturing team at Agenus, Inc. for their support in providing vaccine for this trial.
Funding: This work was supported by the National Cancer Institute Special Program Of Research Excellence (UCSF SPORE, CA097257); American Brain Tumor Association; National Brain Tumor Society; the Accelerated Brain Cancer Cure, Inc.; and individual awards from the National Institute of Neurological Disorders and Stroke – R00 NS078055 and the National Cancer Institute - R01 CA164714
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose
REFERENCES
- 1.Darefsky AS, King JT, Jr., Dubrow R. Adult glioblastoma multiforme survival in the temozolomide era: a population-based analysis of Surveillance, Epidemiology, and End Results registries. Cancer. 2012;118:2163–72. doi: 10.1002/cncr.26494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. The New England journal of medicine. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. The New England journal of medicine. 2014;370:709–22. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 5.Ardon H, Van Gool SW, Verschuere T, Maes W, Fieuws S, Sciot R, et al. Integration of autologous dendritic cell-based immunotherapy in the standard of care treatment for patients with newly diagnosed glioblastoma: results of the HGG-2006 phase I/II trial. Cancer Immunol Immunother. 2012;61:2033–44. doi: 10.1007/s00262-012-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liau LM, Prins RM, Kiertscher SM, Odesa SK, Kremen TJ, Giovannone AJ, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11:5515–25. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
- 7.Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28:4722–9. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phuphanich S, Wheeler CJ, Rudnick JD, Mazer M, Wang H, Nuno MA, et al. Phase I trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol Immunother. 2013;62:125–35. doi: 10.1007/s00262-012-1319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloch O. Immunotherapy for malignant gliomas. Cancer Treat Res. 2015;163:143–58. doi: 10.1007/978-3-319-12048-5_9. [DOI] [PubMed] [Google Scholar]
- 10.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 11.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–8. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 12.Wintterle S, Schreiner B, Mitsdoerffer M, Schneider D, Chen L, Meyermann R, et al. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 2003;63:7462–7. [PubMed] [Google Scholar]
- 13.Berghoff AS, Kiesel B, Widhalm G, Rajky O, Ricken G, Wohrer A, et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol. 2015;17:1064–75. doi: 10.1093/neuonc/nou307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nduom EK, Wei J, Yaghi NK, Huang N, Kong LY, Gabrusiewicz K, et al. PD-L1 expression and prognostic impact in glioblastoma. Neuro Oncol. 2016;18:195–205. doi: 10.1093/neuonc/nov172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bloch O, Crane CA, Kaur R, Safaee M, Rutkowski MJ, Parsa AT. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin Cancer Res. 2013;19:3165–75. doi: 10.1158/1078-0432.CCR-12-3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srivastava PK, Callahan MK, Mauri MM. Treating human cancers with heat shock protein-peptide complexes: the road ahead. Expert Opin Biol Ther. 2009;9:179–86. doi: 10.1517/14712590802633918. [DOI] [PubMed] [Google Scholar]
- 17.Binder RJ, Srivastava PK. Essential role of CD91 in re-presentation of gp96-chaperoned peptides. Proc Natl Acad Sci U S A. 2004;101:6128–33. doi: 10.1073/pnas.0308180101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bloch O, Crane CA, Fuks Y, Kaur R, Aghi MK, Berger MS, et al. Heat-shock protein peptide complex-96 vaccination for recurrent glioblastoma: a phase II, single-arm trial. Neuro Oncol. 2014;16:274–9. doi: 10.1093/neuonc/not203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crane CA, Han SJ, Ahn B, Oehlke J, Kivett V, Fedoroff A, et al. Individual patient-specific immunity against high-grade glioma after vaccination with autologous tumor derived peptides bound to the 96 KD chaperone protein. Clin Cancer Res. 2013;19:205–14. doi: 10.1158/1078-0432.CCR-11-3358. [DOI] [PubMed] [Google Scholar]
- 20.Vogelbaum MA, Jost S, Aghi MK, Heimberger AB, Sampson JH, Wen PY, et al. Application of novel response/progression measures for surgically delivered therapies for gliomas: Response Assessment in Neuro-Oncology (RANO) Working Group. Neurosurgery. 2012;70:234–43. doi: 10.1227/NEU.0b013e318223f5a7. [DOI] [PubMed] [Google Scholar]
- 21.Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190–8. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 22.Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115:3–8. doi: 10.3171/2011.2.jns10998. [DOI] [PubMed] [Google Scholar]
- 23.Weller M, Butowski N, Tran D, Recht L, Lim M, Hirte H, et al. ATIM-03. ACT IV: AN INTERNATIONAL, DOUBLE-BLIND, PHASE 3 TRIAL OF RINDOPEPIMUT IN NEWLY DIAGNOSED, EGFRvIII-EXPRESSING GLIOBLASTOMA. Neuro-Oncology. 2016;18:vi17–vi8. doi: 10.1016/S1470-2045(17)30517-X. [DOI] [PubMed] [Google Scholar]
- 24.Clarke JL, Ennis MM, Yung WK, Chang SM, Wen PY, Cloughesy TF, et al. Is surgery at progression a prognostic marker for improved 6-month progression-free survival or overall survival for patients with recurrent glioblastoma? Neuro Oncol. 2011;13:1118–24. doi: 10.1093/neuonc/nor110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorlia T, Stupp R, Brandes AA, Rampling RR, Fumoleau P, Dittrich C, et al. New prognostic factors and calculators for outcome prediction in patients with recurrent glioblastoma: a pooled analysis of EORTC Brain Tumour Group phase I and II clinical trials. Eur J Cancer. 2012;48:1176–84. doi: 10.1016/j.ejca.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–8. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–77. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishii T, Udono H, Yamano T, Ohta H, Uenaka A, Ono T, et al. Isolation of MHC class I-restricted tumor antigen peptide and its precursors associated with heat shock proteins hsp70, hsp90, and gp96. J Immunol. 1999;162:1303–9. [PubMed] [Google Scholar]
- 29.Srivastava PK. Peptide-binding heat shock proteins in the endoplasmic reticulum: role in immune response to cancer and in antigen presentation. Adv Cancer Res. 1993;62:153–77. doi: 10.1016/s0065-230x(08)60318-8. [DOI] [PubMed] [Google Scholar]
- 30.Brooks WH, Netsky MG, Normansell DE, Horwitz DA. Depressed cell-mediated immunity in patients with primary intracranial tumors. Characterization of a humoral immunosuppressive factor. J Exp Med. 1972;136:1631–47. doi: 10.1084/jem.136.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roszman TL, Brooks WH. Immunobiology of primary intracranial tumours. III. Demonstration of a qualitative lymphocyte abnormality in patients with primary brain tumours. Clin Exp Immunol. 1980;39:395–402. [PMC free article] [PubMed] [Google Scholar]
- 32.Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66:3294–302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 33.Fong B, Jin R, Wang X, Safaee M, Lisiero DN, Yang I, et al. Monitoring of regulatory T cell frequencies and expression of CTLA-4 on T cells, before and after DC vaccination, can predict survival in GBM patients. PLoS One. 2012;7:e32614. doi: 10.1371/journal.pone.0032614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Learn CA, Fecci PE, Schmittling RJ, Xie W, Karikari I, Mitchell DA, et al. Profiling of CD4+, CD8+, and CD4+CD25+CD45RO+FoxP3+ T cells in patients with malignant glioma reveals differential expression of the immunologic transcriptome compared with T cells from healthy volunteers. Clin Cancer Res. 2006;12:7306–15. doi: 10.1158/1078-0432.CCR-06-1727. [DOI] [PubMed] [Google Scholar]
- 35.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England journal of medicine. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. The New England journal of medicine. 2013;369:134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.