Abstract
Tourette syndrome (TS) is a neurodevelopmental disorder characterized by multiple motor and phonic tics. While TS patients have been also shown to exhibit subtle abnormalities of sensorimotor integration and gait, animal models of this disorder are seldom tested for these functions. To fill this gap, we assessed gait and sensorimotor integration in the D1CT-7 mouse, one of the best-validated animal models of TS. D1CT-7 mice exhibit spontaneous tic-like manifestations, which, in line with the clinical phenomenology of TS, are markedly exacerbated by environmental stress. Thus, to verify whether stress may affect sensorimotor integration and gait functions in D1CT-7 mice, we subjected these animals to a 20-min session of spatial confinement, an environmental stressor that was recently shown to worsen tic-like manifestations. Immediately following this manipulation (or no confinement, for controls), animals were subjected to either the sticky-tape task, to test for sensorimotor integration; or a 60-min session in an open field (42 × 42 cm) force-plate actometer for gait analysis. Gait analyses included spatial, temporal, and dynamic (force) parameters. D1CT-7 mice displayed a longer latency to remove a sticky tape, indicating marked impairments in sensorimotor integration; furthermore, these mutants exhibited shortened stride length, increased stride rate, nearly equal early-phase velocity, and higher late-phase velocity. D1CT-7 mice also ran with greater force amplitude than wild-type (WT) littermates. None of these phenotypes was worsened by spatial confinement. These results highlight the potential importance of testing sensorimotor integration and gait functions as a phenotypic correlate of cortical connectivity deficits in animal models of TS.
Keywords: Tourette syndrome, animal models, gait, sensorimotor integration
1. Introduction
Tourette syndrome (TS) is a neurodevelopmental disorder marked by the presence of multiple motor and phonic tics. A diagnostic hallmark of tics is that they are typically preceded by sensory phenomena (SP) - intrusive somatic sensations and premonitory urges that are transiently attenuated by tic execution (Houghton et al., 2014).
Although the neurobiological substrates of TS have not been completely clarified, growing evidence suggests that both tics and SP result from the insufficient inhibition or excessive excitation of different foci within the cortico-striato-thalamo-cortical loops. Specifically, tics may be caused by the activation of ectopic foci in the dorsal striatum (Albin and Mink, 2006), resulting from insufficient interneuronal inhibition and/or excessive output from cortical glutamatergic or nigrostriatal dopaminergic projections. Conversely, SP are posited to reflect the hyperactivation of several cortical areas, ranging from the somatosensory cortex to the supplementary motor area and the anterior cingulate cortex (Wang et al., 2011).
In addition to tics and SP, TS patients show dysfunctions in sensorimotor integration and somatic information processing (Abbruzzese and Berardelli, 2003; Patel et al., 2014; Swerdlow et al., 2001). Given the importance of these domains in motor control, it is not surprising that TS patients also display subtle anomalies of postural control, proprioception, and gait (Lemay et al., 2007; Liu et al., 2014; Nowak et al., 2005), all functions directly regulated by sensory feedback (Frost et al., 2015; Peterka and Loughlin, 2004).
Rodent models are useful tools to study the pathophysiology of TS (Godar et al., 2014); however, analyses of these models have been mostly limited to the testing of tic-like motor manifestations and/or emotional alterations akin to comorbid conditions with TS, such as anxiety. Conversely, sensorimotor integration and gait functions remain poorly explored in animal models of TS. The primary purpose of this study is to fill this gap, by showing the analysis of sensorimotor integration and gait functions in one of the best-validated animal models of TS, the D1CT-7 mouse (Campbell et al., 1999; Nordstrom et al., 2015). This mutant features a neuropotentiating transgene expressed in a subset of neurons harboring the dopamine D1 receptor, localized in the somatosensory cortex, piriform cortex and intercalated nucleus of the amygdala (Campbell et al., 1999). These changes result in a number of phenotypes strikingly reminiscent of TS, including myoclonic axial jerks that are sensitive to all major treatments for TS (Nordstrom and Burton, 2002). Additionally, D1CT-7 mice exhibit locomotor hyperactivity, heightened wall leaping and gnawing (Campbell et al., 1999), as well as increased anxiety-like behaviors (McGrath et al., 1999).
One of the key characteristics of TS lies in its significant fluctuations in severity, which reflect the impact of several contextual stressors. Indeed, stress increases the severity and frequency of tics and SP (Robertson et al., 2002; O'Connor et al., 2003; Eapen et al., 2004; Cohen et al., 2013; Godar and Bortolato, in press). In line with this concept, we recently showed that D1CT-7 mice display an exacerbation in tic-like responses and sensorimotor gating deficits following exposure to mild environmental stress (i.e., a 20-min confinement session within a 10-cm diameter cylinder) (Godar et al., 2016). To verify whether the exacerbation in tic-like responses was accompanied by changes in sensorimotor integration and gait, we also tested whether the same mild environmental stress conditions may affect these parameters.
The secondary purpose of this work is to illustrate in detail how gait analyses can be carried out with a force-plate actometer and associated software (Fowler et al., 2001; Fowler and Muma, 2015). Gait characteristics in mice are affected by the transduction methods (force plate, videography-treadmill, videography-fixed runway) as well as by the behavior-relevant environmental conditions prevailing at the time data are collected. For example, Lepicard et al. (2006) compared the gait of two inbred strains of mice by using an elevated, fixed runway with side walls that were transparent on both sides, opaque on both sides, or transparent on one side and opaque on the other. A railway-mounted video camera recorded images of a mouse's feet as it ran (trotted) on a transparent floor. For both strains, the asymmetrical-wall condition (opaque on one side) elicited the shortest stride length, the lowest stride frequency, and the slowest velocity compared to the other two conditions. Given that the session of data collection was only 5 min (too brief for substantial habituation to occur), the data suggested that gait parameters are influenced by motivational/emotional/cognitive states evoked by exposure to a novel environment. This prior result led us to the decision to include in our gait analysis of the D1CT-7 mouse an early-late (E-L) variable. This allows for the detection of differences in behavior early in a session when novelty and neophobia were maximal, compared to late in the session when substantial adaptation to the open field would be presumed to have occurred.
Overall, the findings presented in this manuscript highlight the value and utility of force-plate actometry and sticky-tape removal tests in the assessment of endophenotypic deficits associated with TS, which may assist in the identification of cross-species paradigms for a more detailed and precise validation of multiple translational aspects in the development of animal models of tic disorders.
2. Materials and methods
2.1 Animals and care
We used 3- to 4-month-old male Balb/cJ mice, weighing 20-30 g. Animals were purchased by Jackson Lab (Bar Harbor, ME, USA), bred in our facilities and genotyped as reported by Campbell et al (1999). Because the pattern of inheritance of D1CT-7 mice is autosomal dominant, wild-type (WT) females were bred with heterozygous D1CT-7 sires; this breeding scheme was selected to standardize maternal behavior. Animals were housed in group cages with ad libitum access to food and water. The room was maintained at 22 C, on a 12:12 h light/dark cycle from 0800 to 2000 h. Mice were tested during their light cycle, between 1200 and 1600h, to minimize potential circadian effects. The mice used for gait analysis were the same animals used for the locomotor activity section of the paper by Godar et al. (2016). All experimental procedures were in compliance with the National Institute of Health guidelines and approved by the Institutional Animal Use Committees of the Universities of Kansas and Utah.
2.2. Spatial confinement
Animals were confined within a clear, bottomless Plexiglas cylinder (10 cm in diameter × 30 cm in height), which was placed in their home cages, deeply embedded in bedding to ensure stability as previously indicated (Godar et al, 2016). Spatial confinement lasted 20 min.
2.3 Sticky-tape test
Deficits in sensorimotor integration were measured using the sticky tape test as previously described (Bouet et al., 2009). Mice were briefly restrained and a circular piece of tape (5 mm in diameter) was placed on the bottom of each forepaw. Animals were then released and the latency to remove each piece of tape was recorded, using a 5-min cut-off time.
2.4. Gait analysis
Apparatus
The apparatus was a square force plate actometer (Fowler et al., 2001; Fowler and Muma, 2015) measuring 42 cm on a side and 30 cm high. The 4 force transducers that supported the load plate at its corners were sampled 100 times/s, giving a temporal resolution of 0.01 s. Force resolution was 0.2 g force, and spatial resolution was about 2 mm. A Pascal program written in-house directed the timing and data-logging processes via a LabMaster interface. After data collection, a scrolling graphics program written in Visual Basic was used to extract selected gait parameters (e.g., stride length, stride rate, etc.) from the data stream. Actometer recording sessions lasted 1 h, and immediately preceding the session, half of the mice were kept for 20 min of confinement in a clear polycarbonate cylinder (10 cm inside diameter, 30 cm high) placed in the home cage as previously described (Godar et al., 2016).
Analytic procedure
Raw actometer data were processed by a Free Pascal program that produced six time series that were displayed as separate horizontal lanes by a scrolling graphics program. The displayed time series included Fz(t), X(t), and Y(t), which represented vertical force, X coordinate, and Y coordinate, respectively (see Fig. 1). On the computer monitor, time series were cross cut by two vertical, controllable cursors (not shown), one on the left and one on the right. The points of intersection between the cursors and the time series data were displayed numerically as the cursors were moved across the face of the time-series data. The time within the recording session was concurrently displayed for both cursors. Thus, the cursors allowed the user to determine the time of occurrence of, and values of, events visible in the data. These data were saved to an open Excel file by one click on a “Save Datum” button. The program user scanned the plotted data and identified large amplitude rhythmic force variations that are characteristic of rodent trotting behavior (see examples in panels A, B, C, and D in Fig. 1). Inside the larger ellipse to the right of panel A, consecutive half strides are designated by the numbers 1-9. Each cycle is a half stride. A rhythmic sequence of five or more half strides constituted a run or “trot” where diagonally opposite feet act in unison to produce alternating stance and swing phases (see Fig. 1). In order to be considered suitable for quantitation, five or more consecutive half strides had to be discerned by inspection. Additional criteria for including a run in the data set for estimation of gait parameters were:
A run had to be reasonably straight. This was judged by the program user by noting the lack of curvature in X(t) and/or Y(t) during the trot sequence of half strides. This criterion ensured that variation in rhythm and other gait parameters would not be the result of changing direction at corners, etc;
A run had to be expressed as a continuous sequence of half strides without any pausing in locomotion;
Rhythmic sequences of force variation, such as those produced by grooming or scratching, were easily excluded from scoring because these in-place behaviors were not accompanied by locomotion (discernible from the X(t) and Y(t) plots having zero slope).
Figure 1.
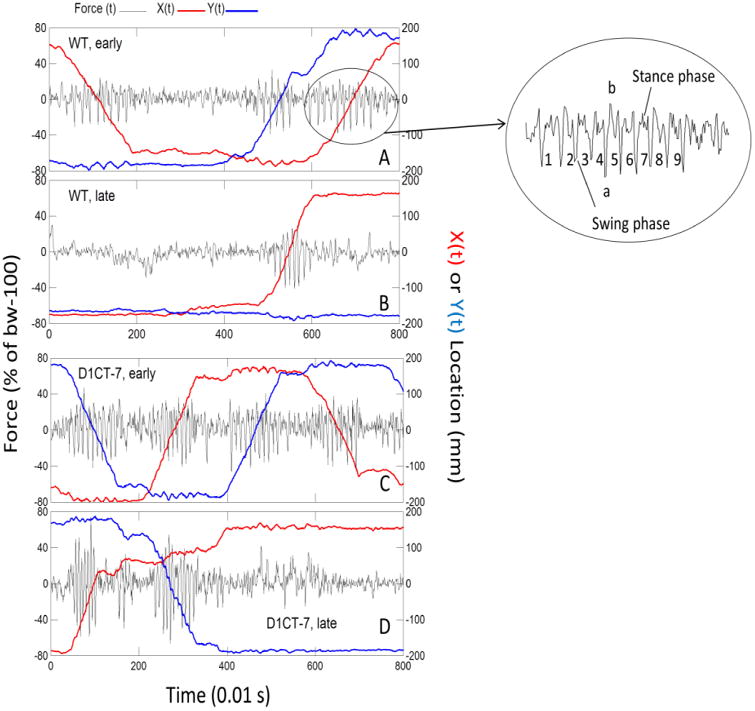
D1CT-7 mice exhibit faster and more forceful strides in force plate actometer tests. Shown here are time series recordings of force and location for one WT mouse (panels A and B) and one D1CT-7 mouse (panels C and D). In each panel, the ordinate on the left is for ground reaction force sampled at 100 samples/s. The black line depicts unsmoothed vertical force variations. Within the ordinate label on the left “bw-100” indicates body weight minus 100. The ordinate on the right gives the scaling of the distance across the floor of the chamber in the X and Y directions. The plotted red line represents X(t), and the blue line represents Y(t). X(t) and Y(t) were subjected to a moving average smooth (averaging kernel = 9). Note that the slopes of X(t) and Y(t) chart the velocity of the run represented by the rhythmic force tracing upon which X(t) and Y(t) are superimposed. Plotting Y(t) by X(t) gives the movement track (not shown here). Panel A: plot of 3 early-phase runs showing ground reaction forces, and corresponding spatial displacements of one WT mouse. The small ellipse is for the third run in Panel A. The enlarged ellipse gives more detail of the force changes during the run and shows the swing and stance phases for 9 half strides (4.5 strides). In the large ellipse, lower case “a” and “b” mark the minimum and maximum, respectively, of this run. Within-run force range (WRFR) is the absolute value of a-b. Panel B: Late-phase data for the same mouse whose early-phase data are in Panel A. In Panel B, note the higher stride rate, greater WRFR, and steeper slope (coded in red) of X(t) compared to Panel A. Panel C: Early-phase data for a D1CT-7 mouse. From left to right the mouse made 4 runs with very little pausing between runs. Note also that the mouse movements reflect one circuit around the actometer chamber in the counter-clockwise direction (-Y(t), +X(t), +Y(t), and –X(t)). The stride rate was faster and the WRFR was larger than for the early-phase WT mouse. Panel D: Late-phase data for the same D1CT-7 mouse whose early phase data are shown in the previous Panel C. The late phase for this D1CT-7 mouse was accompanied by a substantial quickening of locomotor rhythm and higher ground reaction forces of runs.
Data saved in the Excel file were subjected to further processing in SYSTAT in order to calculate stride length, stride rate, velocity, run distance, run duration, distance from the nearest wall, within run force range, and root mean square of run force. Then each of these parameters was averaged across runs for each mouse. Twenty runs were quantified for each mouse (the first 10 [“early”] and last 10 [“late”] trots of the session). The percent of session time required to amass 10 early runs and 10 late runs differed for each mouse. The group means for this variable are shown in Table 1. On the average, the data show that the WT mice produced scorable runs at a slower rate than the D1CT-7 mice. All runs in the 1h session conforming to the inclusion criteria were not scored because of effort costs (an average of ∼1 min per run; ∼11h to score 640 runs) and because an intent of the study was to contrast early and late phases of the exploratory behavior.
Table 1.
D1CT-7 mice show more frequent runs.
| Early phase | Late phase | ||
|---|---|---|---|
| WT | Not confined | 42.48+8.57 | 25.13+4.06 |
| Confined | 24.63+6.77 | 17.45+1.63 | |
| D1CT-7 | Not confined | 6.87+2.75 | 4.46+0.94*** |
| Confined | 5.46+1.81 | 10.95+1.77*** |
Data are mean+SEM of the % of session time used to produce 10 consecutive runs. This dependent variable established the quantitative definition of “Early” and “Late” phases of the recording session. A 3-way ANOVA indicated a significant genotype main effect [F(1,28)=42.53, p<.001] and substantiated the fact that D1CT-7 mice produced qualifying runs at a much higher rate than WT mice in both the early and late phases. Each of the four groups contained 8 mice.
p<0.001, significant genotype effect.
The following variables were calculated:
Stride length, obtained by dividing the distance (in mm) covered by a run by the number of strides in that run for each mouse;
Stride rate, calculated for each trot by dividing the number of half strides by 2 to obtain number of strides and then dividing the number of strides by the duration of the trot to yield stride rate expressed in Hz (Hz is Hertz which means cycles/s);
Velocity, defined as the distance covered by a run divided by the duration of that run and expressed as mm/s (equivalent to the product of stride length and stride rate);
Run distance, calculated as the mean of the within-run distances covered by each mouse;
Run duration, defined as the length of time that the run lasted as measured from the first swing phase to the last swing phase of the run;
Distance from the nearest wall, an index of thigmotactic behaviors as assessed during runs (larger distances designate smaller amounts of thigmotaxis);
Within-run force range (WRFR), which provides an estimate of the amplitude of the ground reaction forces produced during a run. In Fig. 1 see the right side of panel A and the detail in the larger ellipse; the lower case “a” labels the minimum force and lower case “b” designates the maximum force during the run shown. The range is |a-b|. The numbers interleaved with the force tracing in the large ellipse represent the count of 9 consecutive half strides, which is 4.5 strides by contemporary naming conventions;
Root mean square force (RMSF) mathematically takes account of amplitude of force oscillations for the whole run. The calculation for root mean square is the same as that used to obtain standard deviation. The root mean square nomenclature was used here to emphasize the fact that energy expenditure (work performed in a run) is an important attribute of locomotor behavior.
2.5 Statistics
The analysis of latencies to remove the sticky tape was performed by two-way ANOVA, with genotype (WT and D1CT-7) and experimental condition (spatial confinement vs no confinement) as factors.
For gait analysis, the primary statistical tool was analysis of variance (ANOVA). The experimental design dictated 2 genotypes – WT and D1CT-7; 2 pretreatment conditions – 20 min of pre-session spatial confinement versus no confinement, and 2 levels of a repeated-measures factor–“early” versus “late” in the recording session as described above. An initial 3-way ANOVA was performed on each of 8 dependent variables: 1) stride length, 2) stride frequency, 3) velocity, 4) run distance, 5) run duration, 6) distance from the nearest wall, 7) WRFR, and 8) RMSF during runs. For variables 1 through 6, no significant effects were found for spatial confinement (that is, no main effect was detected, and none of the three potential interactions was significant). Therefore, variables 1 through 6 were analyzed with 2-way ANOVAs (i.e., genotype × early vs late). Two 3-way ANOVAs were retained for the two force variables because significant stress treatment effects were detected. In some instances partial eta squared (η2p; Lakens, 2013) was computed to afford effect size comparisons, as follows: η2p = (SSeffect/(SSeffect+SSerror) × 100%; i.e., percent of variance accounted for by the experimental manipulation.
3. Results
3.1. Sticky tape removal
In the sticky-tape task, we found that D1CT-7 animals showed a significant increase in the latency to remove tape (Fig. 2) [F(1,30)=11.75, P<01]. No significant main effect was detected for spatial confinement [F(1,30)=0.27, not significant (NS)]. Likewise, no genotype × restriction interaction was found [F(1,30)=1.28, NS].
Figure 2.
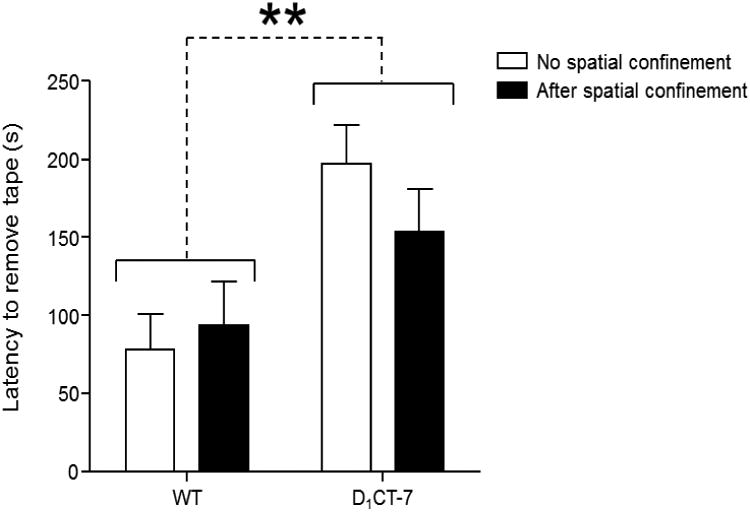
D1CT-7 mice show deficient sensorimotor integration in sticky-tape removal tests. Latencies to remove the sticky tape are represented as means ± SEM. White bars represent mice not exposed to spatial confinement. Black bars represent mice exposed to spatial confinement for 20 min prior to behavioral testing. **p<0.01. Further details for these data are in the text.
3.2.1. Stride Length
As shown in Fig. 3A and illustrated only indirectly in Fig. 1, stride length was significantly and substantially longer in WT than in D1CT-7 mice [F(1,30)=73.87, P<001]. Both types of mice exhibited a significant decline in stride length as a function of early versus late (E-L) [F(1,30)=14.08, P=.001]; the F test for interaction between mouse type and E-L was not significant. The effect size, η2p, was 31.9 % for the E-L effect.
Figure 3.
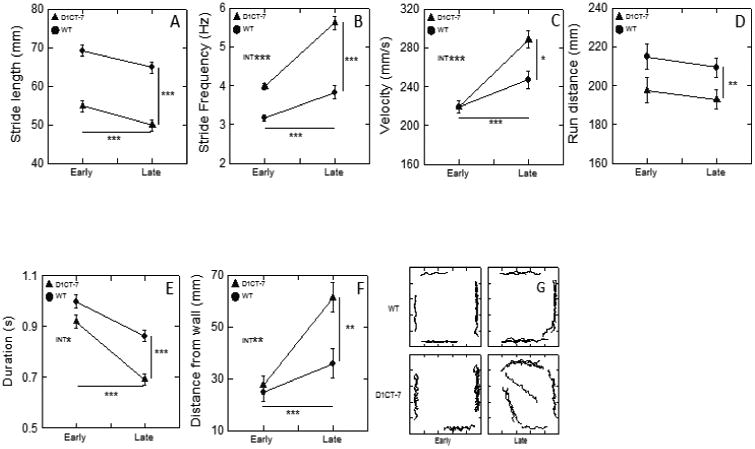
D1CT-7 mice exhibit altered gait and reduced thigmotaxis. These results are for run parameters that were responsive to genotype, and early-late phase effects, but not responsive to the mild stress treatment prior to the recording session. The lines without symbols and accompanying asterisks indicate main effects of 2-way ANOVAs (genotype 2 levels, phase 2 levels). The interaction of genotype and phase is indicated by “INT” written on the face of the graph. Error bars reflect standard error of the mean (SEM). In instances where error bars are not visible, the plot symbol is larger than +/- 1 SEM. *p<0.05, **p<0.01, ***p<0.001. In the ordinate of panel B, Hz means Hertz or cycles/s. Narratives for these data are in the text.
3.2.2 Stride Frequency
In Fig. 1 (A, B, C, and D) stride frequency can be appreciated by noting the time between consecutive half strides (see ellipses in Fig. 1; a half stride is seen as the force tracing for one swing-stance pair). The briefer the time between swing-stance pairs the higher the stride frequency. Statistical analysis of stride frequency (Fig. 3B) indicated that D1CT-7 mice evinced significantly faster limb movements than the WT mice [F(1,30)=88.21, P<.001]. The E-L effect was also highly significant [F(1,30)=98.64, P<.001], as was the interaction between type and the E-L factor [F(1,30)=17.83, P<.001]. E-L effect size, η2p, was 75.6 %, which was over twice the effect size observed for stride length. Stride frequency changed more than stride length as a mouse made behavioral adjustments in its locomotion during the recording session.
3.2.3. Average Velocity per run
As illustrated in Fig. 1 (all panels) by the slopes of the red (X[t]) and blue (Y[t]) tracings, run velocities were higher in the late phase than in the early phase of the recording session. More specifically, compare the ascending red line on the right side of Fig. 1A with the ascending red line shown in Fig. 1B. While D1CT-7 mice had higher velocities than WT mice [F(1,30)=5.10, P<.05, main effect], inspection of Fig. 3C along with a genotype-by-E-L interaction effect [F(1,30)=12.75, P<.001] indicates that the difference between the two mouse types was in the Late time frame, as can be seen in Fig. 1 (compare the slope of the red line in panel B with the slope of the red line earb with the of pane. The E-L effect was clearly evident [F(1,30)=67.11, P<.001]. Velocities early in the session were nearly identical in the face of strong genotype differences in stride length (Fig. 3A) and stride frequency (Fig. 3B). Velocities were higher in the Late phase in both types of mice, with the greater effect occurring for the D1CT-7 mice. This outcome contradicts the common expectation that locomotor behavior generally becomes less energetic as habituation proceeds in an open field setting.
3.2.4. Average run distance
D1CT-7 mice exhibited significantly shorter runs than the WT mice [F(1,30)=8.63, P<.01]. For this dependent variable both the E-L effect and the interaction effect were not statistically significant, an outcome consistent with the data shown in Fig. 3D. It is noteworthy that run distance was the single variable of the eight examined here that was not significantly affected by E-L. This suggests that both types of mice adjusted their locomotion “style” to maintain a nearly constant run distance.
3.2.5. Average duration per run
Overall, run durations (Fig. 3E) were significantly shorter in D1CT-7 mice compared to WT [F(1,30)=23.67, P<.001]. An example of a short-duration run is shown in Fig. 1D far left side. The E-L effect [F(1,30)=66.97, P<.001] was robust for both mouse types, with the mutants exhibiting mildly briefer durations of runs in the late phase [F(1,30)=4.22, P<.05, interaction effect; note non parallel lines in Fig. 3E ].
3.2.6. Distance from the nearest wall: thigmotaxis based only on run events
The D1CT-7 mice positioned their runs further from the walls than the WT mice [F(1,30)=7.11, P=.01, main effect], but there was also a significant interaction between genotype and the E-L factor [F(1,30)=7.46, P=.01], confirming the pattern of means shown in Fig. 3F. The E-L main effect was significant [F(1,30)=29.58, P<.001]. The two types of mice exhibited closely similar thigmotaxic tendencies early in the session, but both types showed substantially lessened thigmotaxis late in the session, with the D1CT-7 mice manifesting the greater change. Fig. 3G shows run trajectories for one WT mouse and one D1CT-7 mouse (both mice in the no-confinement treatment condition) and illustrates diminished thigmotaxis during the late phase of the recording. An example of diminished thigmotaxis for one D1CT-7 mouse can be seen in left half of Fig. 1D.
3.2.7. WRFR
Significantly higher ground reaction forces during runs were observed for the D1CT-7 mice than for the WT mice [F(1,28)=56.75, P<.001; main effect of type] (see Fig. 4A). As shown in Fig. 1, the differences in force production between D1CT-7 mice and WT mice are clearly evident at the individual mouse level of analysis. The effect on force range of the spatial confinement pretreatment was also statistically significant [F(1,28)=15.33, P<.001; main effect of treatment]. The force-amplifying effect induced by spatial confinement can be appreciated by noting in Fig. 4A that all four filled plot symbols have higher forces than the corresponding four means represented by unfilled plot symbols. Force range was markedly higher late in the session compared to early in the session [F(1,28)=65.78, P<.001; main effect of E-L]. In addition, there was also a significant interaction between genotype of mouse and the E-L factor [F(1,28)=22.38, P<.001, interaction effect]. The D1CT-7 mice exhibited substantially greater increases in ground reaction force occasioned by within-session changes than the WT mice (also see Fig. 1).
Figure 4.
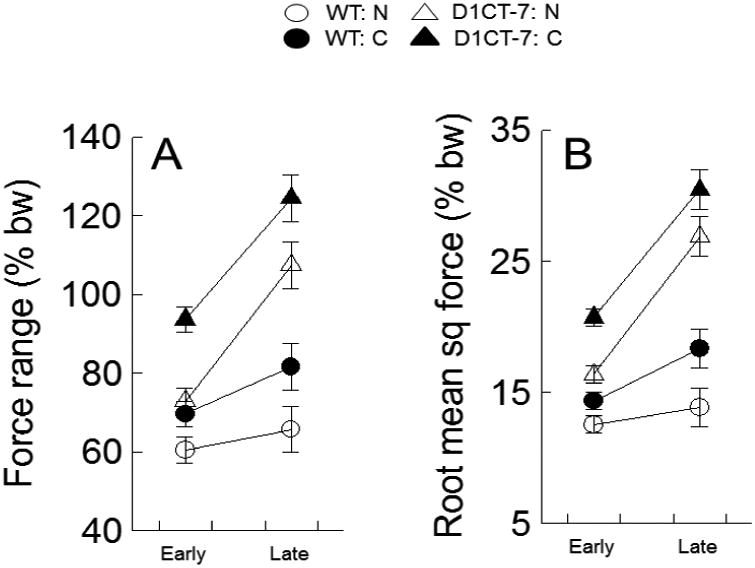
D1CT-7 mice produce late runs with increased peak force and energy output. These group means and SEMs are for the two dependent variables derived from the ground reaction forces recorded for each run. Panel A: within run force range, a measure of peak force amplitude in a run; Panel B: root mean square force, a measure of energy output of a run. Note that force data were normalized to percent of body weight. Error bars represent +/- 1 SEM. See text for the corresponding 3-way ANOVA statistics. Abbreviations: WT: N indicates wild type not confined; WT: C means wild type confined; D1CT-7: N means D1CT-7 not confined; D1CT-7: C indicates D1CT-7 confined; bw means body weight; in the y-axis label for panel B, sq indicates square.
3.2.8. RMSF variations during runs
This variable includes all the force samples in each run, not just 2 values as is the case for the range statistic. The analysis of RMSF (See Fig. 4B) yielded results remarkably similar to those observed for WRFR: [F(1,28)=86.45, P<.001, main effect of genotype], [F(1,28)=14.00, P<.001, main effect of spatial confinement treatment], [F(1,28)=93.13, P<.001, main effect of E-L], and [F(1,28)=31.88, P<.001, genotype by E-L interaction]. Moreover, the effect sizes were generally larger for RMSF than for WRFR (see Table 2). Hence, the RMSF measure differentiated the experimental groups more robustly than was seen for WRFR, although the effect sizes based on WRFR were also quite large by contemporary standards.
Table 2.
Effect size of D1CT-7 genotype, confinement, and habituation for run force and energy output.
| Within run force range | Root mean square force | |
|---|---|---|
| Genotype | 66.9 | 75.5 |
| Pretreatment | 35.4 | 33.3 |
| Early-late | 70.1 | 76.9 |
| Genoytype X Early-late | 44.0 | 53.2 |
Data are % of variance accounted for by the indicated independent variables. The results show that the run force derived measures were robustly affected by D1CT-7 genetic status, mild pre-session spatial confinement and habituation (passage of time in the chamber). For further details, see text.
4. Discussion
The results of this study show that, in line with our hypotheses, D1CT-7 mice exhibited impairments of sensorimotor integration, as assessed by a significant increase in the latency to remove a sticky tape, as well as of gait functions. Compared to WT mice, D1CT-7 littermates exhibited shortened stride length, increased stride rate, nearly equal early-phase velocity, and higher late-phase velocity. The D1CT-7 mice also ran with greater force amplitude than WT mice; of note, qualitative comparisons of individual tracks pointed to a generalized increase in the forcefulness of vertical locomotor behaviors in D1CT-7 even in the absence of lateral locomotion (see, for example, Figs. 1B and 1D), which could likewise be due to greater force amplitude of vertical movements, as well as to micro-tics or related phenomena.
The differences mice in gait parameters between D1CT-7 and WT mice were large, as shown by the effect size indicator (partial eta squared) and by the ease of detecting phenotypic markers at the individual subject level of analysis (see Fig. 1). Furthermore, the late-phase run durations (Fig. 3E) of the D1CT-7 mice were briefer (0.69 +/- 0.02 s) than those of the WT mice (0.860 +/- 0.02 s). Our previous analysis of the locomotor activity in the same mice (Godar et al., 2016; Fig. 4B) revealed that D1CT-7 animals displayed hyperactivity, as measured by distance traveled, with an early-phase distance about twice as big as that of WT counterparts, and relatively flat habituation curves for both genotypes. These data are consistent with previous reports on the lack of locomotor habituation in Balb/Cj mice (Thomsen and Caine, 2011). Taken together, these findings indicate that D1CT-7 mice display a hyper-vigorous movement syndrome with more ballistic high-force and high-frequency limb movements during locomotion in a shorter period of time than the WT mice, with the differences between the two genotypes being greater in the late phase of the 1h session. Although we cannot currently prove a causal nexus between the deficits in sensorimotor integration and these locomotor manifestations, previous findings have extensively shown that sensorimotor feedback is a necessary pre-requisite for motor coordination and adaptation (Shadmehr et al., 2010; Flanders, 2011). Future studies will be needed to evaluate whether the increased stride rate and shortened stride length in D1CT-7 may arise as a phenomenological by-product of altered connectivity between the motor and sensory cortices in these mutants.
The early runs of both WT and D1CT-7 mice were located near the walls of the chamber; although this thigmotactic tendency lessened significantly by the time the late-phase ended, this decline was more pronounced in D1CT-7 mice, resulting in a significant reduction of late-phase thigmotaxis in comparison with WT littermates. This result is in apparent contrast with an earlier report (McGrath et al.,1999), which indicated higher thigmotaxis in D1CT-7 mice in a 40 × 40 cm open field. The potential source of this discrepancy may lie in the different test duration (1 h in the present study vs 15-min in the referenced study). Another key difference between the cited study and ours is that in our analysis the thigmotaxis was based on runs (a topographically distinct class of locomotor behaviors) only, but in the cited work thigmotaxis was based on time spent (includes all behavior regardless of topography) in a peripheral zone of the chamber. Assuming that thigmotaxis may be influenced by the aversion/anxiety for the center of the arena, our data suggest that the hyper-vigorous locomotor patterns of D1CT-7 mice may also signify an aspect of behavioral disinhibition and motor impulsivity. Additional studies are warranted to verify whether this interpretation is supported by alternative behavioral tasks.
We recently documented that D1CT-7 mice subjected to a brief session of spatial confinement in a 10-cm cylinder exhibited a net increase in the frequency of tic-like behaviors and deficits in sensorimotor gating (Godar et al., 2016). In contrast to these findings, the same stressor did not modify either the deficits in sensorimotor integration or most topographical measures of gait (stride length, stride frequency, velocity, run distance, and run duration), indicating that the neurophysiological roots of these variables is at least partially distinct from those of tic-like and gating responses. To the best of our knowledge, no studies have tested whether gait and sensorimotor deficits in TS patients are exacerbated by psychological stress; future research on this critical issue are warranted to ascertain the translational value of our findings. Along a similar line of thought, it should be noted that, although our study is limited by the lack of pharmacological tests with benchmark therapies for TS, such as haloperidol and pimozide, no clinical information is currently available on the effects of these treatments on gait and sensorimotor impairments in TS patients.
The only significant effect of spatial confinement was a higher force output in both genotypes; additionally, D1CT-7 mice had also a higher force output than WT littermates, irrespective of spatial confinement. In line with these results, we previously showed that D1CT-7 mice exhibited higher baseline levels of corticosterone and that confinement stress enhanced the levels of this stress hormone in both genotypes (Godar et al., 2016). Glucocorticoids are known to increase maximal voluntary force production in humans (e.g., Baudry et al., 2014), and enhance spinal motoneuron excitability (Riker et al., 1975; Hall, 1980). However, it is important to reiterate that, in contrast with our previously reported modifications in tic-like behaviors, the effect of mild stress on force measures was only additive (i.e, did not interact) with genotype.
Unlike the stress treatment, in the analysis of forces, the early-late repeated measures factor did interact significantly with genotype. In Fig. 4, the early-late slopes are steeper for D1CT-7 mice (triangles) than for the WT mice (circles) for the ground reaction forces. This result indicates that the D1CT-7 force measures were disproportionately amplified in their late-phase reaction to cumulative experience in the open field. In future studies it may be useful to have corticosterone and/or dopamine measurements during and after the open field test because such an analysis may shed light on the neurochemical mechanisms of the profound differences between early and late phase behavioral measures reported here (e.g., Piazza et al., 1996). It is possible that long sessions (e.g., 1h or more) of high energy locomotor output would be sufficiently stressful to promote elevated corticosterone that, through positive feedback, would sustain or increase the force of locomotor runs as the session continued.
Although speculative, the dramatic differences between WT and D1CT-7 in their ground reaction forces may be related to the hyperactivity of sensorimotor cortex in D1CT-7 mice. This characteristic appears to be phenomenologically similar to the recently-reported reduction in GABAergic inhibitory tone in the somatosensory cortex of TS patients (Puts et al., 2015). The link between somatosensory cortex hyperactivation and gait deficits is supported by at least two lines of evidence: first, the neurons in this area exert a strong inhibitory influence on motor cortex neurons (Rocco-Donovan et al., 2011); second, their firing patterns are synchronized with swing and stance phases of the locomotor stride cycle (Favorov et al., 2015).
Overall, our results unequivocally confirm the hyperactivity phenotype of the D1CT-7 mouse strain and demonstrate profound gait alterations compared to WT mice: stride length is shortened and stride rate is quickened, while ground reaction forces are markedly elevated in D1CT-7 mice relative to controls. As mentioned above, although gait alterations and sensorimotor integration deficits have been described in TS, current knowledge on the phenomenology of these impairments remains rudimentary; thus, any interpretation on the potential translational value of the current funding should be drawn with extreme caution. Nevertheless, these analyses highlight the importance of testing potential deficits of these domains as potential phenotypic correlates of cortical connectivity deficits in animal models of TS, and warrant future studies in other animal models of tic disorders.
Highlights.
Tourette syndrome (TS) patients exhibit abnormal sensorimotor integration and gait
Gait and sensorimotor functions were assessed in the D1CT-7 mouse model of TS
Gait analyses were performed with a force-plate actometer
D1CT-7 mice displayed marked abnormalities in sensorimotor integration and gait
Details are provided on the assessment of multiple gait parameters, including force
Acknowledgments
The present manuscript was partially supported by the National Institute of Health (NIH) grants R01 MH104603 (to MB) and F31 NS093939 (to LJM), a Tourette Association of America Research grant (to MB), and a subaward from the NIH grant P20 GM103638 (to MB). We are grateful to Hunter Strathman for his valuable support in the execution of the experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbruzzese G, Berardelli A. Sensorimotor integration in movement disorders. Mov Disord. 2003;18:231–40. doi: 10.1002/mds.10327. [DOI] [PubMed] [Google Scholar]
- Albin RL, Mink JW. Recent advances in Tourette syndrome research. Trends in neurosciences. 2006;29:175–182. doi: 10.1016/j.tins.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Baudry S, Lanfranco F, Merletti R, Duchateau J, Minetto MA. Effects of short-term dexamethasone administration on corticospinal excitability. Med Sci Sports Exerc. 2014;46:695–701. doi: 10.1249/MSS.0000000000000162. [DOI] [PubMed] [Google Scholar]
- Campbell KM, de Lecea L, Severynse DM, Caron MG, McGrath MJ, Sparber SB, Sun LY, Burton FH. OCD-Like behaviors caused by a neuropotentiating transgene targeted to cortical and limbic D1+ neurons. J Neurosci. 1999;19:5044–53. doi: 10.1523/JNEUROSCI.19-12-05044.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SC, Leckman JF, Bloch MH. Clinical assessment of Tourette syndrome and tic disorders. Neurosci Biobehav Rev. 2013;37:997–1007. doi: 10.1016/j.neubiorev.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eapen V, Fox-Hiley P, Banarjee S, Robertson M. Clinical features and associated psychopathology in a Tourette syndrome cohort. Acta Neurol Scand. 2004;109:255–60. doi: 10.1046/j.1600-0404.2003.00228.x. [DOI] [PubMed] [Google Scholar]
- Favorov OV, Nilaweera WU, Miasnikov AA, Beloozerova IN. Activity of somatosensory-responsive neurons in high subdivisions of SI cortex during locomotion. J Neurosci. 2015;35:7763–76. doi: 10.1523/JNEUROSCI.3545-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanders M. What is the biological basis of sensorimotor integration? Biol Cybern. 2011;104:1–8. doi: 10.1007/s00422-011-0419-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler SC, Birkestrand BR, Chen R, Moss SJ, Vorontsova E, Wang G, Zarcone TJ. A force-plate actometer for quantitating rodent behaviors: illustrative data on locomotion, rotation, spatial patterning, stereotypies, and tremor. J Neurosci Methods. 2001;107:107–24. doi: 10.1016/s0165-0270(01)00359-4. [DOI] [PubMed] [Google Scholar]
- Fowler SC, Muma NA. Use of a force-sensing automated open field apparatus in a longitudinal study of multiple behavioral deficits in CAG140 Huntington's disease model mice. Behav Brain Res. 2015;294:7–16. doi: 10.1016/j.bbr.2015.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost R, Skidmore J, Santello M, Artemiadis P. Sensorimotor control of gait: a novel approach for the study of the interplay of visual and proprioceptive feedback. Front Hum Neurosci. 2015;9:14. doi: 10.3389/fnhum.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godar SC, Bortolato M. What makes you tic? In press. [Google Scholar]
- Godar SC, Mosher LJ, Di Giovanni G, Bortolato M. Animal models of tic disorders: a translational perspective. J Neurosci Methods. 2014;238:54–69. doi: 10.1016/j.jneumeth.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godar SC, Mosher LJ, Strathman HJ, Gochi AM, Jones CM, Fowler SC, Bortolato M. The D1CT-7 mouse model of Tourette syndrome displays sensorimotor gating deficits in response to spatial confinement. Br J Pharmacol. 2016;173:2111–21. doi: 10.1111/bph.13243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ED. Glucocorticoid enhancement of serotonergic facilitation of cat spinal monosynaptic motor neuron excitation. Exp Neurol. 1980;68:589–94. doi: 10.1016/0014-4886(80)90112-0. [DOI] [PubMed] [Google Scholar]
- Houghton DC, Capriotti MR, Conelea CA, Woods DW. Sensory phenomena in Tourette Syndrome: their role in symptom formation and treatment. Curr Dev Disord Rep. 2014;1:245–51. doi: 10.1007/s40474-014-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemay M, Termoz N, Lesperance P, Chouinard S, Rouleau GA, Richer F. Postural control anomalies in children with Tourette syndrome. Exp Brain Res. 2007;179:525–30. doi: 10.1007/s00221-007-0882-7. [DOI] [PubMed] [Google Scholar]
- Lepicard EM, Venault P, Abourachid A, Pellé E, Chapouthier G, Gasc JP. Spatiotemporal analysis of locomotion in BALB/cByJ and C57BL/6J mice in different environmental conditions. Behav Brain Res. 2006;167:365–72. doi: 10.1016/j.bbr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Liu WY, Lin PH, Lien HY, Wang HS, Wong AM, Tang SF. Spatio-temporal gait characteristics in children with Tourette syndrome: a preliminary study. Res Dev Disabil. 2014;35:2008–14. doi: 10.1016/j.ridd.2014.04.025. [DOI] [PubMed] [Google Scholar]
- McGrath MJ, Campbell KM, Veldman MB, Burton FH. Anxiety in a transgenic mouse model of cortical-limbic neuro-potentiated compulsive behavior. Behav Pharmacol. 1999;10:435–43. doi: 10.1097/00008877-199909000-00001. [DOI] [PubMed] [Google Scholar]
- Nordstrom EJ, Bittner KC, McGrath MJ, Parks CR, 3rd, Burton FH. “Hyperglutamatergic cortico-striato-thalamo-cortical circuit” breaker drugs alleviate tics in a transgenic circuit model of Tourette's syndrome. Brain Res. 2015;1629:38–53. doi: 10.1016/j.brainres.2015.09.032. [DOI] [PubMed] [Google Scholar]
- Nordstrom EJ, Burton FH. A transgenic model of comborbid Tourette's syndrome and obsessive-compulsive disorder circuitry. Mol Psychiatry. 2002;7:617–25. doi: 10.1038/sj.mp.4001144. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Rothwell J, Topka H, Robertson MM, Orth M. Grip force behavior in Gilles de la Tourette syndrome. Mov Disord. 2005;20:217–23. doi: 10.1002/mds.20309. [DOI] [PubMed] [Google Scholar]
- O'Connor K, Brisebois H, Brault M, Robillard S, Loiselle J. Behavioral activity associated with onset in chronic tic and habit disorder. Behav Res Ther. 2003;41:241–9. doi: 10.1016/s0005-7967(02)00051-7. [DOI] [PubMed] [Google Scholar]
- Patel N, Jimenez-Shahed J. Simultaneous improvement of tics and parkinsonism after pallidal DBS. Parkinsonism Relat Disord. 2014;20:1022–3. doi: 10.1016/j.parkreldis.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Peterka RJ, Loughlin PJ. Dynamic regulation of sensorimotor integration in human postural control. J Neurophysiol. 91:410–23. doi: 10.1152/jn.00516.2003. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Rougé-Pont F, Deroche V, Maccari S, Simon H, Le Moal M. Glucocorticoids have state-dependent stimulant effects on the mesencephalic dopaminergic transmission. Proc Natl Acad Sci U S A. 1996;93:8716–20. doi: 10.1073/pnas.93.16.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts NA, Harris AD, Crocetti D, Nettles C, Singer HS, Tommerdahl M, Edden RA, Mostofsky SH. Reduced GABAergic inhibition and abnormal sensory symptoms in children with Tourette syndrome. J Neurophysiol. 2015;114:808–17. doi: 10.1152/jn.00060.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riker WF, Jr, Baker T, Okamoto M. Glucocorticoids and mammalian motor nerve excitability. Arch Neurol. 1975;32:688–94. doi: 10.1001/archneur.1975.00490520058009. [DOI] [PubMed] [Google Scholar]
- Robertson MM, Banerjee S, Eapen V, Fox-Hiley P. Obsessive compulsive behaviour and depressive symptoms in young people with Tourette syndrome. A controlled study. Eur Child Adolesc Psychiatry. 2002;11:261–265. doi: 10.1007/s00787-002-0301-3. [DOI] [PubMed] [Google Scholar]
- Rocco-Donovan M, Ramos RL, Giraldo S, Brumberg JC. Characteristics of synaptic connections between rodent primary somatosensory and motor cortices. Somatosens Mot Res. 2011;28:63–72. doi: 10.3109/08990220.2011.606660. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Orban de Xivry JJ, Xu-Wilson M, Shih TY. Temporal discounting of reward and the cost of time in motor control. J Neurosci. 2010;30:10507–16. doi: 10.1523/JNEUROSCI.1343-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Karban B, Ploum Y, Sharp R, Geyer MA, Eastvold A. Tactile prepuff inhibition of startle in children with Tourette's syndrome: in search of an “fMRI friendly” startle paradigm. Biol Psychiatry. 2001;50:578–85. doi: 10.1016/s0006-3223(01)01164-7. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Psychomotor stimulant effects of cocaine in rats and 15 mouse strains. Exp Clin Psychopharmacol. 2011;19:321–41. doi: 10.1037/a0024798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Maia TV, Marsh R, Colibazzi T, Gerber A, Peterson BS. The neural circuits that generate tics in Tourette's syndrome. Am J Psychiatry. 2011;168:1326–37. doi: 10.1176/appi.ajp.2011.09111692. [DOI] [PMC free article] [PubMed] [Google Scholar]


