Abstract
Background
Guidelines recommend variceal screening in patients with cirrhosis to identify varices at high-risk of bleeding requiring primary prophylaxis. Non-invasive criteria to rule out high-risk varices (HRV) will avoid unnecessary endoscopies. Recent Baveno VI criteria define patients with compensated cirrhosis in whom endoscopy can be avoided as those with a liver stiffness (LS) by transient elastography <20 kPa and a platelet count >150,000/mm3.
Aims
To validate Baveno criteria in two cohorts with a different prevalence of HRV and 2) to determine whether alternate parameters not including LS would be equal/more accurate in ruling out HRV.
Methods
Retrospective study evaluating patients with LS >10 kPa who had LS and endoscopy within one year of each other.
Results
The study included 161 patients from a U.S. cohort (14 [9%] with HRV) and 101 patients from an Italian cohort (17 [17%] with HRV). Of patients meeting Baveno criteria (41 in the US, 16 in Italy) none had HRV and therefore 26% (US) and 16% (Italy) endoscopies could have been avoided. Sensitivity and negative predictive value (NPV) were 100%. A stepwise strategy using platelet count >150,000 and MELD=6, increased the number of endoscopies avoided to 54% (US) while maintaining a sensitivity and NPV of 100%. Excellent sensitivity and NPV were validated in the Italian cohort and in another cohort of patients with a clinical diagnosis of cirrhosis.
Conclusions
This study validates Baveno VI criteria, particularly in sites with a low prevalence of HRV and describes a new accurate strategy that does not include LS.
Keywords: Cirrhosis, high-risk varices, non-invasive, transient elastography, endoscopy
Variceal hemorrhage is a complication of cirrhosis that defines decompensation and is associated with significant morbidity and mortality. First variceal hemorrhage can be prevented through the use of non-selective beta-blockers or endoscopic variceal ligation (1). However, not all patients with varices are candidates for the prevention of first variceal hemorrhage. Guidelines recommend prophylactic therapy in patients with high-risk varices (HRV), that is, those that are more likely to bleed, specifically patients with medium/large varices or patients with small varices with red wale marks on their surface or small varices occurring in Child C patients (1, 2)
Determining the presence and size of varices and the presence of red wale marks requires upper endoscopy, an invasive and expensive procedure that is not free of risks. Many studies have looked for non-invasive ways of determining the presence of HRV so as to circumvent the need for screening endoscopy in some patients. Although a number of laboratory/imaging-derived markers have been proposed, the most significant being the platelet/spleen size ratio, these studies have combined patients with both compensated and decompensated cirrhosis (3). Finding noninvasive predictors of the presence of HRV is more relevant in patients with compensated advanced chronic liver disease (cACLD), definition that includes patients with advanced fibrosis/compensated cirrhosis because the prevalence of varices in these patients is significantly lower than in those with decompensated cirrhosis in whom the presence of varices is almost universal (4). More recently, measurements of liver stiffness by transient elastography (TE) have been shown to be useful in discriminating patients with cirrhosis with and without HRV, particularly when combined with variables such as platelet count and spleen size (5-9).
In fact, the most recent Baveno consensus conference (2), based on a body of evidence from the literature and a recent multicenter study in patients with compensated advanced chronic liver diseases (9) defined patients unlikely to have HRV as those with cACLD with a liver stiffness measurement (LSM) less than 20 kPa (determined by TE) and a platelet count> 150,000/mm3. These criteria require validation as they were based on evidence that was mostly acquired in European centers where TE has been available for many more years than in the United States (U.S.) and where the prevalence of obesity (which could alter stiffness measurements) in patients with compensated cirrhosis is lower (10). In fact, TE is not available in most medical centers in the United States and trying to define non-invasive criteria that do not include TE would be desirable.
Our study had two aims: 1) the primary aim was to validate the criteria proposed at the Baveno VI Consensus conference in two cohorts of patients with cACLD, one in the United States and one in Europe and 2) to determine whether alternate parameters (not including TE) would be equally or more predictive than Baveno criteria in ruling out HRV.
Patients and Methods
This is a retrospective cohort study. The U.S. cohort consisted of patients with chronic liver disease that attended the outpatient liver clinics at the Veterans Administration Connecticut Healthcare System (VACHS) in West Haven, CT, U.S. in the period between February 2014 and April 2016 in whom TE was performed. The European cohort consisted of patients with chronic liver disease referred to the outpatient liver clinics at the Division of Gastroenterology, University of Modena and Reggio Emilia, Modena, Italy who had TE performed in the period between May 2010 and April 2016. The present study was approved by the VA Connecticut institutional review board (VA CT IRB) in the USA and by the University of Modena and Reggio Emilia Ethics Committee in Italy. As this is a retrospective study, obtaining informed consent was not applicable.
Patients were included in the study if they had a LSM ≥10 kPa and had laboratory tests and upper endoscopy performed within 12 months of TE. Exclusion criteria were decompensated cirrhosis (defined as the history or presence of overt ascites, overt encephalopathy or variceal hemorrhage); portal or splenic vein thrombosis, history of splenectomy and liver transplantation. Patients with LSM ≥10 were selected because, in the absence of other clinical signs, LSM<10 kPa excludes the presence of cACLD (2) and we wanted to be more inclusive. Patients who had LSM with a success rate <60% or an IQR/median >30% were also excluded.
TE was performed after at least 4 hours of fasting by experienced practitioners. LSMs were performed in the right lobe of liver as previously described and 10 successful measurements were obtained in each patient. In patients who had more than one LSM in the study period, the one with the lowest variability (IQR/median) was selected. LS measurements were performed initially using the M probe but if values were not obtainable because of obesity, they would be performed using the XL probe.
Our first goal was to determine the sensitivity of the Baveno definition (LSM <20 kPa and platelet count >150,000/mm3), that is, its ability in ruling out the presence of HRV and to determine the number of endoscopies that would have been safely avoided by using this definition. Because we did not include Child C patients, HRV were defined as medium/large varices or small varices with red wale marks on their surface (1, 2).
For our second objective, we used data from the U.S. cohort (training cohort) to identify and test other variables that would be helpful in ruling out HRV. We first compared routine laboratory values between patients with and without HRV. For variable(s) that would be most significantly different between groups, the cutoff selected would be the one that would avoid missing even one patient with HRV. Sensitivity, NPV, potential number of endoscopies avoided were then calculated for the following variables: LSM<20 kPa alone, platelet count >150,000 alone, any new variable uncovered with above analysis, combinations of variables in a stepwise approach. Results of the new variables/combination of variables obtained in the U.S. cohort were then validated in the Italian cohort (validation cohort).
Statistical Analysis
Statistical analysis was performed using SPSS package v.22 (SPSS Inc., Chicago, IL, U. S.). Comparisons between groups were performed using Mann-Whitney U test for non-parametric tests and Fisher's exact test for proportions. Because the main objective of the study was to determine parameters or combinations of parameters to rule out HRV, the main results calculated were sensitivity, negative predictive value (NPV) and their 95% confidence intervals (CIs) as well as the number of endoscopies that could have been circumvented. As we were not interested in analyzing specificity/positive predictive value, AUROC curves were not constructed.
Results
United States Cohort
A total of 839 patients were screened at the VACHS in the study period, of which 296 had a LSM ≥10 kPa. Of these, 135 patients were excluded because they did not have an upper endoscopy performed within 1 year of TE. Therefore 161 patients were analyzed. The median time between LSM and endoscopy was 3 months (range 0-363 days) and between LSM and laboratory data was 1 month (0-300 days). There was no significant difference between the LSM measured by M probe vs XL probe in this population (19.6 with M probe vs 21.3 with XL probe). Demographic and clinical characteristics of these patients are shown in Table 1. Notably, only 1 patient was female and the main etiology of cirrhosis was hepatitis C. In patients with hepatitis C, both LSM and upper endoscopy were performed before the initiation of direct acting antiviral therapy. Endoscopically, 106 (65.8%) patients had no gastroesophageal varices, 41 (25.5%) had small varices and 14 (8.6%) had medium/large varices (HRV). There were no patients with varices (any size) and red wale marks. As shown in Table 1, 41/161 (25.5%) patients fulfilled Baveno criteria predicting the absence of HRV and would have circumvented screening endoscopy. None of these patients had medium/large varices, that is, no patient with HRV would have been missed. The sensitivity of Baveno criteria was 100% (95% CI 77%-100%) with a NPV of 100% (95% CI 91%-100%).
Table 1.
Characteristics of patients with compensated cirrhosis (LSM≥10 kPa) in the U.S. Cohort
| Characteristics | U.S. Cohort (N=161) |
|---|---|
| Age-years | 62 (40-80) |
| Male-sex (%) | 99.4 |
| Etiology of liver disease (%) | |
| HCV | 73.3 |
| ETOH | 13.0 |
| NASH/NAFLD | 10.6 |
| Miscellaneous | 3.1 |
| BMI | 29 (17-47) |
| Albumin g/L | 3.6 (2.4-4.6) |
| Bilirubin mg/dl | 0.78 (0.23-3) |
| INR (n=159) | 1.1 (0.9-1.6) |
| Creatinine mg/dL (n=158) | 0.9 (0.6-2.1) |
| AST IU/ml | 46 (14-198) |
| Platelet count × 103/mm3 | 139 (25-383) |
| Child Pugh score (n=159) | 5 (5-9) |
| Child Pugh class (%) (n=159) | |
| A | 92.4 |
| B | 7.6 |
| MELD score (n=156) | 9 (6-17) |
| Liver Stiffness (kPa) | 20.4 (10-75) |
| M probe used (%) | 49.3 |
| Patients with varices (any size) n (%) | 55 (34.1) |
| Patients with high-risk varices n (%) | 14 (8.7) |
| Patients fulfilling Baveno criteria* | 41 (25.5%) |
| Patients fulfilling Baveno criteria who had HRV | 0/41 |
Results are expressed as median (range) if not stated otherwise
Defined as LSM<20 kPa and platelet count >150 × 103/mm3, patients fulfilling the criteria would have circumvented screening endoscopy
** numbers in brackets represent 95% confidence interval
Italian Cohort
This cohort consisted of 101 patients with chronic liver disease with a LS ≥10 kPa. The cohort differed from the U.S. cohort regarding gender (more females); a lower BMI and a higher prevalence of varices and HRV and the fact that all LSMs were obtained using the M probe (Table 2). Endoscopically, 48 (47.5%) patients had no varices, 36 (35.6%) had small varices and 17 (16.8%) had medium/large varices (HRV). The median time between LSM and endoscopy was 2 months (range 0 to 357 days) and between LSM and laboratory data was 2 months (0-273 days). Similar to the U.S. cohort, the only HRV were those that were medium/large-sized. As shown in Table 2, 16/101 (15.8%) patients fulfilled Baveno criteria predicting the absence of HRV and would have circumvented screening endoscopy. None of these patients had medium/large varices, that is, no patient with HRV would have been missed. The sensitivity was 100% [95% CI 80%-100%] with NPV of 100% [95% CI 79%-100%].
Table 2.
Characteristics of patients with compensated cirrhosis (LSM≥10 kPa) in the Italian cohort
| Characteristics | Italian Cohort (n= 101) |
|---|---|
| Age-years | 63 (23-80) |
| Male-sex (%) | 72.3 |
| Etiology of liver disease (%) | |
| HCV | 66.4 |
| ETOH | 11.8 |
| NASH/NAFLD | 3.0 |
| Miscellaneous | 18.8 |
| BMI | 25 (19-30) |
| Albumin g/L | 3.9 (2.5-4.8) |
| Bilirubin mg/dl | 0.96 (0.37-3) |
| INR | 1.17 (0.9-1.8) |
| Creatinine mg/dL | 0.78 (0.44-1.57) |
| AST IU/ml | 44 (10-198) |
| Platelet count × 103/mm3 | 98 (36-340) |
| Child Pugh score | 5 (5-9) |
| Child Pugh class (%) | |
| A | 91.1 |
| B | 8.9 |
| MELD score | 9 (6-16) |
| Liver Stiffness (kPa) | 17.6 (10-75) |
| M probe used (%) | 100 |
| Patients with varices (any size) n (%) | 53 (52.4) |
| Patients with HRV n (%) | 17 (16.8) |
| Patients fulfilling Baveno criteria* | 16 (15.8) |
| Patients fulfilling Baveno criteria who had HRV | 0/16 |
Results are expressed as median (range) if not stated otherwise
Defined as LSM<20 kPa and platelet count >150 × 103/mm3, patients fulfilling the criteria would have circumvented screening endoscopy
** numbers in brackets represent 95% confidence interval
Deconstructing/Refining Baveno criteria
As shown in Table 3 and as analyzed in the U.S. cohort, platelet count and MELD score were the two parameters that most differed between patients with and without HRV. Because MELD was not assessable in 5 patients, in 3 because they were on hemodialysis for chronic kidney disease unrelated to cirrhosis and 2 because they were on anticoagulation, this comparison was made in 156 patients (14 with HRV, and 142 without HRV).
Table 3.
Comparison of the U. S. population with high-risk varices vs the population with low risk varices or no varices (n=156*)
| Characteristics | High Risk varices (n=14) | No/Small varices (n=142) | p |
|---|---|---|---|
| Age- years | 63 (55-68) | 62 (40-80) | 0.820 |
| Male-sex (%) | 100 | 99.3 | 0.907 |
| BMI | 29 (17-43) | 30 (17-47) | 0.605 |
| Etiology of liver disease (%) | 0.763 | ||
| HCV | 73.3 | 73.2 | |
| ETOH | 6.7 | 14.1 | |
| NASH | 13.3 | 9.2 | |
| Miscellaneous | 6.7 | 3.5 | |
| Albumin g/L | 3.3 (2.6-4.3) | 3.6 (2.7-4.6) | 0.950 |
| Bilirubin mg/dl | 1.66 (0.4-3) | 0.74 (0.23-3) | 0.019 |
| INR | 1.2 (1-1.6) | 1.1 (0.9-1.6) | 0.001 |
| Creatinine mg/dL | 0.8 (0.7-1.7) | 0.9 (0.6-2.1) | 0.477 |
| AST IU/ml | 43 (21-181) | 48 (14-198) | 0.605 |
| PLT × 103/mm3 | 80 (36-157) | 145 (25-383) | 0.001 |
| Child Pugh score | 6 (5-8) | 5 (5-8) | 0.075 |
| Child Pugh Class (%) | 0.131 | ||
| A | 80 | 93 | |
| B | 20 | 7 | |
| MELD score | 11 (7-16) | 8 (6-17) | 0.001 |
Results are expressed as median (range) if not stated otherwise
5 patients excluded from this analysis because MELD was not evaluable (see text)
For the cutoff of platelet count, we decided to keep the one established by Baveno criteria (>150,000) because this cutoff has been established not only by Baveno but also by other studies (2,13,14). Because in our U.S cohort, no patient with a MELD of 6 had HRV, while 1/56 patient with a MELD of 7 had HRV, we established the MELD cutoff at a score of 6.
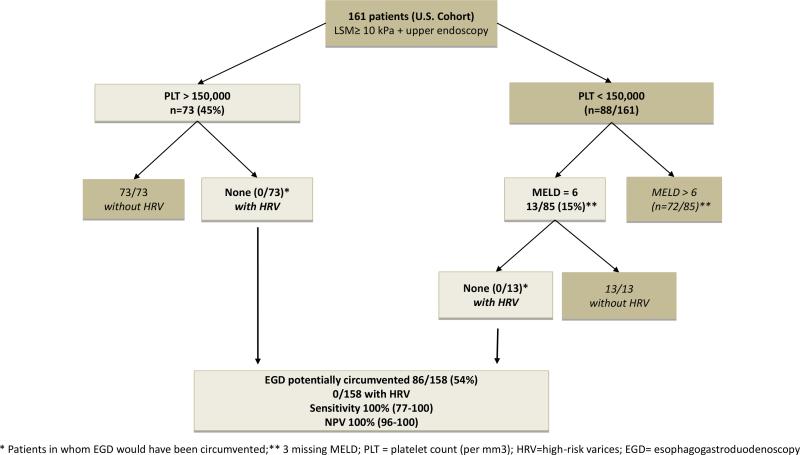
We then analyzed, in the U.S. cohort, the value of (1) platelet count (>150,000/mm3) alone, (2) MELD=6 alone, (3) Baveno criteria/MELD=6, that is, adding patients who did not fulfill Baveno criteria but had a MELD=6 as being unlikely to have HRV, and (4) platelet count/MELD, that is, adding patients with platelet count <150,000 but had a MELD=6 as being unlikely to have HRV) (Figure 1). The results of these analyses (Table 4A) shows that the strategy that avoided the largest number of endoscopies (86/158 or 54%) without missing any patient with HRV (sensitivity 100%, 95% CI 77-100%; NPV 100%, 95% CI 96-100%) was the one that selected patients with platelet count >150,000 and added those with a MELD=6 among those who had a platelet count <150,000 (Figure 1).
Figure 1.
New stepwise strategy for ruling out high risk varices in patients with compensated cirrhosis using only platelet count and MELD score.
Table 4.
Refining Baveno criteria
| A U.S. cohort (n=161) – 9% HRV |
B Italian Cohort (n=101) – 17% HRV |
|||||||
|---|---|---|---|---|---|---|---|---|
| EGDs avoided | HRV | Sensitivity | NPV | EGDs avoided | HRV | Sensitivity | NPV | |
| Baveno criteria | 41 (26%) | 0/14 | 100% (77-100) | 100% (91-100) | 16 (16%) | 0/17 | 100% (80-100) | 100% (79-100) |
| PLT >150 | 73 (45%) | 0/14 | 100% (77-100) | 100% (95-100) | 19 (19%) | 1*/17 (1%) | 94% (71-100) | 95% (75-100) |
| MELD =6 | 29 (19%) | 0/14 | 100% (77-100) | 100% (88-100) | 16 (16%) | 0/17 | 100% (80-100) | 100% (79-100) |
| Baveno/MELD=6 | 60 (38%) | 0/14 | 100% (77-100) | 100% (94-100) | 28 (28%) | 0/17 | 100% (80-100) | 100% (88-100) |
| PLT >150/MELD=6 | 86 (54%) | 0/14 | 100% (77-100) | 100% (96-100) | 30 (30%) | 1*/17 (1%) | 94% (71-100) | 97% (83-100) |
HRV= High risk varices; EGD= esophagogastroduodenoscopy; PLT= platelet count (×1000/mm3); Baveno/MELD adds patients who do not fulfill Baveno criteria (PLT<150 and LSM >20) but have a MELD <7; PLT/MELD adds patients with a platelet count <150,000 but have a MELD =6
patient with PLT 340, LSM 22.5 and medium-sized varices
In the Italian cohort (Table 4B) with a higher prevalence of HRV the best strategy was also the platelet/MELD strategy, although in this cohort the number of endoscopies avoided was lower (30/101 or 30%) and one patient would have been missed (sensitivity 94%, 95% CI 71-100%; NPV 97%, CI 83-100%). The missed patient had an LSM of 22.5 kPa, platelet count of 340,000/mm3, had medium-sized varices and no clear explanation for the unexpectedly high platelet count.
Because patients in these cohorts were selected using TE, we analyzed a third cohort of patients from the outpatient liver clinics at the Division of Gastroenterology, University of Modena and Reggio Emilia, Modena, Italy, who had a clinical diagnosis of compensated cirrhosis (by laboratory tests and imaging) but who did not have TE performed to see if we could further validate this new strategy. This cohort consisted of 146 patients with compensated (no ascites, no encephalopathy, no variceal hemorrhage) cirrhosis, 34 (23%) of whom had HRV. Of these, 39 (27%) met the platelet/MELD criteria and could have avoided endoscopy and only 1 patient with HRV (platelet count = 159,000/mm3) would have been missed for a sensitivity of 97% (95%CI 85-100) and NPV of 98% (95%CI 85-100).
Discussion
Identifying patients with a low probability of having high-risk gastroesophageal varices (i.e. those requiring prophylactic therapy) is important to be able to circumvent the performance of screening endoscopies thus saving time, costs and, importantly, possible risks associated with the procedure. This is particularly relevant for patients with cACLD who have a low probability of having high-risk varices (HRV) (4). In this sense, the Baveno VI consensus workshop (2), based on a large body of evidence and a recent multicenter study (9) took a big step forward by defining patients having a low probability of having HRV as those with cACLD, a liver stiffness <20 kPa and a platelet count >150,000/mm3.
We were able to validate these criteria in two separate cohorts with cACLD (defined as a liver stiffness ≥10 kPa and no decompensating events): one in a male Veteran population with a high prevalence of obesity in the U.S. and one in a general population in northern Italy. Because prevalence affects the predictive value of any test, we chose two cohorts with different prevalence of varices/HRV. The U.S. cohort had a lower prevalence of varices (34%) and of HRV (9%) compared to the Italian cohort (52% varices, 17% HRV). Despite these differences, none of the patients that met Baveno criteria in either cohort were found to have HRV. However, endoscopy would have been circumvented in a larger number of patients in the U.S. cohort (26% vs. 16%). The sensitivity of the definition was 100% in both cohorts with a 100% NPV. Because the number of patients included is not large, lower limit of the confidence intervals for the NPV indicate that, theoretically, up to 9% (in the low prevalence cohort) to 21% (in the higher prevalence cohort) could have HRV.
Differences in the prevalence of varices between the two cohorts are not explicable since they were both selected using the same criteria. Since this is a retrospective study, we cannot be sure that these were unselected patients. In fact, our third cohort of patients, in whom a clinical diagnosis of cirrhosis had been established clinically and who did not have TE performed, had an even larger prevalence of varices.
In a recently published retrospective study that included 310 patients with an even lower prevalence of HRV (5%), 33% of patients met Baveno criteria (and could have circumvented endoscopy) but they missed 2 patients with HRV for a sensitivity of 87% and NPV 98%, lower than our results (11). Notably, one of the 2 misclassified patients had had a splenectomy and excluding this patient could have made results comparable to ours.
It must be mentioned that in the Baveno consensus, ruling in cACLD required a LSM>15 kPa so there is a possibility that patients without cACLD could have been included in our study. We wanted to be inclusive so as not to miss any patient with cACLD. We performed an analysis (results not shown) restricted to patients with LSM >15 (122 in the U.S. cohort and 70 in the Italian cohort). As expected, the prevalence of varices was a bit higher (10% in the U.S. cohort, 24% in the Italian cohort) but the number of endoscopies avoided was essentially unchanged (27% and 19%, respectively).
The second objective of our study was to identify additional or alternate parameters that would increase the number of endoscopies that could be circumvented and that could be applicable in sites where TE is not available. Platelet count and MELD score were the most likely candidates as they were significantly different between those with and without HRV.
Platelet count alone has been shown to be a strong predictor of HRV in studies that have included patients with both compensated and decompensated cirrhosis with a suboptimal diagnostic accuracy (12, 13). In patients with compensated cirrhosis, one study showed that none of 8 patients with large varices had a platelet count above 150,000/mm3 (14) while in another study this occurred in 1/13 patients with medium/large varices for a negative predictive value of 99% (15). These findings are not unlike our results where none of 14 patients with HRV in the U.S. cohort and only 1/17 in the Italian cohort had a platelet count >150,000/mm3.
Although MELD score has been explored as a predictor of varices, these studies have all included a significant proportion of patients with decompensated cirrhosis (13, 16, 17). To our knowledge this is the first study that shows the value of MELD in predicting HRV in patients with compensated cirrhosis. We found that none of 14 patients with HRV in the U.S. cohort and none of 17 patients with HRV in the Italian cohort had a MELD score of 6. However, the number of endoscopies avoided just using the MELD score is quite low.
Adding MELD=6 to Baveno criteria, the number of endoscopies that could have been avoided increased from 26% to 38% in the U.S. cohort and from 16% to 28% in the Italian cohort, while maintaining the same sensitivity and NPV. However this requires TE and this is not widely available.
In fact, the largest proportion of endoscopies that could be avoided resulted from a stepwise combination of a platelet count >150,000 and a MELD =6 (in those with a platelet count <150,000) as depicted in the Figure. With this combination, 54% of endoscopies could be avoided (compared to 26% using Baveno criteria) while maintaining a sensitivity and NPV of 100%. Results from our U.S. training cohort were validated in the Italian cohort in whom we found that we could avoid 30% of endoscopies (compared to 16% using Baveno criteria) but with a somewhat lower but still acceptable sensitivity of 94% and a NPV of 97% because one patient with HRV was missed. Because one could argue that the two original cohorts were selected based on TE, we analyzed a third cohort of patients with a clinical diagnosis of compensated cirrhosis in whom TE was not performed and were able to further validate this strategy.
The study is retrospective and the interval between endoscopy and LSM was as large as 12 months. However, per recent guidance (1) the minimal interval for repeat screening endoscopy in patients with no or small varices is one to two years which indicates that patients are unlikely to develop HRV in this interval. Sample size was not large, most patients were male and most patient had untreated hepatitis C. Therefore, our new strategy requires validation in a larger number of patients with a more equal gender distribution and in the growing population of patients with cirrhosis due to nonalcoholic steatohepatitis.
In conclusion, this study validates non-invasive criteria defined by the Baveno consensus workshop in the identification of patients with compensated cirrhosis unlikely to have HRV requiring prophylactic therapy and who can therefore safely avoid screening endoscopy. Recognizing that TE is not widely available in the U.S. and worldwide, we also found that stepwise combination of platelet count>150,000/mm3 and a MELD score =6 has the potential of avoiding more endoscopies particularly in centers with a low prevalence of high-risk varices. This simple model requires validation in other larger cohorts with different etiologies of cirrhosis.
Key points.
Non-invasive tests are useful in ruling out high-risk varices (HRV) in patients with compensated cirrhosis
Using Baveno VI criteria we correctly identified 100% of patients who could avoid screening endoscopy and up to 26% of endoscopies could have been safely avoided
Where transient elastography is available, Baveno VI criteria is the most validated strategy and the risk of missing HRV is minimal
Where transient elastography is not available, lab-based non-invasive tests (platelet count >150,000/mm3 followed by MELD=6 in a stepwise strategy) may circumvent more endoscopies while maintaining a high sensitivity and NPV, but results must be validated in larger cohorts
Acknowledgments
Financial Support: Yale Liver Center NIH P30 DK34989
Abbreviations
- HRV
High-risk varices
- cACLD
compensated advanced chronic liver disease
- TE
Transient Elastography
- LSM
Liver stiffness measurement
- NPV
Negative predictive value
- CI
Confidence interval
- MELD
Model for end-stage liver disease
Footnotes
Conflict of interest
No conflict of interest
References
- 1.Garcia-Tsao G, Abraldes J, Berzigotti A, et al. Portal Hypertensive Bleeding in Cirrhosis: Risk Stratification, Diagnosis and Management - 2016 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2016 doi: 10.1002/hep.28906. [DOI] [PubMed] [Google Scholar]
- 2.De Franchis R, BAVENO VIF. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63(3):743–52. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 3.Giannini EG, Zaman A, Kreil A, et al. Platelet count/spleen diameter ratio for the noninvasive diagnosis of esophageal varices: results of a multicenter, prospective, validation study. Am J Gastroenterol. 2006;101(11):2511–9. doi: 10.1111/j.1572-0241.2006.00874.x. [DOI] [PubMed] [Google Scholar]
- 4.Kovalak M, Lake J, Mattek N, et al. Endoscopic screening for varices in cirrhotic patients: data from a national endoscopic database. Gastrointest Endosc. 2007;65(1):82–8. doi: 10.1016/j.gie.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 5.Kim BK, Han KH, Park JY, et al. A liver stiffness measurement-based, noninvasive prediction model for high-risk esophageal varices in B-viral liver cirrhosis. Am J Gastroenterol. 2010;105(6):1382–90. doi: 10.1038/ajg.2009.750. [DOI] [PubMed] [Google Scholar]
- 6.Berzigotti A, Seijo S, Arena U, et al. Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology. 2013;144(1):102–11. e1. doi: 10.1053/j.gastro.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Ding NS, Nguyen T, Iser DM, et al. Liver stiffness plus platelet count can be used to exclude high-risk oesophageal varices. Liver Int. 2016;36(2):240–5. doi: 10.1111/liv.12916. [DOI] [PubMed] [Google Scholar]
- 8.Augustin S, Millan L, Gonzalez A, et al. Detection of early portal hypertension with routine data and liver stiffness in patients with asymptomatic liver disease: a prospective study. J Hepatol. 2014;60(3):561–9. doi: 10.1016/j.jhep.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 9.Abraldes JG, Bureau C, Stefanescu H, et al. Noninvasive tools and risk of clinically significant portal hypertension and varices in compensated cirrhosis: The “Anticipate” study. Hepatology. 2016 doi: 10.1002/hep.28824. [DOI] [PubMed] [Google Scholar]
- 10.Berzigotti A, Garcia-Tsao G, Bosch J, et al. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology. 2011;54(2):555–61. doi: 10.1002/hep.24418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maurice JB, Brodkin E, Arnold F, et al. Validation of the Baveno VI criteria to identify low risk cirrhotic patients not requiring endoscopic surveillance for varices. J Hepatol. 65(5):899–905. doi: 10.1016/j.jhep.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 12.Sharma P, Mishra SR, Kumar M, et al. Liver and spleen stiffness in patients with extrahepatic portal vein obstruction. Radiology. 2012;263(3):893–99. doi: 10.1148/radiol.12111046. [DOI] [PubMed] [Google Scholar]
- 13.Tafarel JR, Tolentino LH, Correa LM, et al. Prediction of esophageal varices in hepatic cirrhosis by noninvasive markers. Eur J Gastroenterol Hepatol. 2011;23(9):754–8. doi: 10.1097/MEG.0b013e3283488a88. [DOI] [PubMed] [Google Scholar]
- 14.Qamar AA, Grace ND, Groszmann RJ, et al. Platelet count is not a predictor of the presence or development of gastroesophageal varices in cirrhosis. Hepatology. 2008;47(1):153–9. doi: 10.1002/hep.21941. [DOI] [PubMed] [Google Scholar]
- 15.Sanyal AJ, Fontana RJ, Di Bisceglie AM, et al. The prevalence and risk factors associated with esophageal varices in subjects with hepatitis C and advanced fibrosis. Gastrointest Endosc. 2006;64(6):855–64. doi: 10.1016/j.gie.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Zambam De Mattos A, Alves De Mattos A, Daros LF, et al. Aspartate aminotransferase-to-platelet ratio index (APRI) for the non-invasive prediction of esophageal varices. Ann Hepatol. 12(5):810–4. [PubMed] [Google Scholar]
- 17.Burton JR, JR., Liangpunsakul S, Lapidus J, et al. Validation of a multivariate model predicting presence and size of varices. J Clin Gastroenterol. 2007;41(6):609–15. doi: 10.1097/01.mcg.0000225669.84099.04. [DOI] [PubMed] [Google Scholar]