Abstract
Parkinson’s disease (PD) is a progressive and debilitating neurodegenerative disorder that affects over one million people in the United States. Previous studies, carried out in young adult rats, have shown that calcitriol, the active metabolite of vitamin D, can be neuroprotective in 6-hydroxydopamine (6-OHDA) models of PD. However, as PD usually affects older individuals, the ability of calcitriol to promote dopaminergic recovery was examined in lesioned young adult (4 month old), middle-aged (14 month old) and aged (22 month old) rats. Animals were given a single injection of 12 μg 6-OHDA into the right striatum. Four weeks later they were administered vehicle or calcitriol (1.0 μg/kg, s.c.) once a day for eight consecutive days. In vivo microdialysis experiments were carried out three weeks after the calcitriol or vehicle treatments to measure potassium and amphetamine evoked overflow of DA from both the left and right striata. In control animals treated with 6-OHDA and vehicle there were significant reductions in evoked overflow of DA on the lesioned side of the brain compared to the contralateral side. The calcitriol treatments significantly increased evoked overflow of DA from the lesioned striatum in both the young adult and middle-aged rats. However, the calcitriol treatments did not significantly augment DA overflow in the aged rats. Postmortem tissue levels of striatal DA were also increased in the young and middle-aged animals, but not in the aged animals. In the substantia nigra, the calcitriol treatments led to increased levels of DA in all three age groups. Thus, the effects of calcitriol were similar in the young adult and middle-aged animals, but in the aged animals the effects of calcitriol were diminished. These results suggest that calcitriol may help promote recovery of dopaminergic functioning in injured nigrostriatal neurons; however, the effectiveness of calcitriol may be reduced in aging.
Keywords: Aging, Striatum, Substantia Nigra, Dopamine, Calcitriol, 6-OHDA
1. Introduction
Increasing evidence indicates that vitamin D has significant actions in the brain including regulation of calcium homeostasis (Gezen-Ak et al., 2011; Groves et al., 2014; Zanatta et al., 2012), modulation of neurotrophic factors (Neveu et al., 1994a; Sanchez et al., 2002; Saporito et al., 1994; Veenstra et al., 1997a) and neurotransmitter systems (Cass et al., 2012; Jiang et al., 2014; Sonnenberg et al., 1986), immunomodulation (Fernandes de Abreu et al., 2009; Kesby et al., 2011), and neuroprotection (Garcion et al., 2002; Groves et al., 2014; Kesby et al., 2011). The active metabolite of vitamin D3 is calcitriol (1,25-dihydroxyvitamin D3), which can cross the blood brain barrier to a limited extent (Gascon-Barre and Huet, 1983; Pardridge et al., 1985). In addition, the brain itself may be able to synthesize calcitriol (Eyles et al., 2005; Neveu et al., 1994b). The effects of calcitriol are mediated through genomic pathways, via the vitamin D receptor (VDR), and nongenomic pathways, via membrane bound VDR’s or membrane-associated rapid response steroid binding receptors (Fernandes de Abreu et al., 2009; Garcion et al., 2002; Groves et al., 2014). Both types of receptors are found in the adult brain (Cui et al., 2013; Eyles et al., 2005, 2014; Pendyala et al., 2012; Prufer et al., 1999; Stumpf and O’Brien, 1987). Together these studies suggest that calcitriol has significant effects in the brain.
Parkinson’s disease (PD) is a neurodegenerative disorder that is likely due in part to the progressive degeneration of nigrostriatal dopamine (DA) neurons. Several studies have linked deficiencies in vitamin D, or changes in the vitamin D receptor, with an increased risk of developing PD (Butler et al., 2011; Evatt et al., 2008; Newmark and Newmark, 2007). In animal models of PD, calcitriol has been shown to have beneficial effects. Using 6-hydroxydopamine (6-OHDA) models of PD, several studies have demonstrated that calcitriol can partially protect against the behavioral, neurochemical and histological effects of the toxin (Kim et al., 2006; Sanchez et al., 2009; Smith et al., 2006; Wang et al., 2001). Similarly, neuroprotective effects of calcitriol have been reported in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of PD (Kim et al., 2006). Two studies have also reported that calcitriol administration, starting 3 to 4 weeks after lesioning with 6-OHDA, was able to help restore tyrosine hydroxylase (TH) positive cells in the lesioned substantia nigra (Sanchez et al., 2009) and DA release and tissue levels in the lesioned nigrostriatal system (Cass et al., 2014). In addition, calcitriol has also been reported to be neuroprotective in several in vitro studies that have examined dopaminergic toxins or cells (for example see: Ibi et al., 2001; Jang et al., 2014, 2015b; Orme et al., 2013; Shinpo, 2000). Together, these studies demonstrate that calcitriol has neuroprotective and dopaminergic promoting properties in vitro, and in animal models of PD.
Although calcitriol has been shown to have beneficial effects in animal models of PD, previous studies have used young adult animals in their studies. PD is primarily a disease of the elderly, and there can be differences in how young and aged animals respond to pharmacological treatments. Thus, the present experiments were designed to examine and compare the ability of calcitriol to promote restoration of DA overflow and tissue content of DA in young adult (4 month old), middle-aged (14 month old) and aged (22 month old) rats previously lesioned with 6-OHDA. In vivo microdialysis was used to evaluate basal extracellular levels of DA and its primary metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA), and stimulus-evoked overflow of DA, from the striatum of rats treated with 6-OHDA and calcitriol. Postmortem tissue levels of DA in the striatum and substantia nigra were also evaluated to further asses the effects of calcitriol in the three age groups.
2. Materials and methods
2.1. Animals
Young adult (4 months old, 303 – 366 g), middle-aged (14 months old, 461 – 536 g) and aged (22 months old, 405 – 499 g) male Fischer-344 rats were obtained from Harlan Laboratories (Indianapolis, IN). The animals were housed in pairs under a 12-hr light-dark cycle with food and water freely available. All animal use procedures were approved by the Animal Care and Use Committee at the University of Kentucky and were in strict accordance with National Institutes of Health guidelines. Every effort was made to reduce the number of animals used and to minimize their pain and discomfort.
2.2. 6-OHDA Injections
Animals were anesthetized with isoflurane (2.0–2.5% as needed) and positioned in a stereotaxic apparatus. Using sterile procedures, the skull was exposed, and a small hole drilled in the skull over the right striatum (1.0 mm anterior to bregma, 2.8 mm lateral from midline). A microliter syringe with a 26 gauge blunt tapered needle was used to inject 12 μg 6-OHDA (Sigma-Aldrich, St. Louis, MO) into the striatum 5.0 mm below the surface of the cortex. The 6-OHDA was injected in a total of 4 μl 0.9 % saline with 0.1% ascorbic acid (pH 5.5) at a rate of 0.5 μl/min. The needle was left in place for an additional 5 minutes following the injection before being withdrawn. Gelfoam was placed in the burr hole, the incision closed with wound clips, and the animals allowed to recover.
2.3. Calcitriol Treatment
Four weeks after the 6-OHDA injections the animals were injected once daily for eight consecutive days with calcitriol (1.0 μg/kg/day) or vehicle. All injections were administered subcutaneously. The calcitriol (Sigma Chemical Co., St. Louis, MO) was first dissolved in propylene glycol at a concentration of 100 μg/ml. For injections the calcitriol in propylene glycol was diluted into 0.9% saline so that the final volume given was 1 ml/kg of body weight. Vehicle treated animals were injected with propylene glycol diluted in 0.9% saline.
2.4. In Vivo Microdialysis
Microdialysis studies were conducted three weeks after the final calcitriol or vehicle treatment. The rats were anesthetized with urethane (1.25–1.50 g/kg, i.p.) and positioned in a stereotaxic frame. Body temperature was maintained at 37°C with a heating pad coupled to a rectal thermometer. Microdialysis probes (CMA/11 probes, 3.0 mm length of dialysis membrane; CMA/Microdialysis, Acton, MA) were slowly lowered into both the left and right striata (0.0 mm anterior to bregma, 3.0 mm lateral from midline, tip of probe 6.3 mm below the surface of the brain). The probes were perfused at a rate of 1.2 μl/min with artificial cerebrospinal fluid containing 145 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, 1.0 mM MgCl2, 0.2 mM ascorbic acid, and 2.0 mM NaH2PO4 (pH 7.4). Dialysate fractions were collected at 20-min intervals. Following a two-hour equilibration period and the collection of 3 baseline fractions, DA overflow was stimulated by increasing the potassium concentration in the perfusate to 100 mM (NaCl reduced to 47.7 mM) for a single 20-min fraction, and then two hours later by adding 100 μM d-amphetamine to the perfusate for a single 20-min fraction. Five final fractions with normal artificial cerebrospinal fluid were collected following the d-amphetamine stimulation. Dialysate samples were immediately frozen on dry ice and stored at −80°C until assayed for DA, DOPAC and HVA.
2.5. Tissue Collection and HPLC Analysis
After collecting the dialysate fractions the urethane-anesthetized animals were killed by decapitation and their brains rapidly removed and chilled in ice-cold saline. A coronal slice of brain 2 mm thick at the level of the dialysis probes was made with the aid of an ice-chilled brain mold (Rodent Brain Matrix, ASI Instruments, Warren, MI). The location of all dialysis probes was confirmed to be centered in the dorsal striatum at the level of, or just rostral to, the crossing of the anterior commissure. The site of the intrastriatal injection was also visible and was confirmed to be located in the dorsal striatum. The striatum was then dissected from each half of the slice. The substantia nigra was dissected from both sides of a 2 mm thick coronal slice through the midbrain. The tissue pieces were placed in preweighed vials, weighed, and frozen on dry ice. Samples were stored at −80°C until assayed for DA by high performance liquid chromatography (HPLC) as previously described (Cass et al., 2003). For dialysis samples, 20 μl of the dialysate was injected directly onto the column.
2.6. Data Analysis
Dialysis probes were calibrated in vitro prior to use to determine acceptable probes (recovery of DA at least 12%). However, values were not corrected for in vitro recoveries as uncorrected values may be better correlated to true values (Glick et al., 1994). Basal levels of DA and metabolites were defined as the average value in the three fractions preceding stimulation by excess potassium. Dialysis data were expressed as nM concentration of DA or metabolite in the dialysate and, for evoked overflow, as the total amount of DA in the dialysate above baseline following stimulation with potassium or amphetamine. Tissue levels of DA were expressed as ng/g wet weight of tissue. Results were analyzed statistically using analyses of variance (ANOVA) followed by Newman-Keuls tests for post hoc comparisons.
3. Results
3.1. Baseline Values for Parameters Measured
Table 1 shows the baseline values of all parameters on the non-lesioned side of the control animals. Basal microdialysate DA levels did not change over age. However, basal DOPAC levels decreased with age (one-way ANOVA, F(2, 20) = 6.83, p < 0.01). Newman-Keuls post hoc comparisons indicated that DOPAC levels in middle-aged and aged animals decreased by 15% (p < 0.05) and 28% (p < 0.01), respectively, compared to the young animals. While all of the other parameters tended to decrease with age, the decreases did not reach statistical significance.
Table 1.
Baseline control values (left side of vehicle treated animals) for the neurochemical parameters examined in the rats used in this study.
| Parameter | Age Group
|
||
|---|---|---|---|
| Young | Middle-Aged | Aged | |
| Basal DA (nM) | 4.77 ± 0.45 | 5.12 ± 0.44 | 4.95 ± 0.66 |
| Basal DOPAC (nM) | 1631 ± 110 | 1379 ± 58* | 1175 ± 83* |
| Basal HVA (nM) | 929 ± 57 | 844 ± 35 | 778 ± 39 |
| Potassium-evoked overflow of DA (pg) | 950 ± 57 | 914 ± 117 | 853 ± 142 |
| Amphetamine-evoked overflow of DA (pg) | 776 ± 54 | 745 ± 73 | 725 ± 82 |
| Striatal DA content (ng/g tissue wt.) | 13,966 ± 846 | 13,574 ± 757 | 11,656 ± 677 |
| Nigral DA content (ng/g tissue wt.) | 877 ± 75 | 815 ± 60 | 776 ± 54 |
Rats were injected daily with vehicle for 8 consecutive days starting 4 weeks after a right, unilateral 6-OHDA lesion. Microdialysis and tissue harvesting were performed 3 weeks after the vehicle injections. Values are mean ± SEM for 7–8 rats per group.
p < 0.05 vs. young animals (one-way ANOVA followed by Newman-Keuls post hoc comparisons).
3.2. Dialysate Levels of DA and Metabolites
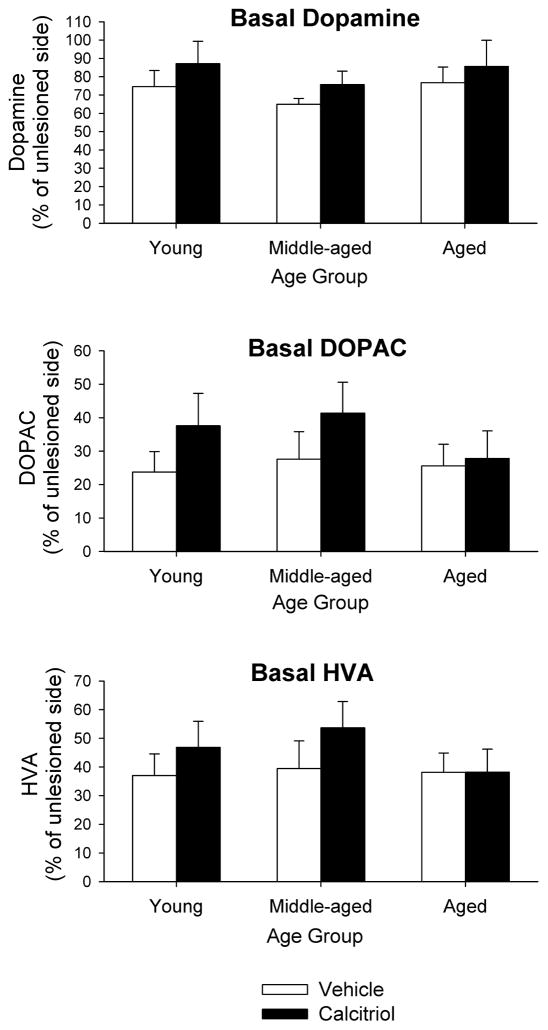
Basal extracellular dialysate levels of DA and metabolites on the lesioned side of the brain, expressed as percentage of the unlesioned side, are shown in Fig. 1. Basal levels of DA on the lesioned side ranged from 65–87% of the unlesioned side. A two-factor ANOVA revealed no statistically significant differences in basal DA levels between groups or treatments. For basal extracellular DOPAC the levels on the lesioned side ranged from 24–41% of the unlesioned side, and for basal extracellular HVA the levels on the lesioned side ranged from 37–53% of the unlesioned side (Fig. 1). Similar to the results for basal DA levels, two-factor ANOVAs indicated no statistically significant differences in basal DOPAC or HVA levels between groups or treatments.
Fig. 1.
Basal dialysate levels of DA, DOPAC and HVA from the striatum of young adult, middle-aged and aged animals injected in the right striatum with 6-OHDA followed four weeks later with eight days of calcitriol treatment. Microdialysis experiments were performed three weeks after the end of the calcitriol injections. The results are expressed as percentage of DA, DOPAC or HVA on the lesioned side compared to the contralateral, unlesioned side. Values shown are mean ± SEM from 7–8 animals per group.
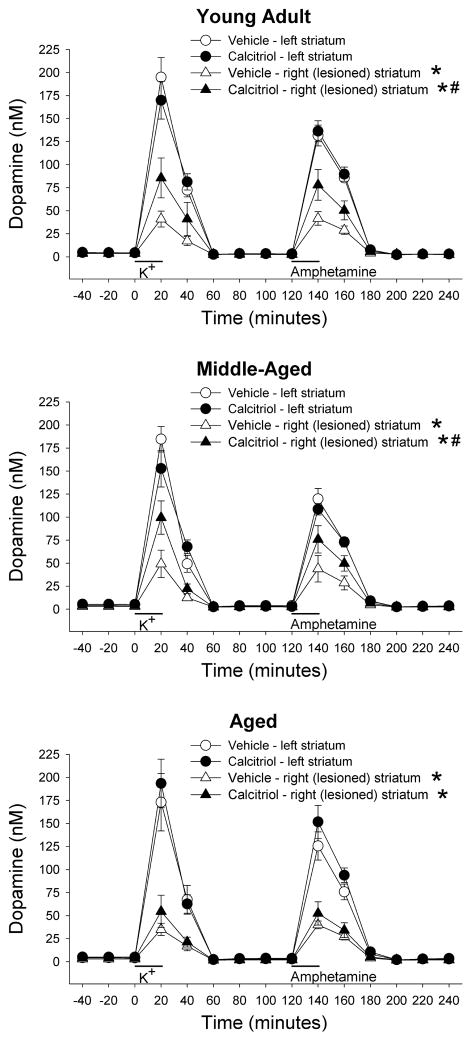
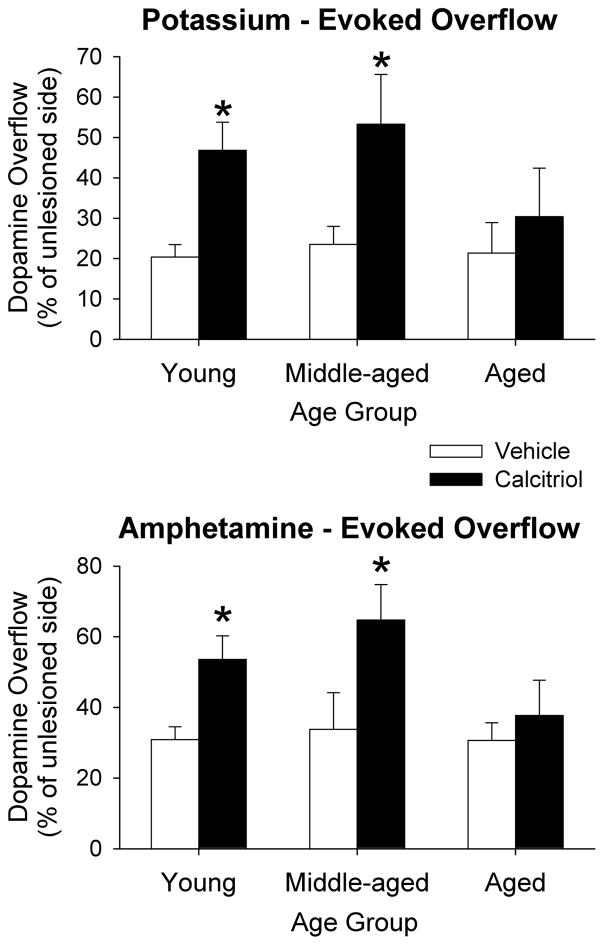
Figure 2 shows the time course data for DA levels from the in vivo microdialysis experiments for the three age groups. The data in each age group was analyzed using a mixed ANOVA with side of brain and time of dialysis sample collection as within factors, and treatment group as a between factor. Significant side of brain by treatment group interactions were found for the young adult (F(1, 14) = 4.84, p < 0.05) and middle-aged (F(1, 14) = 6.35, p < 0.05) animals. Newman-Keuls post-hoc comparisons indicated that within each age and treatment group, overall DA levels on the lesioned side were lower than those from the contralateral side (p < 0.01 for all groups). In addition, on the lesioned side, DA levels from the groups treated with calcitriol were greater than those from the vehicle treated group for both the young adult and middle-aged animals (p < 0.05 for both). However, there was not a significant difference in DA levels on the lesioned side of the aged animals between the vehicle and calcitriol treated groups. In order to facilitate comparison of the evoked overflow of DA between the groups and ages the data were converted to total amount of DA in the dialysate fractions above basal levels following stimulation by excess potassium or d-amphetamine, and then expressed as percentage of DA on the lesioned side compared to the unlesioned side (Fig. 3). In the vehicle treated rats, potassium-evoked DA overflow was reduced on the lesioned side of the brain compared to the contralateral side by 80%, 76% and 79% in the young, middle-aged and aged animals, respectively. In the calcitriol treated rats the decreases in potassium-evoked DA overflow on the lesioned side were 53%, 47% and 70% in the young, middle-aged and aged animals, respectively. The data were analyzed using two-way ANOVA with age and treatment group as between factors. A significant interaction was found (F(2, 40) = 4.02, p < 0.05), and Newman-Keuls post-hoc comparisons indicated that the potassium- evoked overflow of DA on the lesioned side of the brain was significantly greater in both the young adult and middle-aged animals treated with calcitriol compared to the groups treated with vehicle (p < 0.05 for both ages). Amphetamine-evoked DA overflow was reduced on the lesioned side of the brain in the vehicle treated rats by 69%, 66% and 69% in the young, middle-aged and aged animals, respectively. In the calcitriol treated rats the decreases in amphetamine-evoked DA overflow on the lesioned side were 46%, 34% and 66% in the young, middle-aged and aged animals, respectively. Similar to above, a two-way ANOVA indicated a significant age by treatment group interaction (F(2, 40) = 3.24, p < 0.05), and Newman-Keuls post-hoc comparisons indicated that the amphetamine-evoked overflow of DA on the lesioned side of the brain was significantly greater in the young adult and middle-aged animals treated with calcitriol compared to the groups treated with vehicle (p < 0.05 for both ages). In the aged animals there was not a significant difference between the vehicle and calcitriol treated groups for either potassium- or amphetamine-evoked overflow of DA.
Fig. 2.
Time course of dialysate levels of DA from the left and right striata of animals injected in the right striatum with 6-OHDA four weeks prior to treatment with vehicle or calcitriol. Microdialysis experiments were performed three weeks after the last vehicle or calcitriol injection. Excess potassium (100 mM) was included in the perfusate for 20-min starting at 0 min (horizontal bar above K+), and 100 μM amphetamine was included in the perfusate for 20-min starting at 120 min (horizontal bar above Amphetamine). Values shown are mean ± SEM from 7–8 animals per group. * p < 0.01 vs. left striatum of same group, # p < 0.05 vs. right striatum of vehicle treated group (mixed ANOVA with side of brain and time of dialysis sample collection as within factors, and treatment group as a between factor; followed by Newman-Keuls post-hoc comparisons).
Fig. 3.
Potassium-evoked and amphetamine-evoked overflow of DA from the striatum of young adult, middle-aged and aged animals injected in the right striatum with 6-OHDA four weeks prior to treatment with vehicle or calcitriol. Microdialysis experiments were performed three weeks after the last vehicle or calcitriol injection. The results are expressed as percentage of DA overflow on the lesioned side compared to the contralateral, unlesioned side. Values shown are mean ± SEM from 7–8 animals per group. * p < 0.05 vs. vehicle treated group of the same age (two-way ANOVA with age and treatment group as between factors; followed by Newman-Keuls post-hoc comparisons).
3.3. Tissue Levels of DA
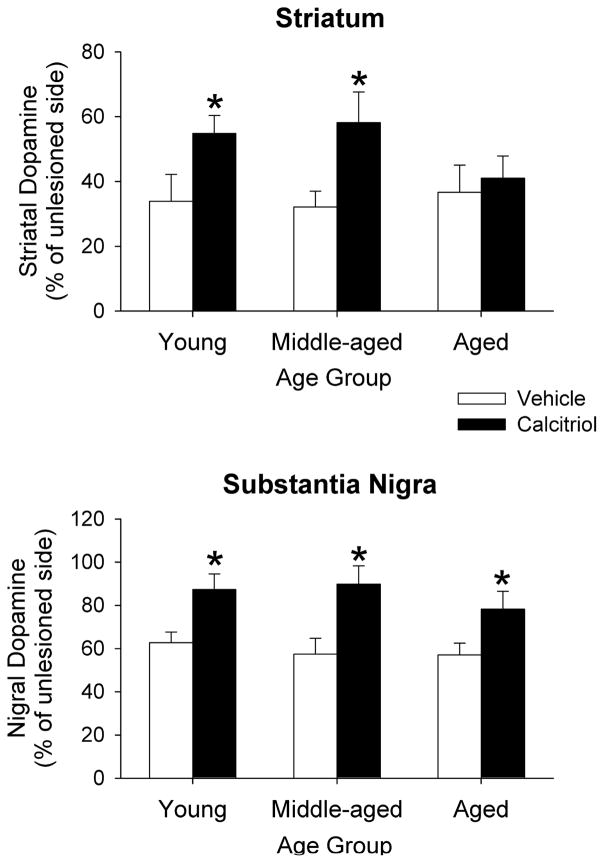
The 6-OHDA treatments and the systemic injections of calcitriol affected postmortem levels of DA in all age groups in both the striatum and substantia nigra (Fig. 4). In the young adult, middle-aged and aged rats treated with vehicle, striatal DA levels were decreased in the respective age groups by 66%, 68% and 64% on the lesioned side compared to the contralateral side. In the calcitriol treated rats striatal DA levels were decreased by 45%, 42% and 59% in the young, middle-aged and aged groups on the lesioned side. The data were analyzed using two-way ANOVA with age and treatment group as between factors. A significant interaction was found (F(2, 40) = 3.49, p < 0.05), and Newman-Keuls post-hoc comparisons indicated that in the young adult and middle-aged groups the decrease in DA levels in the calcitriol treated animals was significantly less than the decrease in the vehicle treated animals (p < 0.05 for both groups). The calcitriol treatments had no significant effect on striatal DA levels in the aged animals. In the substantia nigra, decreases in DA on the lesioned side of the vehicle treated rats were 38% in the young adult, 43% in the middle-aged and 43% in the aged groups. In the calcitriol treated rats nigral DA levels on the lesioned side were decreased by 13%, 10% and 22% in the young, middle-aged and aged groups. A two-way ANOVA indicated a significant age by treatment group interaction (F(2, 40) = 3.25, p < 0.05), and Newman-Keuls post-hoc comparisons indicated that in all three age groups the decrease in DA levels in the calcitriol treated animals was significantly less than the decrease in the vehicle treated animals (p < 0.05 for all three groups).
Fig. 4.
Postmortem tissue levels of DA from the striatum and substantia nigra of young adult, middle-aged and aged animals injected in the right striatum with 6-OHDA four weeks prior to treatment with vehicle or calcitriol. Tissue was harvested three weeks after the last vehicle or calcitriol injection. The results are expressed as percentage of striatal or nigral DA on the lesioned side compared to the contralateral, unlesioned side. Values shown are mean ± SEM from 7–8 animals per group. * p < 0.05 vs. vehicle treated group of the same age (two-way ANOVA with age and treatment group as between factors; followed by Newman-Keuls post-hoc comparisons).
4. Discussion
The present experiments were designed to examine the ability of calcitriol to promote restoration of DA overflow and tissue content of DA in young adult, middle-aged and aged rats that had previously been lesioned with 6-OHDA. The calcitriol treatments led to increases in potassium and amphetamine evoked overflow of striatal DA, and to increases in striatal and nigral tissue levels of DA, on the lesioned side in both the young adult and middle-aged rats. However, in the aged rats the calcitriol treatments had no statistically significant effect on evoked overflow or tissue content of striatal DA. However, nigral levels of DA did increase on the lesioned side of the aged rats treated with calcitriol. These results suggest that calcitriol may be able to help promote recovery of dopaminergic functioning in injured nigrostriatal neurons; however, the effectiveness of calcitriol may be reduced in aging. It should also be pointed out that 6-OHDA produces an acute injury of dopaminergic neurons, not a slow progressive loss as is seen in PD, and thus our results may not translate directly to the clinical situation of patients with PD
In both humans and monkeys, striatal DA levels are often reported to decrease during normal aging (Haycock et al., 2003; Irwin et al., 1994; Kish et al., 1992; Wenk et al., 1989). However, this is not always the case with rodents, as some studies report significant decreases in aged Fischer-344 rats (Ling et al., 2000; Marshall and Altar, 1986; Marshall and Rosenstein, 1990), while other reports indicate little to no significant differences (Cass et al., 2005; Hebert et al., 1998; Stanford et al., 2003). In the current study we report a significant decrease in basal microdialysate levels of DOPAC in the aged rats, and a trend for a decrease in the aged rats for basal HVA levels, evoked overflow of DA, and tissue content of DA. There was no effect of aging on basal extracellular DA levels, suggesting that compensatory responses (Robinson et al., 1994; Schwarting and Huston, 1996, Zigmond et al., 1990) are able to maintain normal basal release. Similarly, on the lesioned side of the brain, compensatory mechanisms are likely maintaining relatively normal basal DA levels while basal metabolite levels are reduced to a greater extent. It should also be kept in mind that our control values for aging in this study were obtained from the non-lesioned side of 6-OHDA treated animals. Nonetheless, our current results suggest that any age-related decreases in the nigrostriatal dopaminergic system in Fischer-344 rats are minimal.
In the young adult and middle-aged animals the calcitriol treatments led to increases in both potassium- and amphetamine-evoked overflow of DA. Potassium-evoked stimulation reflects calcium-dependent release of DA (vesicular, exocytotic release of DA), while amphetamine-evoked stimulation reflects calcium-independent release of DA (cytosolic release of DA). The fact that both potassium and amphetamine-evoked overflow of DA were increased following calcitriol treatment suggests that the calcitriol was promoting recovery of both calcium-dependent and calcium-independent release of DA. Baseline release of DA was not significantly affected by the 6-OHDA lesions, or the calcitriol treatments, likely due to compensatory responses aimed at maintaining normal extracellular levels of DA (Robinson et al., 1994; Schwarting and Huston, 1996, Zigmond et al., 1990). However, under the conditions of stimulation that examine extent of release from vesicular and cytosolic stores of DA, the evoked DA was increased suggesting that both vesicular and cytosolic pools of DA were increased by the calcitriol treatments.
The present study examined the ability of calcitriol to restore dopaminergic parameters in previously lesioned animals. However, several studies have demonstrated the ability of calcitriol, administered prior to lesioning, to protect against the effects of dopaminergic toxins in young adult animals (Kim et al., 2006; Sanchez et al., 2009; Smith et al., 2006; Wang et al., 2001). It is thus possible that the protective effects of calcitriol (i.e. given before the neurotoxin lesion) may be more pronounced than the restorative effects in aged animals.
The mechanisms by which calcitriol promotes recovery of dopaminergic processes after lesioning have not been defined. Several reports have demonstrated that calcitriol can lead to increases in expression or release of GDNF in various cell lines (Naveihan et al., 1996a; Orme et al., 2013; Verity et al., 1999) and to upregulation of endogenous GDNF levels in the brain (Cass et al., 2012; Sanchez et al., 2002, 2009). GDNF can promote dopaminergic recovery by several possible mechanisms including its effects on DA synthesis, storage, release, and number of functional terminals (Bourque and Trudeau, 2000; Cass and Peters, 2010; Hebert et al., 1996; Pothos et al., 1998; Salvatore et al., 2004). Thus, calcitriol-induced upregulation of GDNF could be one possible mechanism for calcitriol’s effects on dopaminergic systems. Other neurotrophic factors could also be involved as calcitriol has been shown to regulate several trophic factors and receptors (Naveilhan et al., 1996b; Neveu et al., 1994a; Saporito et al., 1994; Veenstra et al., 1997a, 1997b). Interestingly, several studies have reported that upregulation of brain trophic factors or trophic activity may be reduced in aged animals (Kaseloo et al., 1996; Ling et al., 2000; Yurek and Fletcher-Turner, 2000, 2001), and that aged animals can have a reduced response to exogenous factors (Date et al., 1993; Fox et al., 2001; Schneider, 1992). This could help explain the reduced effectiveness of the calcitriol treatments in the striatum of the aged animals. Another possible mechanism could be calcitriol-induced increases in tyrosine hydroxylase expression and/or activation (Cui et al., 2015; Puchacz et al., 1996), which could lead to increased stores and release of DA. Calcitriol and vitamin D also have numerous others affects in the brain which could alter the functional status of neurons, such as reducing the production of free radicals or upregulating free radical scavenging systems (Garcion et al., 1998, 1999; Ibi et al., 2001; Shinpo et al., 2000), modulating neuro-immunological function (Fernandes de Abreu et al., 2009; Harms et al., 2011; Kesby et al., 2011) and regulating neuronal calcium signaling (Brewer et al., 2006; de Viragh et al., 1989). Thus, there are numerous mechanisms that could be involved with calcitriol’s effects on dopaminergic neurons. Further studies will be needed to determine the mechanisms at work, and what differences may account for the reduced effects observed in the aged animals.
The dose of calcitriol and the time course followed in this study were based on previously published reports. The 1 μg/kg/day dose of calcitriol was found to be optimal in studies with young adult rats, with higher doses possibly leading to adverse effects (Cass et al., 2012). In addition, a seven to eight day treatment schedule has been found to be effective in numerous studies examining calcitriol’s ability to modulate brain chemistry and physiology (Cass et al., 2012, 2014; Sanchez et al., 2002, 2009; Wang et al., 2000, 2001). The microdialysis studies were performed three weeks after the final calcitriol treatment for a couple of reasons: 1) it is a time point in which significant changes in evoked overflow and content of DA have been found in young adult normal, and lesioned, rats following calcitriol administration (Cass et al., 2012, 2014); and 2) since it is possible that the effects of calcitriol may be partially mediated by increased GDNF levels, the three week time period has been reported to lead to changes in stimulus evoked release and content of DA in animals following treatment with exogenous GDNF (Cass and Peters, 2010; Hebert and Gerhardt, 1997; Hebert et al., 1996). It should be kept in mind that the previous calcitriol studies examined young adult animals, and different treatment doses and schedules may be able to produce an improved outcome in the aged animals.
In the present study the 6-OHDA was administered directly into the striatum, and there was greater depletion of DA in the striatum than in the substantia nigra. This suggests that damage to striatal DA terminals is greater than damage to cell bodies in the substantia nigra. Thus, the extent of terminal damage in the aged animals, compared to the younger animals, may be more difficult to overcome, leading to a partial recovery in the younger animals but not in the aged animals. The less extensive damage in the nigra may allow for improved recovery in the aged as well as younger rats.
Although in the aged rats the calcitriol had no significant effect on dopaminergic parameters in the striatum, it did increase tissue DA levels in the substantia nigra. Several studies have pointed out the important role of nigral DA in regulating motor performance (Andersson et al., 2006; Gerhardt et al., 2002; Pruett and Salvatore, 2013; Robertson and Robertson, 1989; Salvatore et al., 2009; Trevitt et al., 2001). Thus, the positive effects of calcitriol in the substantia nigra of the aged animals, while not as pronounced as the overall effects in the two younger age groups, suggests that calcitriol may be beneficial even in older individuals.
While this study found a less robust effect of calcitriol in aged animals compared to young adult and middle-aged animals, there are studies that support positive effects of calcitriol in the brains of aged animals, particularly concerning cognitive function. For example, in aged rats, vitamin D and calcitriol supplementation have led to improved hippocampal function and reduced cognitive decline (Brewer et al., 2006; Briones and Darwish, 2012; Latimer et al., 2014), while in vitamin D deficient rats, the brains of aged animals show increased levels of nitrosative stress, and changes in glucose metabolism and mitochondrial proteins that may increase the risk for cognitive decline (Keeney et al., 2013). Similarly, studies in elderly humans indicate lower cognitive performance in individuals with vitamin D deficiencies (Kueider et al., 2016; Llewellyn et al., 2010; Przybelski and Binkley, 2007). These studies support that vitamin D and calcitriol have beneficial effects in the aged brain.
The current results suggest that calcitriol may help promote recovery of neurotoxin-injured dopaminergic systems in animals; although the effects may be less pronounced in aged compared to younger adult animals. Several studies with PD patients have associated vitamin D insufficiency, or variations in the vitamin D receptor, with an increased risk of developing PD (Butler et al., 2011; Evatt et al., 2008; Newmark and Newmark, 2007; Peterson, 2014); and lower levels of vitamin D have also been associated with non-motor symptoms of PD (Jang et al., 2015a; Kwon et al., 2016; Peterson et al., 2013), suggesting a possible link between vitamin D levels and PD. Additionally, a published vitamin D intervention study has demonstrated positive effects of vitamin D supplementation on clinical rating scales in PD patients (Suzuki et al., 2013), providing clinical evidence for a potential therapeutic role for vitamin D or calcitriol. However, not all studies have found an association between vitamin D levels and PD (Petersen et al., 2014; Shrestha et al., 2016). Thus, the link between low levels of vitamin D and PD is controversial, and may only apply to some individuals with PD. However, taken together, the animal studies provide evidence that calcitriol may have protective and restorative effects on damaged dopaminergic neurons, and the clinical studies indicate that in some cases low levels of vitamin D may be associated with PD. These results suggest that additional studies on the effects of calcitriol on the brain are warranted.
The effects of calcitriol were studied in rats previously lesioned with 6-OHDA
Calcitriol increased dopamine in the striatum of young and middle-aged rats
Calcitriol did not increase dopamine in the striatum of aged rats
Calcitriol increased nigral tissue dopamine levels in all three age groups
The dopaminergic effects of calcitriol are diminished in lesioned, aged rats
Acknowledgments
This work was supported in part by the United States National Institutes of Health (Grant number NS60924). Neither of the authors have a conflict of interest of any type in association with this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson DR, Nissbrandt H, Bergquist F. Partial depletion of dopamine in substantia nigra impairs motor performance without altering striatal dopamine neurotransmission. Eur J Neurosci. 2006;24:617–624. doi: 10.1111/j.1460-9568.2006.04953.x. [DOI] [PubMed] [Google Scholar]
- Brewer LD, Porter NM, Kerr DS, Landfield PW, Thibault O. Chronic 1α,25-(OH)2vitamin D3 treatment reduces Ca2+-mediated hippocampal biomarkers of aging. Cell Calcium. 2006;40:277–286. doi: 10.1016/j.ceca.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Briones TL, Darwish H. Vitamin D mitigates age-related cognitive decline through the modulation of pro-inflammatory state and decrease in amyloid burden. J Neuroinflammation. 2012;9:244. doi: 10.1186/1742-2094-9-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque MJ, Trudeau LE. GDNF enhances the synaptic efficacy of dopaminergic neurons in culture. Eur J Neurosci. 2000;12:3172–3180. doi: 10.1046/j.1460-9568.2000.00219.x. [DOI] [PubMed] [Google Scholar]
- Butler MW, Burt A, Edwards TL, Zuchner S, Scott WK, Martin ER, Vance JM, Wang L. Vitamin D receptor gene as a candidate gene for Parkinson disease. Ann Hum Genet. 2011;75:201–210. doi: 10.1111/j.1469-1809.2010.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass WA, Peters LE. Neurturin effects on nigrostriatal dopamine release and content: comparison with GDNF. Neurochem Res. 2010;35:727–734. doi: 10.1007/s11064-010-0128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass WA, Harned ME, Peters LE, Nath A, Maragos WF. HIV-1 protein Tat potentiation of methamphetamine-induced decreases in evoked overflow of dopamine in the striatum of the rat. Brain Res. 2003;984:133–142. doi: 10.1016/s0006-8993(03)03122-6. [DOI] [PubMed] [Google Scholar]
- Cass WA, Peters LE, Smith MP. Reductions in spontaneous locomotor activity in aged male, but not female, rats in a model of early Parkinson’s disease. Brain Res. 2005;1034:153–161. doi: 10.1016/j.brainres.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Cass WA, Peters LE, Fletcher AM, Yurek DM. Evoked dopamine overflow is augmented in the striatum of calcitriol treated rats. Neurochem Int. 2012;60:186–191. doi: 10.1016/j.neuint.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass WA, Peters LE, Fletcher AM, Yurek DM. Calcitriol promotes augmented dopamine release in the lesioned striatum of 6-hydroxydopamine treated rats. Neurochem Res. 2014;39:1467–1476. doi: 10.1007/s11064-014-1331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Pelekanos M, Liu PY, Burne THJ, McGrath JJ, Eyles DW. The vitamin D receptor in dopamine neurons; its presence in human substantia nigra and its ontogenesis in rat midbrain. Neuroscience. 2013;236:77–87. doi: 10.1016/j.neuroscience.2013.01.035. [DOI] [PubMed] [Google Scholar]
- Cui X, Pertile R, Liu P, Eyles DW. Vitamin D regulates tyrosine hydroxylase expression: N-cadherin a possible mediator. Neuroscience. 2015;304:90–100. doi: 10.1016/j.neuroscience.2015.07.048. [DOI] [PubMed] [Google Scholar]
- Date I, Yoshimoto Y, Imaoka T, Miyoshi Y, Gohda Y, Furuta T, Asari S, Ohmoto T. Enhanced recovery of the nigrostriatal dopaminergic system in MPTP-treated mice following intrastriatal injection of basic fibroblast growth factor in relation to aging. Brain Res. 1993;621:150–154. doi: 10.1016/0006-8993(93)90312-b. [DOI] [PubMed] [Google Scholar]
- de Viragh PA, Haglid KG, Celio MR. Parvalbumin increases in the caudate putamen of rats with vitamin D hypervitaminosis. Proc Natl Acad Sci USA. 1989;86:3887–3890. doi: 10.1073/pnas.86.10.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evatt ML, DeLong MR, Khazai N, Rosen A, Triche S, Tangpricha V. Prevalence of vitamin D insufficiency in patients with Parkinson disease and Alzheimer disease. Arch Neurol. 2008;65:1348–1352. doi: 10.1001/archneur.65.10.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1α-hydroxylase in human brain. J Clin Neuroanat. 2005;29:21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Eyles DW, Liu PY, Josh P, Cui X. Intracellular distribution of the vitamin D receptor in the brain: comparison with classic target tissues and redistribution with development. Neuroscience. 2014;268:1–9. doi: 10.1016/j.neuroscience.2014.02.042. [DOI] [PubMed] [Google Scholar]
- Fernandes de Abreu DA, Eyles D, Feron F. Vitamin D, a neuro-immunomodulator: implications for neurodegenerative and autoimmune diseases. Psychoneuroendocrinology. 2009;34S:S265–S277. doi: 10.1016/j.psyneuen.2009.05.023. [DOI] [PubMed] [Google Scholar]
- Fox CM, Gash DM, Smoot MK, Cass WA. Neuroprotective effects of GDNF against 6-OHDA in young and aged rats. Brain Res. 2001;896:56–63. doi: 10.1016/s0006-8993(00)03270-4. [DOI] [PubMed] [Google Scholar]
- Garcion E, Sindji L, Montero-Menei C, Andre C, Brachet P, Darcy F. Expression of inducible nitric oxide synthase during rat brain inflammation: regulation by 1,25-dihydroxyvitamin D3. Glia. 1998;22:282–294. [PubMed] [Google Scholar]
- Garcion E, Sindji L, Leblondel G, Brachet P, Darcy F. 1,25-Dihydroxyvitamin D3 regulates the synthesis of γ-glutamyl transpeptidase and glutathione levels in rat primary astrocytes. J Neurochem. 1999;73:859–866. doi: 10.1046/j.1471-4159.1999.0730859.x. [DOI] [PubMed] [Google Scholar]
- Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13:100–105. doi: 10.1016/s1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- Gascon-Barre M, Huet PM. Apparent [3H]1,25-dihydroxyvitamin D3 uptake by canine and rodent brain. Am J Physiol. 1983;244:E266–E271. doi: 10.1152/ajpendo.1983.244.3.E266. [DOI] [PubMed] [Google Scholar]
- Gerhardt GA, Cass WA, Yi A, Zhang Z, Gash DM. Changes in somatodendritic but not terminal dopamine regulation in aged rhesus monkeys. J Neurochem. 2002;80:168–177. doi: 10.1046/j.0022-3042.2001.00684.x. [DOI] [PubMed] [Google Scholar]
- Gezen-Ak D, Dursun E, Yilmazer S. The effects of vitamin D receptor silencing on the expression of LVSCC-A1C and LVSCC-A1D and the release of NGF in cortical neurons. PLoS ONE. 2011;6(3):e17553. doi: 10.1371/journal.pone.0017553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick SD, Dong N, Keller RW, Jr, Carlson JN. Estimating extracellular concentrations of dopamine and 3,4-dihydroxyphenylacetic acid in nucleus accumbens and striatum using microdialysis: relationships between in vitro and in vivo recoveries. J Neurochem. 1994;62:2017–2021. doi: 10.1046/j.1471-4159.1994.62052017.x. [DOI] [PubMed] [Google Scholar]
- Groves NJ, McGrath JJ, Burne THJ. Vitamin D as a neurosteroid affecting the developing and adult brain. Annu Rev Nutr. 2014;34:117–141. doi: 10.1146/annurev-nutr-071813-105557. [DOI] [PubMed] [Google Scholar]
- Harms LR, Burne THJ, Eyles DW, McGrath JJ. Vitamin D and the brain. Best Pract Res Clin Endocinol Metab. 2011;25:657–669. doi: 10.1016/j.beem.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Haycock JW, Becker L, Ang L, Furukawa Y, Hornykiewicz O, Kish SJ. Marked disparity between age-related changes in dopamine and other presynaptic dopaminergic markers in human striatum. J Neurochem. 2003;87:574–585. doi: 10.1046/j.1471-4159.2003.02017.x. [DOI] [PubMed] [Google Scholar]
- Hebert MA, Gerhardt GA. Behavioral and neurochemical effects of intranigral administration of glial cell line-derived neurotrophic factor on aged Fischer 344 rats. J Pharmacol Exp Ther. 1997;282:760–768. [PubMed] [Google Scholar]
- Hebert MA, Gerhardt GA. Normal and drug-induced locomotor behavior in aging: comparison to evoked DA release and tissue content in Fischer 344 rats. Brain Res. 1998;797:42–54. doi: 10.1016/s0006-8993(98)00370-9. [DOI] [PubMed] [Google Scholar]
- Hebert MA, Van Horne CG, Hoffer BJ, Gerhardt GA. Functional effects of GDNF in normal rat striatum: presynaptic studies using in vivo electrochemistry and microdialysis. J Pharmacol Exp Ther. 1996;279:1181–1190. [PubMed] [Google Scholar]
- Ibi M, Sawada H, Nakanishi M, Kume T, Katsuki H, Kaneko S, Shimohama S, Akaike A. Protective effects of 1α,25-(OH)2D3 against the neurotoxicity of glutamate and reactive oxygen species in mesencephalic culture. Neuropharmacology. 2001;40:761–771. doi: 10.1016/s0028-3908(01)00009-0. [DOI] [PubMed] [Google Scholar]
- Irwin I, DeLanney LE, McNeill T, Chan P, Forno LS, Murphy JM, Jr, Di Monte DA, Sandy MS, Langston JW. Aging and the nigrostriatal dopamine system: a non-human primate study. Neurodegeneration. 1994;3:251–265. [PubMed] [Google Scholar]
- Jang W, Kim HJ, Li H, Jo KD, Lee MK, Song SH, Yang HO. 1,25-Dyhydroxyvitamin D3 attenuates rotenone-induced neurotoxicity in SH-SY5Y cells through induction of autophagy. Biochem Biophys Res Comm. 2014;451:142–147. doi: 10.1016/j.bbrc.2014.07.081. [DOI] [PubMed] [Google Scholar]
- Jang W, Park J, Kim JS, Youn J, Oh E, Kwon KY, Jo KD, Lee MK, Kim HT. Vitamin D deficiency in Parkinson’s disease patients with orthostatic hypotension. Acta Neurol Scand. 2015a;132:242–250. doi: 10.1111/ane.12390. [DOI] [PubMed] [Google Scholar]
- Jang W, Park HH, Lee KY, Lee YJ, Kim HT, Koh SH. 1,25-Dyhydroxyvitamin D3 attenuates L-DOPA-induced neurotoxicity in neural stem cells. Mol Neurobiol. 2015b;51:558–570. doi: 10.1007/s12035-014-8835-1. [DOI] [PubMed] [Google Scholar]
- Jiang P, Zhang LH, Cai HL, Li HD, Liu YP, Tang MM, Dang RL, Zhu WY, Xue Y, He X. Neurochemical effects of chronic administration of calcitriol in rats. Nutrients. 2014;6:6048–6059. doi: 10.3390/nu6126048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaseloo PA, Lis A, Asada H, Barone TA, Plunkett RJ. In vitro assessment of neurotrophic activity from the striatum of aging rats. Neurosci Lett. 1996;218:157–160. doi: 10.1016/s0304-3940(96)13146-3. [DOI] [PubMed] [Google Scholar]
- Keeney JT, Förster S, Sultana R, Brewer LD, Latimer CS, Cai J, Klein JB, Porter NM, Butterfield DA. Dietary vitamin D deficiency in rats from middle to old age leads to elevated tyrosine nitration and proteomics changes in levels of key proteins in brain: implications for low vitamin D-dependent age-related cognitive decline. Free Radic Biol Med. 2013;65:324–334. doi: 10.1016/j.freeradbiomed.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesby JP, Eyles DW, Burne THJ, McGrath JJ. The effects of vitamin D on brain development and adult brain function. Mol Cell Endocrinol. 2011;347:121–127. doi: 10.1016/j.mce.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Kim JS, Ryu SY, Yun I, Kim WJ, Lee KS, Park JW, Kim YI. 1α,25-Dihydroxyvitamin D3 protects dopaminergic neurons in rodent models of Parkinson’s disease through inhibition of microglial activation. J Clin Neurol. 2006;2:252–257. doi: 10.3988/jcn.2006.2.4.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish SJ, Shannak K, Rajput A, Deck JH, Hornykiewicz O. Aging produces a specific pattern of striatal dopamine loss: implications for the etiology of idiopathic Parkinson’s disease. J Neurochem. 1992;58:642–648. doi: 10.1111/j.1471-4159.1992.tb09766.x. [DOI] [PubMed] [Google Scholar]
- Kueider AM, Tanaka T, An Y, Kitner-Triolo MH, Palchamy E, Ferrucci L, Thambisetty M. State- and trait-dependent associations of vitamin-D with brain function during aging. Neurobiol Aging. 2016;39:38–45. doi: 10.1016/j.neurobiolaging.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon KY, Jo KD, Lee MK, Oh M, Kim EN, Park J, Kim JS, Youn J, Oh E, Kim HT, Oh MY, Jang W. Low serum vitamin D levels may contribute to gastric dysmotility in de novo Parkinson’s disease. Neurodegener Dis. 2016;16:199–205. doi: 10.1159/000441917. [DOI] [PubMed] [Google Scholar]
- Latimer CS, Brewer LD, Searcy JL, Chen KC, Popović J, Kraner SD, Thibault O, Blalock EM, Landfield PW, Porter NM. Vitamin D prevents cognitive decline and enhances hippocampal synaptic function in aging rats. Proc Natl Acad Sci USA. 2014;111:E4359–E4366. doi: 10.1073/pnas.1404477111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling ZD, Collier TJ, Sortwell CE, Lipton JW, Vu TQ, Robie HC, Carvey PM. Striatal trophic activity is reduced in the aged rat brain. Brain Res. 2000;856:301–309. doi: 10.1016/s0006-8993(00)01945-4. [DOI] [PubMed] [Google Scholar]
- Llewellyn DJ, Lang IA, Langa KM, Muniz-Terrera G, Phillips CL, Cherubini A, Ferrucci L, Melzer D. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med. 2010;170:1135–1141. doi: 10.1001/archinternmed.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JF, Altar CA. Striatal dopamine uptake and swim performance of the aged rat. Brain Res. 1986;379:112–117. doi: 10.1016/0006-8993(86)90262-3. [DOI] [PubMed] [Google Scholar]
- Marshall JF, Rosenstein AJ. Age-related decline in rat striatal dopamine metabolism is regionally homogeneous. Neurobiol Aging. 1990;11:131–137. doi: 10.1016/0197-4580(90)90046-3. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, Neveu I, Wion D, Brachet P. 1,25-Dihydroxyvitamin D3, an inducer of glial cell line-derived neurotrophic factor. NeuroReport. 1996a;7:2171–2175. doi: 10.1097/00001756-199609020-00023. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, Neveu I, Baudet C, Funakoshi H, Wion D, Brachet P, Metsis M. 1,25-Dihydroxyvitamin D3 regulates the expression of the low-affinity neurotrophin receptor. Mol Brain Res. 1996b;41:259–268. doi: 10.1016/0169-328x(96)00103-9. [DOI] [PubMed] [Google Scholar]
- Neveu I, Naveilhan P, Baudet C, Brachet P, Metsis M. 1,25-Dihydroxyvitamin D3 regulates NT-3, NT-4 but not BDNF mRNA in astrocytes. NeuroReport. 1994a;6:124–126. doi: 10.1097/00001756-199412300-00032. [DOI] [PubMed] [Google Scholar]
- Neveu I, Naveilhan P, Menaa C, Wion D, Brachet P, Garabedian M. Synthesis of 1,25-dihydroxyvitamin D3 by rat brain macrophages in vitro. J Neurosci Res. 1994b;38:214–220. doi: 10.1002/jnr.490380212. [DOI] [PubMed] [Google Scholar]
- Newmark HL, Newmark J. Vitamin D and Parkinson disease – a hypothesis. Mov Disord. 2007;22:461–468. doi: 10.1002/mds.21317. [DOI] [PubMed] [Google Scholar]
- Orme RP, Bhangal MS, Fricker RA. Calcitriol imparts neuroprotection in vitro to midbrain dopaminergic neurons by upregulating GDNF expression. PLoS ONE. 2013;8(4):e62040. doi: 10.1371/journal.pone.0062040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM, Sakiyama R, Coty WA. Restricted transport of vitamin D and A derivatives through the rat blood-brain barrier. J Neurochem. 1985;44:1138–1141. doi: 10.1111/j.1471-4159.1985.tb08735.x. [DOI] [PubMed] [Google Scholar]
- Pendyala G, Ninemire C, Fox HS. Protective role for the disulfide isomerase PDIA3 in methamphetamine neurotoxicity. PLoS ONE. 2012;7(6):e38909. doi: 10.1371/journal.pone.0038909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen MS, Bech S, Christiansen DH, Schmedes AV, Halling J. The role of vitamin D levels and vitamin D receptor polymorphism on Parkinson’s disease in the Faroe Islands. Neurosci Lett. 2014;561:74–79. doi: 10.1016/j.neulet.2013.12.053. [DOI] [PubMed] [Google Scholar]
- Peterson AL. A review of vitamin D and Parkinson’s disease. Maturitas. 2014;78:40–44. doi: 10.1016/j.maturitas.2014.02.012. [DOI] [PubMed] [Google Scholar]
- Peterson AL, Murchison C, Zabetian C, Leverenz JB, Watson GS, Montine T, Carney N, Bowman GL, Edwards K, Quinn JF. Memory, mood, and vitamin D in persons with Parkinson’s disease. J Parkinsons Dis. 2013;3:547–555. doi: 10.3233/JPD-130206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothos EN, Davila V, Sulzer D. Presynaptic recording of quanta from midbrain dopamine neurons and modulation of the quantal size. J Neurosci. 1998;18:4106–4118. doi: 10.1523/JNEUROSCI.18-11-04106.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruett BS, Salvatore MF. Nigral GFRα1 infusion in aged rats increases locomotor activity, nigral tyrosine hydroxylase, and dopamine content in synchronicity. Mol Neurobiol. 2013;47:988–999. doi: 10.1007/s12035-013-8397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prufer K, Veenstra TD, Jirikowski GF, Kumar R. Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. J Chem Neuroanat. 1999;16:135–145. doi: 10.1016/s0891-0618(99)00002-2. [DOI] [PubMed] [Google Scholar]
- Przybelski RJ, Binkley NC. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys. 2007;460:202–205. doi: 10.1016/j.abb.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Puchacz E, Stumpf WE, Stachowiak EK, Stachowiak MK. Vitamin D increases expression of the tyrosine hydroxylase gene in adrenal medullary cells. Mol Brain Res. 1996;36:193–196. doi: 10.1016/0169-328x(95)00314-i. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Robertson HA. Evidence that L-dopa-induced rotational behavior is dependent on both striatal and nigral mechanisms. J Neurosci. 1989;9:3326–3331. doi: 10.1523/JNEUROSCI.09-09-03326.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Mocsary Z, Camp DM, Whishaw IQ. Time course of recovery of extracellular dopamine following partial damage to the nigrostriatal dopamine system. J Neurosci. 1994;14:2687–2696. doi: 10.1523/JNEUROSCI.14-05-02687.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore MF, Zhang JL, Large DM, Wilson PE, Gash CR, Thomas TC, Haycock JW, Bing G, Stanford JA, Gash DM, Gerhardt GA. Striatal GDNF administration increases tyrosine hydroxylase phosphorylation in the rat striatum and substantia nigra. J Neurochem. 2004;90:245–254. doi: 10.1111/j.1471-4159.2004.02496.x. [DOI] [PubMed] [Google Scholar]
- Salvatore MF, Pruett BS, Spann SL, Dempsey C. Aging reveals a role for nigral tyrosine hydroxylase ser31 phosphorylation in locomotor activity generation. PLoS ONE. 2009;4(12):e8466. doi: 10.1371/journal.pone.0008466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez B, Lopez-Martin E, Segura C, Labandeira-Garcia JL, Perez-Fernandez R. 1,25-Dihydroxyvitamin D3 increases striatal GDNF mRNA and protein expression in adult rats. Mol Brain Res. 2002;108:143–146. doi: 10.1016/s0169-328x(02)00545-4. [DOI] [PubMed] [Google Scholar]
- Sanchez B, Relova JL, Gallego R, Ben-Batalla I, Perez-Fernandez R. 1,25-Dihydroxyvitamin D3 administration to 6-hydroxydopamine-lesioned rats increases glial cell line-derived neurotrophic factor and partially restores tyrosine hydroxylase expression in substantia nigra and striatum. J Neurosci Res. 2009;87:723–732. doi: 10.1002/jnr.21878. [DOI] [PubMed] [Google Scholar]
- Saporito MS, Brown ER, Hartpence KC, Wilcox HM, Vaught JL, Carswell S. Chronic 1,25-dihydroxyvitamin D3-mediated induction of nerve growth factor mRNA and protein in L929 fibroblasts and in adult rat brain. Brain Res. 1994;633:189–196. doi: 10.1016/0006-8993(94)91539-3. [DOI] [PubMed] [Google Scholar]
- Schneider JS. Effects of age on GM1 ganglioside-induced recovery of concentrations of dopamine in the striatum in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice. Neuropharmacology. 1992;31:185–192. doi: 10.1016/0028-3908(92)90030-s. [DOI] [PubMed] [Google Scholar]
- Schwarting RKW, Huston JP. Unilateral 6-hydroxydopamine lesions of meso-striatal dopamine neurons and their physiological sequelae. Prog Neurobiol. 1996;49:215–266. doi: 10.1016/s0301-0082(96)00015-9. [DOI] [PubMed] [Google Scholar]
- Shinpo K, Kikuchi S, Sasaki H, Moriwaka F, Tashiro K. Effect of 1,25-dihydroxyvitamin D3 on cultured mesencephalic dopaminergic neurons to the combined toxicity caused by L-buthionine sulfoximine and 1-methyl-4-phenylpyridine. J Neurosci Res. 2000;62:374–382. doi: 10.1002/1097-4547(20001101)62:3<374::AID-JNR7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Shrestha S, Lutsey PL, Alonso A, Huang X, Mosley TH, Jr, Chen H. Serum 25-hydroxyvitamin D concentrations in mid-adulthood and Parkinson’s disease risk. Mov Disord. 2016;31:972–978. doi: 10.1002/mds.26573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MP, Fletcher-Turner A, Yurek DM, Cass WA. Calcitriol protection against dopamine loss induced by intracerebroventricular administration of 6-hydroxydopamine. Neurochem Res. 2006;31:533–539. doi: 10.1007/s11064-006-9048-4. [DOI] [PubMed] [Google Scholar]
- Sonnenberg J, Luine VN, Krey LC, Christakos S. 1,25-Dihydroxyvitamin D3 treatment results in increased choline acetyltransferase activity in specific brain nuclei. Endocrinology. 1986;118:1433–1439. doi: 10.1210/endo-118-4-1433. [DOI] [PubMed] [Google Scholar]
- Stanford JA, Vorontsova E, Surgener SP, Gerhardt GA, Fowler SC. Aged Fischer 344 rats exhibit altered orolingual motor function: relationships with nigrostriatal neurochemical measures. Neurobiol Aging. 2003;24:259–266. doi: 10.1016/s0197-4580(02)00083-0. [DOI] [PubMed] [Google Scholar]
- Stumpf WE, O’Brien LP. 1,25(OH)2 vitamin D3 sites of action in the brain. An autoradiographic study. Histochemistry. 1987;87:393–406. doi: 10.1007/BF00496810. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Yoshioka M, Hashimoto M, Murakami M, Noya M, Takahashi D, Urashima M. Randomized, double-blind, placebo-controlled trial of vitamin D supplementation in Parkinson disease. Am J Clin Nutr. 2013;97:1004–1013. doi: 10.3945/ajcn.112.051664. [DOI] [PubMed] [Google Scholar]
- Trevitt JT, Carlson BB, Nowend K, Salamone JD. Substantia nigra pars reticulata is a highly potent site of action for the behavioral effects of the D1 antagonist SCH 23390 in the rat. Psychopharmacology. 2001;156:32–41. doi: 10.1007/s002130100708. [DOI] [PubMed] [Google Scholar]
- Veenstra TD, Windebank AJ, Kumar R. 1,25-Dihydroxyvitamin D3 regulates expression of N-myc, c-myc, protein kinase C, and transforming growth factor-β2 in neuroblastoma cells. Biochem Biophys Res Commun. 1997a;235:15–18. doi: 10.1006/bbrc.1997.6718. [DOI] [PubMed] [Google Scholar]
- Veenstra TD, Londowski JM, Windebank AJ, Brimijoin S, Kumar R. Effects of 1,25-dihydroxyvitamin D3 on growth of mouse neuroblastoma cells. Dev Brain Res. 1997b;99:53–60. doi: 10.1016/s0165-3806(96)00196-4. [DOI] [PubMed] [Google Scholar]
- Verity AN, Wyatt TL, Lee W, Hajos B, Baecker PA, Eglen RM, Johnson RM. Differential regulation of glial cell line-derived neurotrophic factor (GDNF) expression in human neuroblastoma and glioblastoma cell lines. J Neurosci Res. 1999;55:187–197. doi: 10.1002/(SICI)1097-4547(19990115)55:2<187::AID-JNR6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chiang YH, Su TP, Hayashi T, Morales M, Hoffer BJ, Lin SZ. Vitamin D3 attenuates cortical infarction induced by middle cerebral arterial ligation in rats. Neuropharmacology. 2000;39:873–880. doi: 10.1016/s0028-3908(99)00255-5. [DOI] [PubMed] [Google Scholar]
- Wang JY, Wu JN, Cherng TL, Hoffer BJ, Chen HH, Borlongan CV, Wang Y. Vitamin D3 attenuates 6-hydroxydopmaine-induced neurotoxicity in rats. Brain Res. 2001;904:67–75. doi: 10.1016/s0006-8993(01)02450-7. [DOI] [PubMed] [Google Scholar]
- Wenk GL, Pierce DJ, Struble RG, Price DL, Cork LC. Age-related changes in multiple neurotransmitter systems in the monkey brain. Neurobiol Aging. 1989;10:11–19. doi: 10.1016/s0197-4580(89)80005-3. [DOI] [PubMed] [Google Scholar]
- Yurek DM, Fletcher-Turner A. Lesion-induced increase of BDNF is greater in the striatum of young versus old rat brain. Exp Neurol. 2000;161:392–396. doi: 10.1006/exnr.1999.7274. [DOI] [PubMed] [Google Scholar]
- Yurek DM, Fletcher-Turner A. Differential expression of GDNF, BDNF, and NT-3 in the aging nigrostriatal system following a neurotoxic lesion. Brain Res. 2001;891:228–235. doi: 10.1016/s0006-8993(00)03217-0. [DOI] [PubMed] [Google Scholar]
- Zanatta L, Goulart PB, Goncalves R, Pierozan P, Winkelmann-Duarte EC, Woehl VM, Pessoa-Pureur R, Silva FRMB, Zamoner A. 1α,25-dihydroxyvitamin D3 mechanism of action: modulation of L-type calcium channels leading to calcium uptake and intermediate filament phosphorylation in cerebral cortex of young rats. Biochim Biophys Acta. 2012;1823:1708–1719. doi: 10.1016/j.bbamcr.2012.06.023. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Abercrombie ED, Berger TW, Grace AA, Stricker EM. Compensations after lesions of central dopaminergic neurons: some clinical and basic implications. Trends Neurosci. 1990;13:290–295. doi: 10.1016/0166-2236(90)90112-n. [DOI] [PubMed] [Google Scholar]