Abstract
Objective
To systematically review the literature pertaining to rapid-rate repetitive transcranial magnetic stimulation (rTMS) compared with sham therapy for the treatment of a major depressive episode in order to arrive at qualitative and quantitative conclusions about the efficacy of rapid-rate rTMS.
Methods
MEDLINE, the Cochrane Library, the metaRegister of Controlled Trials and abstracts from scientific meetings were searched for the years 1966 until July 2003. The search terms “transcranial magnetic stimulation” and “transcranial magnetic stimulation AND depression” were used. Eighty-seven randomized controlled trials investigating the efficacy of rTMS were referenced on MEDLINE. Nineteen of these involved treatment of a major depressive episode, and these were reviewed. Six met more specific inclusion criteria including the use of rapid-rate stimulation, application to the left dorsolateral prefrontal cortex, evaluation with the 21-item Hamilton Rating Scale for Depression (HAM-D) and use of an intent-to-treat analysis. Scores on the 21-item HAM-D after treatment and standard deviations were extracted from each article for treatment and control subjects. A random-effects model was chosen for the meta-analysis, and the weighted mean difference was used as a summary measure.
Results
Six studies that met the inclusion criteria were identified and included in the meta-analysis. Two of these reported a significantly greater improvement in mood symptoms in the treatment versus the sham group. When combined in the meta-analysis, the overall weighted mean difference was –1.1 (95% confidence interval –4.5 to 2.3), and the results of a test for heterogeneity were not significant (χ25 = 5.81, p = 0.33).
Conclusions
This meta-analysis suggests that rapid-rate rTMS is no different from sham treatment in major depression; however, the power within these studies to detect a difference was generally low. Randomized controlled trials with sufficient power to detect a clinically meaningful difference are required.
Medical subject headings: depression, meta-analysis, physical stimulation, transcranial magnetic stimulation, treatment outcome
Abstract
Objectif
Recenser systématiquement les publications qui traitent de la magnétostimulation transcrânienne répétitive (MSTr) rapide comparativement à une thérapie simulée pour traiter un épisode dépressif majeur afin de tirer des conclusions qualitatives et quantitatives sur l'efficacité de la MSTr rapide.
Méthodes
On a recherché dans MEDLINE, la Cochrane Library, le metaRegister of Controlled Trials et des résumés de réunions scientifiques de 1966 jusqu'à juillet 2003 les expressions «transcranial magnetic stimulation» et «transcranial magnetic stimulation AND depression». MEDLINE contenait des références sur 87 essais contrôlés randomisés portant sur l'efficacité de la MSTr. Dix-neuf de ces études portaient sur le traitement d'un épisode dépressif majeur et ont été analysées. Six satisfaisaient à des critères d'inclusion plus spécifiques, y compris la stimulation rapide, l'application au cortex préfrontal dorsolatéral gauche, l'évaluation au moyen de l'échelle de dépression de Hamilton à 21 questions et l'utilisation d'une analyse de l'intention de traiter. Pour chaque article, on a extrait des résultats de l'échelle de dépression de Hamilton à 21 questions après le traitement et les écarts types pour les sujets traités et les sujets témoins. Un modèle d'effets aléatoires a été retenu pour la méta-analyse et la différence moyenne pondérée a servi de mesure sommaire.
Résultats
Six études satisfaisaient aux critères d'inclusion et ont été incluses dans la méta-analyse. Deux de ces études ont signalé une amélioration beaucoup plus marquée des symptômes thymiques chez les sujets traités par rapport à ceux qui ont reçu la thérapie stimulée. Combinée à la méta-analyse, la différence moyenne pondérée globale s'est établie à –1,1 (intervalle de confiance à 95 % –4,5 à 2,3) et les résultats d'un essai d'hétérogénéité n'étaient pas significatifs (χ25 = 5,81, p = 0,33).
Conclusions
Cette méta-analyse indique que la MSTr rapide n'est pas différente du traitement simulé dans des cas de dépression majeure, mais la capacité de ces études à détecter une différence était généralement faible. Des essais contrôlés randomisés comportant une capacité suffisante pour détecter une différence cliniquement significative s'imposent.
Introduction
Transcranial magnetic stimulation (TMS) has recently emerged as a possible treatment for depression. It is a noninvasive method of brain stimulation in which magnetic fields are used to induce electric currents in the cerebral cortex, thereby depolarizing neurons.1 An effective TMS device was first built in 1985 by Anthony Barker at the University of Sheffield in England.1 It was designed as a neurodiagnostic tool that activated neurons in the motor cortex and produced an evoked potential in muscle tissue. More focused magnetic fields were then used to map cortical regions involved in the functions of memory, vision and muscle control.2,3,4 Pascual-Leone et al5,6 described epileptic seizures and increased excitability of neurons with high-frequency repetitive TMS (rTMS). These effects are reminiscent of electroconvulsive therapy (ECT), with the potential to remedy the putative defects of neuronal activation in depression.7 Both rTMS and ECT use electrical energy to induce neuropsychiatric change; however, the magnetic fields in TMS are unaffected by the high impedance of the skull and, thus, TMS can be applied relatively painlessly to conscious patients without the need for sedation.1
Single-pulse TMS was first used as a possible therapeutic tool for depression in 1993.1 Since then, depression continues to be the most commonly studied psychiatric condition in the application of rTMS.7 The dorsolateral prefrontal cortex (DLPFC) has been the primary area of interest for stimulation for 2 reasons. First, networks of brain regions including the prefrontal, cingulate, parietal and temporal cortical regions, as well as parts of the striatum, thalamus and hypothalamus, are thought to regulate mood. Second, this region is the most accessible for treatment with rTMS of the areas thought to be important in mood disturbances.7
The first open studies using TMS in depression involved single-pulse stimulators at frequencies lower than 0.3 Hz.8,9,10 The large, circular coil was positioned over the vertex, stimulating frontal and parietal areas bilaterally. Conca et al11 reported an augmentation of the speed of response to antidepressant medication with an open trial of 2 weeks' duration of single-pulse TMS.
Once devices that produced rTMS became available, they essentially replaced single-pulse generators. George et al12 were the first to administer rapid-rate rTMS to the left DLPFC in a series of 6 patients. Rapid-rate or fast rTMS is generally defined as a stimulation frequency greater than 1 Hz.13 The Hamilton Rating Scale for Depression (HAM-D) was used to evaluate response, in which a decrease in score indicates improvement in depressive symptoms.14 A drop of 50% has been considered to indicate response by some authors,15 whereas others have considered a drop of 6 points to be clinically meaningful16 (possible scores range from 0 to 66). In the study by George et al,12 depression scores significantly decreased after treatment with rTMS. Many open trials followed. Figiel et al17 found that 21 of 56 patients experienced at least a 60% decline in their HAM-D score after 5 days of treatment with daily rapid-rate rTMS. Triggs et al18 found an average decrease of 41% in HAM-D scores in 10 patients with medication-resistant major depression using fast rTMS. In contrast, 1 open trial did not find antidepressant activity with rTMS.19
These open studies must be interpreted with caution. It is well known that depression is a condition that is highly susceptible to the placebo response, with rates ranging from 30% to 50% in drug trials.20,21 Device-based treatments, like rTMS, may result in even higher placebo response rates because of the elaborate rituals and sophisticated technology involved.22 Fortunately, several randomized controlled trials (RCTs) have now been conducted to investigate the efficacy of rTMS in the treatment of depression. The objectives of this paper are to review the literature systematically to arrive at qualitative conclusions about the efficacy of rapid-rate rTMS compared with sham therapy in treating a major depressive episode and to assess the results quantitatively in the form of a meta-analysis.
Methods
A review of the literature was performed using the database MEDLINE. The phrases “transcranial magnetic stimulation” and “transcranial magnetic stimulation AND depression” were used with and without the limits “review” and “randomized controlled trial.” The Cochrane database of controlled trials (www.cochrane.org) and the metaRegister of Controlled Trials (www.controlled-trials.com/mrct) were also used to locate articles. Review articles were obtained and the references scanned for further RCTs. Abstracts from several scientific meetings including those of the Society of Biological Psychiatry (2002, 2003) and the American Psychiatric Association (2000, 2001, 2002, 2003) were searched.
The following inclusion criteria were based on principles outlined in the Cochrane Reviewers' Handbook 4.1.423 and the Users' Guides to the Medical Literature.24
Criteria pertaining to study validity: (1) a randomized parallel or crossover design with sham control, (2) evidence of allocation concealment (investigators could not predict to which group patients were randomly allocated), (3) investigators and patients were blinded to whether patients were receiving the treatment or sham therapy, (4) use of an intent-to-treat analysis (ensures that data for all randomly allocated patients are analyzed at the completion of the study and is essential for validating the randomization process) and (5) if a crossover design was used, then a test for interaction or carryover effect must be shown to be nonsignificant (if not done or significant, only the data from the first phase of the study were examined).
Criteria pertaining to the subjects: (1) adults with a major depressive episode, meeting the criteria of the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV).25
Criteria pertaining to the intervention: (1) rapid rTMS, frequency ≥ 10 Hz, (2) application over the left DLPFC, (3) duration of 5–10 days, (4) intensity of ≥ 80% (defined as a percentage of the intensity required to cause a muscle contraction of the abductor pollicis brevis) and (5) the sham condition similar in each study, with the coil angled between 45° and 90° from the scalp.
Criteria pertaining to the outcome: (1) data reported in a usable form for the meta-analysis, with post-treatment scores with standard deviations, (2) the 21-item HAM-D14was chosen as the primary outcome measure to be included in the meta-analysis, because it is a well-validated scale and was used in most studies in this systematic review.
Several exclusion criteria were established: (1) open trials, (2) studies investigating primary psychotic disorders, major depressive episodes with psychotic features, or other psychiatric illnesses, (3) studies targeting specific populations such as the elderly or children (because these groups may introduce clinical heterogeneity in the causes and prognosis of a major depressive episode) and (4) studies investigating rTMS in combination with the initiation of a medication.
The meta-analysis was performed using RevMan 4.1 for Windows, according to guidelines in the Cochrane Reviewers' Handbook 4.1.4.23 The weighted mean difference summary measure was used, because the data were continuous and the identical outcome measure was used across studies. Using this approach, only patients within the same trial are compared with each other. A random-effects model was chosen, because it is generally more conservative than a fixed-effects model. A random-effects model produces wider confidence intervals and is based on the premise that 1 true answer does not exist.
In order to determine whether combining the results was appropriate, a test for heterogeneity was performed. This test is generally of low power; however, if significant it indicates that a large amount of variation exists in the outcomes of the combination of studies and that combining them may not provide valid results. Significance was set at p < 0.05.
Results
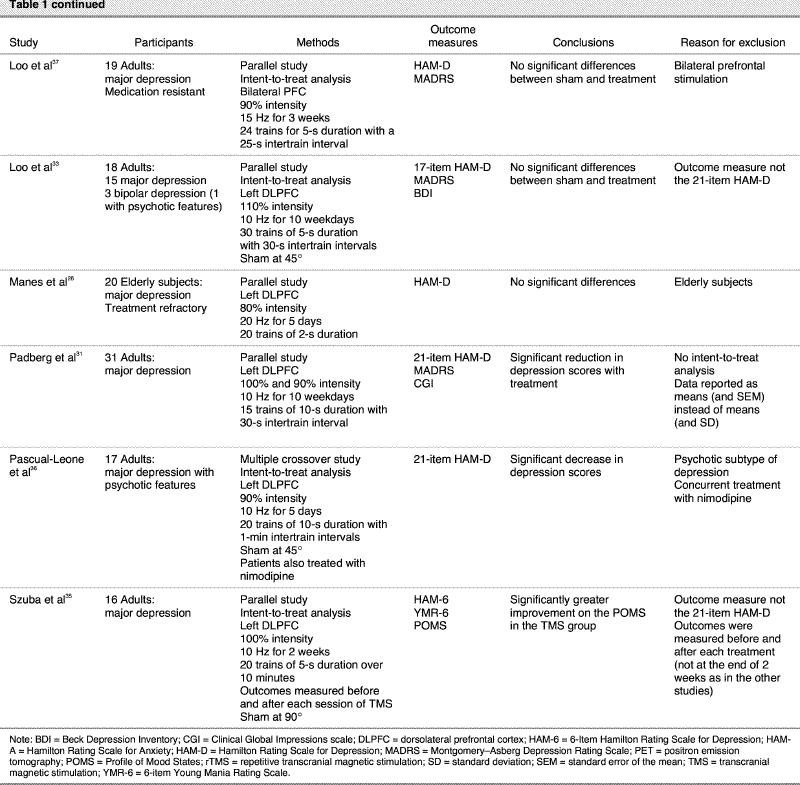
No studies were obtained through the Cochrane Collaboration database or the metaRegister of Controlled Trials. No RCTs were found in the abstracts of scientific meetings. When the phrase “transcranial magnetic stimulation” was entered, 2379 articles were found to be referenced on MEDLINE. When this was limited to human RCTs, 87 articles were listed. Several of these articles involved rTMS in the treatment of psychiatric disorders other than depression, including schizophrenia, mania, bipolar disorder and post-traumatic stress disorder. Nineteen RCTs used rTMS versus placebo for depression. Thirteen of these were excluded (Table 1). Of these 13 excluded articles, 1 specifically involved an elderly population.26 Six studies did not employ an intent-to-treat analysis.27,28,29,30,31,32 Of these 6 studies, 1 involved concurrently starting treatment with a medication,28 another involved right-sided stimulation,29 1 reported data in a different scale as opposed to the 21-item HAM-D,30 and 1 did not report data in a usable form.31 Two other studies used a scale other than the preferred 21-item HAM-D.33,34 One study measured acute mood changes before and after each treatment rather than after 5–10 days of treatment and used a different outcome measure as opposed to the 21-item HAM-D.35 One study involved subjects with a psychotic depression, over 5 months of a multiple crossover design, with concurrent treatment with nimodipine.36 Two studies were excluded based on methods that were different from those used in the other studies. One used bilateral prefrontal stimulation,37 and the other used neuronavigation with positron emission tomography (PET) in order to localize the DLPFC.38
Table 1

Table 1 continued
After these exclusions, 6 studies remained that met the strict inclusion criteria (Table 2).15,16,39,40,41,42 Three of these were crossover trials, and all had examined the effects of treatment order. One of these found no significant effect for treatment order.39 In contrast, Kimbrell et al40found an effect for order in 1 of the outcome measures, the Beck Depression Inventory, and Eschweiler et al16 found interactions between treatment group and order. Therefore, a conservative approach was taken, and only the data from the first phase of all 3 crossover studies were analyzed. The other 3 of the 6 included studies had a parallel design.15,41,42 Three of the 6 studies compared fast, slow and sham rTMS, but the data from the slow treatment were excluded from the meta-analysis.15,40,42
Table 2

Three studies indicated a significantly greater improvement in mood symptoms in the treatment group compared with the sham group.15,16,39 George et al39 reported a 5-point improvement on the HAM-D in the treatment group versus a 3-point worsening in the control group. Similarly, Eschweiler et al16 reported a decrease of 5.4 points on the HAM-D compared with an increase of 1.6 points in the sham group. Although George et al15 reported significantly more responders in the active versus the sham group, when comparing per cent change on the HAM-D there were no statistically significant differences between the fast rTMS group compared with the sham group. Avery et al41 reported that although the sample was small, the data suggested greater improvement in the group that received rTMS. They noted a 10.5-point drop on the HAM-D in the treatment group compared with a 4.5-point drop in the control group. In contrast, Kimbrell et al40 and Padberg et al42 did not find any clinically meaningful antidepressant efficacy of rapid rTMS.
A meta-analysis was conducted of the 6 studies that met the inclusion criteria (Fig. 1). Only the data from the first phase of the crossover trials were used along with the parallel trials. The result of the χ2 test for heterogeneity was not significant (χ25 = 5.81, p = 0.33), indicating that combining these studies was appropriate. The overall weighted mean difference was –1.1 (95% confidence interval [CI] –4.5 to 2.3). As this confidence interval includes zero, it is not a statistically significant result. The test for overall effect was also not statistically significant (z = 0.62, p = 0.5). Therefore, there was no evidence to suggest that rapid rTMS was any better than placebo.
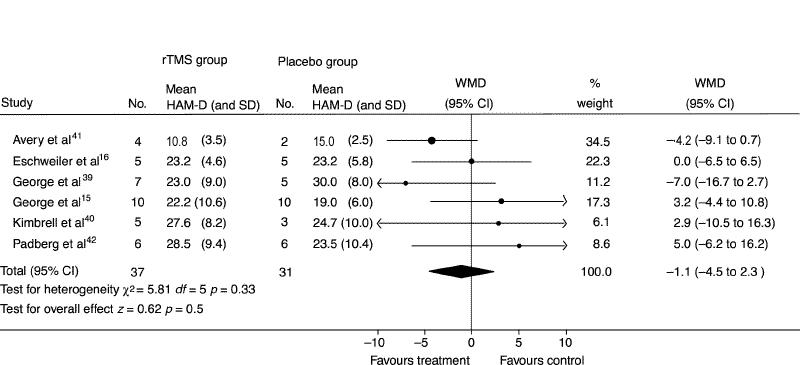
Fig. 1: Meta-analysis using a random-effects model and the weighted mean difference (WMD) of the efficacy of repetitive transcranial magnetic stimulation (rTMS) versus placebo as a treatment for depression measured with the Hamilton Rating Scale for Depression (HAM-D).
Discussion
The results of this meta-analysis indicate that rapid-rate rTMS does not appear to be any more efficacious than sham therapy in treating depression. These results are in partial agreement with those of a meta-analysis by Martin et al43 who found an effect in favour of rTMS after 2 weeks of treatment (standardized mean difference = –0.35), but no significant difference at a 2-week follow-up. Martin et al43 included 14 RCTs and concluded that the trials were generally of low quality and provided insufficient evidence to support the use of rTMS in the treatment of depression. These results are in contrast with those of another meta-analysis conducted by McNamara et al.44 They found a beneficial effect of rTMS compared with placebo when 5 RCTs were included in a meta-analysis, with a difference in the rate of improvement between the treated and control group of 43% (95% CI 25%–61%). The results of a test for heterogeneity were not significant. This meta-analysis included 2 of the studies excluded from the present study. One of these involved rTMS given to the right DLPFC using a low frequency,29 and the other, a study with a multiple crossover design, included patients with psychotic features and treatment with nimodipine.36Therefore, the present study includes more homogeneous conditions of rTMS delivery and clinical presentation of patients. In addition, the present study used data in a continuous form instead of dichotomizing the responses, thereby conserving information.45 Several new studies have been published since the meta-analysis by McNamara et al44 was conducted, and these were included in the present meta-analysis.
There are several possible explanations for the lack of benefit found for rTMS versus sham therapy in the present meta-analysis. The first, and most obvious, is that this is a valid result and rTMS is no more efficacious than placebo. A second possibility is that the most effective combination of parameters of rTMS has not been delineated. Even in the 6 studies combined in this meta-analysis, there were differences in the frequency, intensity, duration of train of pulses and days of treatment. These parameters are analogous to the dosage of a medication. If the therapeutic dosage is unknown, establishing efficacy may be difficult, if not impossible. In addition, the optimal site for the delivery of rTMS for depression has not been found. There is no evidence that the DLPFC is the best location. However, almost all of the studies encountered in this systematic review used a procedure described by Pascual-Leone et al36 in which the DLPFC is located by inducing muscle contractions in the abductor pollicis brevis and moving 5 cm anterior to this site. This method does not take into account variability in head size or shape.7 In fact, studies using post hoc localization by magnetic resonance imaging (MRI) of coil position found considerable variability in coil distance from the middle prefrontal gyrus.46,47 When the DLPFC was localized by PET before stimulation, Herwig et al38 found a moderate improvement in depressive symptoms indicating that accurate localization is important.
A further confounding variable is the sham condition. Tilting the coil off the scalp is meant to stimulate the skin to reproduce the tactile sensation of real TMS along with the acoustic effects. However, inadequate tilting may in fact stimulate the cortex, exerting possible therapeutic effects.48,49 This would reduce any possible differences in the treatment versus control groups, resulting in “negative” trials. Another difficulty arises in that the sensation of sham versus active rTMS may differ slightly, which could essentially unblind subjects in crossover trials.7
Another possible explanation for these negative findings is the low power in the 6 trials combined in the meta-analysis. For example, performing a sample size calculation based on the following assumptions, (1) a minimal clinically important decrease of 6 points on the HAM-D, (2) a standard deviation of 8 (an estimate from the 6 studies in this meta-analysis), (3) an alpha level of 0.05 and (4) power of 0.80, yields 56 subjects needed in a single trial (for formula see Friedman et al50). This is slightly fewer than the total of 68 subjects included in the 6 trials examined in this meta-analysis, indicating that the power of each of these trials was significantly below that needed to detect a difference of this magnitude in the HAM-D. Therefore, if this difference cannot be detected because of low power, a trial may produce negative results even if there really is a difference between the treatment and control groups (type II error).
The intention of this meta-analysis was to determine the efficacy of rTMS compared with sham therapy. The comparison of rTMS with ECT has also been investigated with open51,52 and controlled53 protocols. Unfortunately, these studies could not be included in this meta-analysis, because the question of whether rTMS is different from ECT is a separate issue. A future review and meta-analysis may look at comparing rTMS with ECT. Although studies comparing rTMS with ECT have suggested favourable results for rTMS with no statistically significant differences between these treatments, these studies are not designed to determine equivalence. In other words, “no significant difference” does not mean these treatments are equal. Perhaps future studies with the much larger sample sizes needed to determine equivalence might be helpful in clarifying this question.
In conclusion, when rTMS was first introduced, several open trials suggested a possible antidepressant effect. The RCTs that followed provided mixed results. This meta-analysis of 6 small, but generally well-designed, studies found that rapid-rate rTMS was no more efficacious than sham therapy in treating adults with a major depressive episode. Heterogeneity in these 6 studies was not significant, indicating that combining the data was appropriate. Further RCTs with larger samples and sufficient power are needed in which the DLPFC is precisely localized and the parameters of rTMS are of sufficient intensity, frequency and duration. In addition, true sham conditions must be delineated. These steps would help answer the question of whether rTMS is efficacious in treating depression.
Acknowledgments
The author would like to acknowledge the following individuals for their comments on this manuscript: Dr. Jeff Mahon, Dr. Kelly Zarnke, Dr. Joe Pellizzari and Dr. Peter Williamson.
Footnotes
Competing interests: None declared.
Correspondence to: Dr. Jennifer L. Couturier, Department of Psychiatry, University of Western Ontario, London Health Sciences Centre, 346 South St., London ON N6A 4G5; jlcoutur@uwo.ca
Submitted Sept. 8, 2003; Revised Jan. 27, 2004; Accepted Mar. 17, 2004
References
- 1.Hasey G. Transcranial magnetic stimulation in the treatment of mood disorder: a review and comparison with electroconvulsive therapy. Can J Psychiatry 2001;46:720-7. [DOI] [PubMed]
- 2.Pascual-Leone A, Wasserman EM, Grafman J, Hallett M. The role of the dorsolateral prefrontal cortex in implicit procedural learning. Exp Brain Res 1996;107:479-85. [DOI] [PubMed]
- 3.Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans A. Transcranial magnetic stimulation during positron emission tomography: a new method for studying connectivity of the human cerebral cortex. J Neurosci 1997;17:3178-84. [DOI] [PMC free article] [PubMed]
- 4.Cohen LG, Sato S, Rose D, Kufta C, Bandinelli S, Hallett M. Correlation of transcranial magnetic stimulation (TCMS), direct cortical stimulation (DCS) and somatosensory evoked potentials (SEP) for mapping of hand motor representation area (HMRA) [abstract]. Neurology 1989;39(Suppl 1):375.
- 5.Pascual-Leone A, Houser CM, Reese K, Shotland LI, Grafman J, Sato S, et al. Safety of rapid-rate transcranial magnetic stimulation in normal volunteers. Electroenceph Clin Neurophysiol 1993;89:120-30. [DOI] [PubMed]
- 6.Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial stimulation of the human motor cortex. Brain 1994;117:847-58. [DOI] [PubMed]
- 7.Wassermann EM, Lisanby SH. Therapeutic application of repetitive transcranial magnetic stimulation: a review. Clin Neurophysiology 2001;112:1367-77. [DOI] [PubMed]
- 8.Hoflich G, Kasper S, Hufnagel A, Ruhrmann S, Moller HJ. Application of transcranial magnetic stimulation in treatment of drug-resistant major depression: a report of two cases. Hum Psychopharmacol 1993;8:361-5.
- 9.Grisaru N, Yarovslavsky U, Abarbanel J, Lamberg T, Belmaker RH. Transcranial magnetic stimulation in depression and schizophrenia. Eur Neuropsychopharmacol 1994;4:287-8.
- 10.Kolbinger HM, Hoflich G, Hufnagel A, Moller HJ, Kasper S. Transcranial magnetic stimulation (TMS) in the treatment of major depression: a pilot study. Hum Psychopharmacol 1995;10:305-10.
- 11.Conca A, Koppi S, Konig P, Swoboda E, Krecke N. Transcranial magnetic stimulation: A novel antidepressive strategy? Neuropsychobiology 1996;34:204-7. [DOI] [PubMed]
- 12.George MS, Wassermann EM, Williams WA, Callahan A, Ketter TA, Basser P, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport 1995;6:1853-6. [DOI] [PubMed]
- 13.George MS, Lisanby SH, Sackeim HA. Transcranial magnetic stimulation: applications in neuropsychiatry. Arch Gen Psychiatry 1999;56:300-11. [DOI] [PubMed]
- 14.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56-62. [DOI] [PMC free article] [PubMed]
- 15.George MS, Nahas Z, Molloy M, Speer AM, Oliver NC, Li X, et al. A controlled trial of daily left prefrontal cortex TMS for treating depresssion. Biol Psychiatry 2000;48:962-70. [DOI] [PubMed]
- 16.Eschweiler GW, Wegerer C, Schlotter W, Spandl C, Stevens A, Bartels M, et al. Left prefrontal activation predicts therapeutic effects of repetitive transcranial magnetic stimulation (rTMS) in major depression. Psychiatry Res 2000;99:161-72. [DOI] [PubMed]
- 17.Figiel GS, Epstein C, McDonald WM, Amazon-Leece J, Figiel L, Saldivia A, et al. The use of rapid-rate transcranial magnetic stimulation (rTMS) in refractory depressed patients. J Neuropsychiatry Clin Neurosci 1998;10:20-5. [DOI] [PubMed]
- 18.Triggs WJ, McCoy KJ, Greer R, Rossi F, Bowers D, Kortenkamp S, et al. Effects of left frontal transcranial magnetic stimulation on depressed mood, cognition, and corticomotor threshold. Biol Psychiatry 1999;45:1440-6. [DOI] [PubMed]
- 19.Schouten EAM, d'Alfonso AAL, Nolen WA, De Haan DHF, Wijkstra J, Kahn RS. Mood improvement from transcranial magnetic stimulation. Am J Psychiatry 1999;156:669 [DOI] [PubMed]
- 20.Brown W. Placebo as a treatment for depression. Neuropsychopharmacology 1994;10:265-9. [DOI] [PubMed]
- 21.Schatzberg A, Kraemer HC. Use of placebo control groups in evaluating efficacy of treatment of unipolar major depression. Biol Psychiatry 2000;47:736-44. [DOI] [PubMed]
- 22.Kaptchuk TJ, Goldman P, Stone DA, Stason WB. Do medical devices have enhanced placebo effects? J Clin Epidemiol 2000;53:786-92. [DOI] [PubMed]
- 23.Clarke M, Oxnan AD, editors. Cochrane Reviewers' Handbook 4.1.4 [updated Oct 2001]. In: The Cochrane Library; Issue 4, 2001. Oxford: Update Software.
- 24.Guyatt G, Rennie D, editors. Users' guides to the medical literature: a manual for evidence-based clinical practice. Chicago: American Medical Association; 2002.
- 25.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: the Association; 1994.
- 26.Manes F, Jorge R, Morcuende M, Yamada T, Paradiso S, Robinson RG. A controlled study of repetitive transcranial magnetic stimulation as a treatment of depression in the elderly. Int Psychogeriatr 2001;13:225-31. [DOI] [PubMed]
- 27.Garcia-Toro M, Mayol A, Arnillas H, Capllonch I, Ibarra O, Crespi M, et al. Modest adjunctive benefit with transcranial magnetic stimulation in medication-resistant depression. J Affect Disord 2001;64:271-5. [DOI] [PubMed]
- 28.Garcia-Toro M, Pascual-Leone A, Romera M, Gonzalez A, Mico J, Ibarra O, et al. Prefrontal repetitive transcranial magnetic stimulation as add on treatment in depression. J Neurol Neurosurg Psychiatry 2001;71:546-8. [DOI] [PMC free article] [PubMed]
- 29.Klein E, Kreinin I, Chistyakov A, Koren D, Mecz L, Marmur S, et al. Therapeutic efficacy of right prefrontal slow repetitive transcranial magnetic stimulation in major depression. Arch Gen Psychiatry 1999;56:315-20. [DOI] [PubMed]
- 30.Boutros NN, Gueorguieva R, Hoffman RE, Oren DA, Feingold A, Berman R. Lack of therapeutic effect of a 2-week sub-threshold transcranial magnetic stimulation course for treatment-resistant depression. Psychiatry Res 2002;113:245-54. [DOI] [PubMed]
- 31.Padberg F, Zwanzger P, Keck ME, Kathmann N, Mikhaiel P, Ella R, et al. Repetitive transcranial magnetic stimulation (rTMS) in major depression: relation between efficacy and stimulation intensity. Neuropsychopharmacology 2002;27:638-45. [DOI] [PubMed]
- 32.Hoppner J, Schulz M, Irmisch G, Mau R, Schlafke D, Richter J. Antidepressant efficacy of two different rTMS procedures: high frequency over left versus low frequency over right prefrontal cortex compared with sham stimulation. Eur Arch Psychiatry Clin Neurosci 2003;253:103-9. [DOI] [PubMed]
- 33.Loo CK, Mitchell P, Sachdev P, McDarmont B, Parker G, Gandevia S. Double-blind controlled investigation of transcranial magnetic stimulation for the treatment of resistant major depression. Am J Psychiatry 1999;156:946-8. [DOI] [PubMed]
- 34.Berman RM, Narasimhan M, Sanacora G, Miano AP, Hoffman RE, Hu XS, et al. A randomized clinical trial of repetitive transcranial magnetic stimulation in the treatment of major depression. Biol Psychiatry 2000;47:332-7. [DOI] [PubMed]
- 35.Szuba MP, O'Reardon JP, Rai AS, Snyder-Kastenberg J, Amsterdam JD, Gettes DR, et al. Acute mood and thyroid stimulating hormone effects of transcranial magnetic stimulation in major depression. Biol Psychiatry 2001;50:22-7. [DOI] [PubMed]
- 36.Pascual-Leone A, Rubio B, Pallardo F, Catala MD. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet 1996;348:233-7. [DOI] [PubMed]
- 37.Loo CK, Mitchell PB, Croker VM, Malhi GS, Wen W, Gandevia SC, et al. Double-blind controlled investigation of bilateral prefrontal transcranial magnetic stimulation for the treatment of resistant major depression. Psychol Med 2003;33:33-40. [DOI] [PubMed]
- 38.Herwig U, Lampe Y, Juengling D, Wunderlich A, Walter H, Spitzer M, et al. Add-on rTMS for treatment of depression: a pilot study using stereotaxic coil-navigation according to PET data. J Psychiatr Res 2003;37:267-75. [DOI] [PubMed]
- 39.George MS, Wassermann EM, Kimbrell TA, Little JT, Williams W, Danielson AL, et al. Mood improvement following daily left prefrontal repetitive transcranial magnetic stimulation in patients with depression: a placebo-controlled crossover trial. Am J Psychiatry 1997;154:1752-6. [DOI] [PubMed]
- 40.Kimbrell TA, Little JT, Dunn RT, Frye MA, Greenberg BD, Wassermann EM, et al. Frequency dependence of antidepressant response to left prefrontal repetitive transcranial magnetic stimulation (rTMS) as a function of baseline cerebral glucose metabolism. Biol Psychiatry 1999;46:1603-13. [DOI] [PubMed]
- 41.Avery DH, Claypoole K, Robinson L, Neumaier JF, Dunner DL, Scheele L, et al. Repetitive transcranial magnetic stimulation in the treatment of medication-resistant depression: preliminary data. J Nerv Ment Dis 1999;187:114-7. [DOI] [PubMed]
- 42.Padberg F, Zwanzger P, Thoma H, Kathmann N, Haag C, Greenberg BD, et al. Repetitive transcranial magnetic stimulation (rTMS) in pharmacotherapy-refractory major depression: comparative study of fast, slow and sham rTMS. Psychiatry Res 1999;88:163-71. [DOI] [PubMed]
- 43.Martin JL, Barbanoj MJ, Schlaepfer TE, Thompson E, Perez V, Kulisevsky J. Repetitive transcranial magnetic stimulation for the treatment of depression. Br J Psychiatry 2003;182:480-91. [DOI] [PubMed]
- 44.McNamara B, Ray JL, Arthurs OJ, Boniface S. Transcranial magnetic stimulation for depression and other psychiatric disorders. Psychol Med 2001;31:1141-6. [DOI] [PubMed]
- 45.Streiner DL. Breaking up is hard to do: the heartbreak of dichotomizing continuous data. Can J Psychiatry 2002;47:262-6. [DOI] [PubMed]
- 46.Kozel FA, Nahas Z, deBrux C, Molloy M, Lorberbaum JP, Bohning D, et al. How the distance from coil to cortex relates to age, motor threshold and possibly the antidepressant response to repetitive transcranial magnetic stimulation. J Neuropsychiatry Clin Neurosci 2000;12:376-84. [DOI] [PubMed]
- 47.McConnell KA, Nahas Z, Shastri A, Lorberbaum JP, Kozel FA, Bohning DE, et al. The transcranial magnetic stimulation motor threshold depends on the distance from coil to underlying cortex: a replication in healthy adults comparing two methods of assessing the distance to cortex. Biol Psychiatry 2001;49:454-9. [DOI] [PubMed]
- 48.Loo CK, Taylor JL, Gandevia SC, McDarmont BN, Mitchell PB, Sachdev PS. Transcranial magnetic stimulation (TMS) in controlled treatment studies: Are some “sham” forms active? Biol Psychiatry 2000;47:325-31. [DOI] [PubMed]
- 49.Lisanby SH, Gutman D, Luber B, Schroeder C, Sackeim HA. Physiological effects of sham TMS: intracerebral measurements of the induced electric field and the induction of motor evoked potentials. Biol Psychiatry 2001;49:460-3. [DOI] [PubMed]
- 50.Friedman LM, Furberg CD, DeMets DL. Fundamentals of clinical trials. New York: Springer-Verlag; 1998. p. 109-12.
- 51.Grunhaus L, Dannon PN, Schreiber S, Dolberg OH, Amiaz R, Ziv R, et al. Repetitive transcranial magnetic stimulation is as effective as electroconvulsive therapy in the treatment of nondelusional major depressive disorder: an open study. Biol Psychiatry 2000;47:314-24. [DOI] [PubMed]
- 52.Janicak PG, Dowd SM, Martis B, Alam D, Beedle D, Krasuski J, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: preliminary results of a randomized trial. Biol Psychiatry 2002;51:659-67. [DOI] [PubMed]
- 53.Grunhaus L, Schreiber S, Dolberg OT, Polak D, Dannon PN. A randomized controlled comparison of electroconvulsive therapy and repetitive transcranial magnetic stimulation in severe and resistant nonpsychotic major depression. Biol Psychiatry 2003;53:324-31. [DOI] [PubMed]


