Abstract
It has been argued that clinical depression is accompanied by reductions in cortical excitability of the left prefrontal cortex (PFC). In support of this, repetitive transcranial magnetic stimulation (rTMS), which is a method of enhancing cortical excitability, has shown antidepressant efficacy when applied over the left PFC, although the overall therapeutic effects remain inconclusive. The cerebral pathophysiology of depression is, however, not limited to dysfunctions in the PFC, thus, targeting alternative brain regions with rTMS may provide new therapeutic windows in the treatment of depression. Evidence from electroencephalography and lesion studies suggests that not only is the left PFC involved in depression but also the parietal cortex and cerebellum. Furthermore, rTMS over the parietal cortex and the cerebellum has been found to improve mood and emotional functioning, at least in healthy volunteers. We have integrated these findings in an rTMS-oriented theoretical framework for the neurobiology of low mood and depression. To establish the possible therapeutic efficacy of this model, whereby, for example, the application of slow rTMS over the right parietal cortex and fast rTMS over the cerebellum may be beneficial in different subtypes of depression, clinical rTMS studies that target the parietal cortex and cerebellum are warranted.
Medical subject headings: cerebellum, depression, mood, parietal cortex, prefrontal cortex, transcranial magnetic stimulation
Abstract
On a soutenu que des réductions de l'excitabilité corticale du cortex préfrontal gauche (CPF) accompagnent la dépression clinique. À l'appui de cette affirmation, on a montré l'effet antidépresseur efficace de la magnétostimulation transcrânienne répétitive (MSTr), moyen d'améliorer l'excitabilité corticale, appliquée au CPF gauche, même si les effets thérapeutiques globaux demeurent non concluants. La pathophysiologie cérébrale de la dépression n'est toutefois pas limitée aux dysfonctions du CPF et c'est pourquoi l'application de la MSTr à d'autres régions du cerveau pourrait offrir de nouveaux moyens de traiter la dépression. Les données électroencéphalographiques et tirées d'études de lésions indiquent que non seulement le CPF gauche intervient dans la dépression, mais aussi le cortex pariétal et le cervelet. De plus, la MSTr appliquée au cortex pariétal et au cervelet améliore la thymie et l'adaptation affective, du moins chez des volontaires en bonne santé. Nous avons intégré ces constatations dans un cadre théorique axé sur la MSTr pour la neurobiologie de l'hypothymie et de la dépression. Pour déterminer l'efficacité thérapeutique possible du modèle lorsque, par exemple, l'application d'une MSTr lente au cortex pariétal droit et d'une MSTr rapide au cervelet peut se révéler bénéfique dans différents sous-types de dépression, des études cliniques sur la MSTr appliquée au cortex pariétal et au cervelet sont justifiées.
Introduction
Epidemiologic studies report that clinical depression has an annual prevalence varying from 1% to 6% in community samples worldwide.1 Estimates of the prevalence for 1999 in the general population have shown a sharp increase in morbidity (2.1%–7.6%), making depression one of the most common mental disorders in industrialized countries.2 Most treatment protocols involve the administration of pharmacologic agents, ranging from tricyclic antidepressants to selective serotonin reuptake inhibitors with or without psychotherapeutic support. Electroconvulsive therapy (ECT), on the other hand, is usually reserved only for those patients whose condition is refractory to any kind of treatment. Although its antidepressant efficacy is quite high, reaching a remission rate of 64%–84% in patients diagnosed with nonpsychotic depression treated with bitemporal ECT, the application is invasive and is accompanied by transient physical and sometimes permanent cognitive side effects.
Repetitive transcranial magnetic stimulation (rTMS), a noninvasive technique based on focal electromagnetic induction, was introduced in 1985. Hofflich et al3 were the first to report beneficial effects for this procedure after applying rTMS over the left prefrontal cortex (PFC) in 2 patients with refractory depression. Currently, the most widely used rTMS treatment protocol involves the modulation of neural excitability of the left PFC. Although recent reviews4,5 indicate that PFC rTMS does indeed possess antidepressant efficacy, the available data and results remain heterogeneous and inconclusive.
There is also evidence for a role for the parietal cortex and the cerebellum in the complex neurocircuitry underlying emotion and mood regulation, which is dysfunctional in depression.6,7 The aims of the current article are to discuss rTMS studies that have targeted the PFC in the treatment of depression and to consider recent research in the field of affective neuroscience investigating the antidepressant efficacy of rTMS when applied over the right parietal cortex and the medial cerebellum. Moreover, these findings are discussed within an rTMS-oriented framework for emotional processing in depression.
Transcranial magnetic stimulation
The underlying working principles of TMS are based on 2 physical laws that were originally formulated by Ampère and by Faraday. The first law refers to the generation of a magnetic field, using an electric current, and the second law describes the generation of an electric current through an alternating magnetic field.5 Essentially, TMS involves applying a brief magnetic pulse or train of pulses, the latter called repetitive TMS (rTMS), to the scalp using a coil of wire.8 When the magnetic field of the magnetic pulse alternates rapidly enough, a secondary electric current is induced that alters the local electric field near conductive nerve tissue. This secondary current, however, needs to create a strong transmembrane potential for neurons to depolarize and generate an action potential. The effects of rTMS depend on the stimulation parameters, which include frequency and intensity. By convention, slow rTMS (≤ 1 Hz) is mostly applied to reduce neural excitability,9 whereas fast rTMS (≥ 10 Hz) is applied to enhance neural excitability.10 Speer et al11 demonstrated these opposite effects of slow and fast rTMS over the dorsolateral PFC by measuring decreases and increases in regional cerebral blood flow, respectively. The second parameter, stimulation intensity, is usually calculated as a percentage relative to the motor threshold (MT). By applying TMS over the motor cortex, muscle contractions, usually of the abductor pollicus brevis (APB), can be evoked. The MT is the lowest stimulation intensity needed to induce APB twitches in 5 of 10 consecutive trials.12 Stimulation intensities exceeding the MT are referred to as suprathreshold rTMS, whereas the term subthreshold rTMS designates stimulation intensities below MT. The average maximum field strength of TMS is about 1.5–2.5 T, but this strength decays exponentially with distance. As a result, only superficial brain tissue will be affected directly. It has also been argued that the effects of subthreshold rTMS are more local, whereas suprathreshold rTMS results in more widespread and distant transsynaptic effects.13 TMS is capable of modulating networks that are functionally connected. This was demonstrated in neuroimaging studies by Fox et al14 and Paus et al,15 which show distant rTMS effects in such neural networks. rTMS may be used not only to investigate the pathophysiology underlying mood disorders but also to “normalize” disrupted activity in the cerebral cortex.
Prefrontal rTMS and depression
rTMS research on depression started with the premise of a dysfunctional PFC in this condition.16,17 Two related concepts have been postulated concerning the relation between prefrontal activity and depression. It is argued by most researchers and clinicians that depression involves a hypoactive left PFC, but some suggest that a right rather than left PFC dominance in activity is the culprit. A recent meta-analysis by Kozel and George18 tested the antidepressant efficacy of fast rTMS over the left PFC. They reported statistically significant effect sizes, as well as measurable clinical improvement. The antidepressant efficacy of slow rTMS over the right PFC has also been investigated. Klein et al19 demonstrated that slow rTMS over the right PFC was more effective than sham stimulation, providing evidence for the idea of a distorted homeostasis between right and left PFC activity in depression. Because rTMS was performed on patients who were receiving or starting a medication protocol, this clinical trial was a “potentiation” study. Recently, Burt et al5 conducted a meta-analysis for both left and right PFC rTMS across 3 categories of studies in clinically depressed patients. The categories included (1) open and noncontrolled trials, (2) controlled designs and (3) comparisons with ECT. Reductions in the Hamilton Rating Scale for Depression (HAM-D)20 were taken to be the dependent variables of interest. Both open and controlled trials showed an antidepressant response initially, but the overall clinical significance was small for both left and right PFC rTMS.
Studies that compared rTMS with ECT indicated that the duration of stimulation might be an important factor for achieving such clinical significance. In another meta-analysis, McNamara et al21 also reported beneficial effects of rTMS over the PFC, but the overall clinical significance of rTMS treatment in depression is, according to those authors, rather unconvincing. rTMS research on depression is, however, still in its infancy and the extremely large stimulation parameter range is just beginning to be explored. Burt et al,5 for instance, suggested that the extension of the rTMS treatment sessions beyond the traditional 1–2 weeks might result in more pronounced antidepressant effects. Several other reasons could underlie the ambivalence of current findings, and it is likely that an effective treatment protocol has not yet been developed. Parameter settings such as stimulation frequency might also play an important role in establishing more clinically relevant outcomes. For instance, fast rTMS uses frequencies between 10 and 20 Hz, whereas for slow rTMS frequencies of around 1 Hz are used. The total amount of pulses applied is, however, of crucial importance: upgrading stimulation frequencies to 2 Hz for slow and 25 Hz for fast rTMS should not only result in more pronounced effects but may also reduce interindividual variability.22
Thus, intensification of stimulation parameters could be a first step,23 but attempts to target other brain regions involved in the pathophysiology of depression with rTMS to explore its therapeutic efficacy might prove to be even more important.
Introducing alternative brain regions into clinical rTMS studies
As noted here, the antidepressant efficacy of PFC rTMS is still inconclusive, and alternative treatment tools, such as nervus vagus stimulation (VNS) and deep brain stimulation (DBS), are currently being explored in preclinical and clinical trials.24 However, apart from the largely unexplored parameter range, rTMS studies have not yet investigated other brain regions involved in the pathophysiology of depression. We would like to propose alternative brain areas as targets for rTMS treatment in depression. Recent research suggests improvements in mood and emotional functioning with the application of rTMS over the medial cerebellum25 and the right parietal cortex.26 We will discuss further the plausibility of a neurobiological framework of low mood and depression involving the cerebellum and right parietal cortex.
The parietal cortex: evidence for a left prefrontal–right parietal depression circuit
There is evidence from lesion and neuroimaging studies for the involvement of the parietal cortex in depression.7,27 In particular, a hypoactive right parietal cortex has been associated with depression. However, depending on whether depression is comorbid with anxiety, right parietal hyperactivity has also been observed.28 This can be explained by the fact that the right parietal cortex is involved in arousal:6 hypoarousal is linked to depression, whereas hyperarousal is associated with anxiety. Problematically, a highly complex picture emerges when depression is comorbid with anxiety, which is often the case.29
A well-known biochemical marker for depression is cortisol. Presumably because of a hyperactive hypothalamic–pituitary–adrenal (HPA) axis, depressive as well anxious subjects often demonstrate basal levels of this stress-related hormone that are higher than normal.30 Moreover, Belanoff et al31 recently demonstrated that the cortisol-receptor antagonist mifepristone was effective in the treatment of psychotic depression.
Furthermore, Schutter et al32 found that higher basal levels of cortisol are associated with reductions in functional connectivity between the left prefrontal and the right parietal cortex. This functional connectivity between different cortical brain regions can be measured using electroencephalography. Electroencephalographic coherence analysis33 may provide valuable insights into the neurobiological mechanisms of depression. Cerebral atrophy, for instance, which has been reported in depression,34 is accompanied by a breakdown in functional corticocortical connectivity.35
Recently, an rTMS experiment was conducted to investigate the involvement of the right parietal cortex in depression. In healthy human subjects, the application of 2-Hz rTMS over the right parietal cortex for 20 minutes continuously resulted in statistically significant decreases in self- reported, attentional and psychophysiologic indices of depressive mood compared with placebo.26
The fact that reductions in several indices of depressive mood have been found in healthy control subjects after a single session of rTMS suggests a possible antidepressant efficacy, although it must be noted that rTMS-induced effects on mood might differ between healthy controls and clinically depressed subjects. These findings, however, are consistent with those of a study by Schutter et al32 that showed that high levels of cortisol are related to reductions in functional connectivity between the left prefrontal and the right parietal cortex. Moreover, there are indications that the antidepressant properties of the steroid hormone testosterone36 are associated with increases in functional connectivity in this left prefrontal–right parietal circuit.37 We think that these data, when taken together with the observation made by Jing and Takigawa38 that functional connectivity between the left prefrontal and right parietal brain regions is enhanced by left PFC rTMS, support the need for clinical rTMS trials in depression targeting the right parietal cortex.
In summary, recent research from multiple disciplines suggests that the functional connectivity between the left prefrontal and right parietal cortex plays a role in depressive mood. Moreover, the antidepressant effects of rTMS studies over the left PFC and recently observed improvements in mood and emotional functioning after rTMS over the right parietal cortex might both involve increased functional connectivity between these cortical brain areas.39,40
The cerebellum
For decades, the cerebellum has been thought to be predominantly involved in motor performance and cognitive operations. Recently, however, a growing body of evidence indicates that the cerebellum is also involved in emotion. The first evidence for cerebellar involvement in emotion came from the work of Robert G. Heath during the early fifties. Although his initial work predominantly involved the electrical stimulation of the septum, he then began research on stimulation of the cerebellum, thinking that it might provide a better entry to the emotional circuitry of the brain. Several cerebellar pacemaker studies by Heath41 and by Heath et al42 did indeed demonstrate positive effects on mood and personality in patients with psychiatric illness after electrical stimulation of the cerebellum. Moreover, Schmahmann and Sherman43 provided clinical support for the role of the cerebellum and particularly the vermis in the regulation of emotion and mood. Given its modulatory role on emotion, the midline cerebellar vermis together with the fastigial nucleus and the flocculonodular lobe have been designated the limbic cerebellum.44 Furthermore, additional evidence for the involvement of the cerebellum in mood disorders, such as depression, was provided by structural magnetic resonance imaging studies. Unipolar depression is not only associated with volumetric reductions of the frontal lobes but also of the cerebellum.45 Leroi et al46 recently found further evidence for this cerebellum–depression relation. In a study of patients with degenerative cerebellar diseases, comprehensive psychiatric evaluations revealed that depressive disorders were associated with cerebellar degeneration. Starkstein et al47 found evidence for a relation between cerebrovascular lesions in the posterior brain structures, including the cerebellum, and depression. Beyer and Krishnan48 recently concluded after reviewing the literature that depression is associated with abnormalities in the frontal lobes, the basal ganglia and the cerebellum. Given the structural deviations of the cerebellum, functional abnormalities are likely to be present also. However, reports of functional cerebellar deviances are lacking, most probably because of the practice of using the cerebellum as a reference for cerebral perfusion and activation patterns.44 Interestingly, however, studies by Schmahmann and Pandya49 and by Middleton and Strick50 demonstrated that the cerebellum and the PFC are anatomically linked in a bidirectional fashion. The first loop consists of the thalamus, which receives efferent input from the cerebellum and projects to the PFC. The circuit is closed via prefrontal projections back to the cerebellum via the pontine nucleus of the brain stem.49,50 Recently, Schutter et al25 investigated the existence of the assumed projection from the medial cerebellum to the PFC in healthy human subjects using fast rTMS and electroencephalography. rTMS targeting the medial part of the cerebellum indeed modulated ongoing electrical activity in the PFC. Interestingly, in the latter study, elevations in mood and alertness were reported spontaneously after medial cerebellar stimulation exclusively. Animal studies have provided support for cerebellar modulation via the mesencephalic reticular formation of EEG patterns and levels of alertness.51 The findings of Schutter et al25 not only provide the first evidence for a cerebelloprefrontal link in humans, but the observed increases in mood are also a further indication for a role of the cerebellum in regulation of affect. Because the cerebellum has efferent pathways to the substantia nigra, and depression has been linked to deficiencies in the biogenic monoamines, cerebellar rTMS in the study by Schutter et al25 might have stimulated dopamine release, resulting in the observed changes in PFC activity and the elevations in alertness and mood. In support of this, Keck et al52 applied 20-Hz rTMS in rats over the left frontal cortex and observed increases in the release of dopamine in both the mesolimbic and the mesostriatal system. Because there is evidence for dopaminergic effects of cerebellar stimulation,53 it is likely that the elevations in alertness and mood after cerebellar rTMS in the study by Schutter et al25 may be explained in terms of dopaminergic stimulation. In sum, the evidence suggests a role for the cerebellum in clinical depression, and mood improvements after fast cerebellar rTMS have recently been shown in healthy volunteers. Therefore, fast cerebellar rTMS might be explored as method for the treatment of clinical depression.
An rTMS-oriented theoretical framework for the treatment of depression
Until now, the PFC has been the main target in the investigation of the therapeutic application of rTMS in depression. It has been argued that enhanced turnover of dopamine in particular in the subcortical structures may contribute largely to the beneficial effects of rTMS. Although PFC rTMS has demonstrated antidepressant properties, its overall clinical efficacy is not yet clear. This might be the result of the lack of insight regarding the precise physiologic mechanisms by which rTMS establishes its effects, as well as the difficult type of patient population that receives stimulation, because these subjects normally have failed to benefit from all conventional treatment. Furthermore, stimulation parameters could also play an important role in the inconsistencies found and the lack of therapeutic efficacy.54 Given the high interindividual variability in both functional as well as structural anatomy, the use of higher frequency rates and longer stimulation times should induce more stable and stronger effects.22 The problematic issue of safety, however, puts a constraint on the stimulation parameter settings that can be used in humans.4 However, triggering seizures or inducing kindling through magnetic stimulation is not very easy to accomplish, at least in healthy subjects.55 Further exploring the possibilities of the safe use of rTMS at more intense stimulation parameters, such as higher frequency rates and longer duration, might be worthwhile.56
As noted, alterations in dopamine levels seem to be involved in the pathogenesis of depression;57 there is, in particular, a growing body of evidence for the association between depression and lowered dopaminergic activity.58 Thus, it is of interest that animal research indicates that the antidepressant effects of left PFC rTMS are probably caused by enhanced dopaminergic activity.52 Further evidence for enhanced dopaminergic activity after rTMS was recently provided by Zangen and Hyodo.59 Interestingly, however, these authors showed that stimulation over the caudal cortex caused a greater increase in dopamine levels than stimulation over the frontal cortex, which led them to suggest that the caudal parts of the cortex could have a greater potential for establishing antidepressant efficacy. This evidence from animal research suggests that the PFC might not be the ideal target location for rTMS in depression. There is evidence for the involvement of interconnected structures in depression, including the parietal cortex,30,31 the cerebellum and various subcortical nuclei.42 Unfortunately, direct subcortical stimulation is not yet an issue for rTMS research, because of the fast decay of the magnetic field with distance. On the basis of findings from direct stimulation studies carried out in human healthy volunteers,25,26 patients42 and animals,52 it is, however, reasonable to assume that the clinical application of rTMS over the parietal cortex and cerebellum might be a worthwhile venture. Currently, clinical slow and fast rTMS treatment studies in depressed patients targeting the right parietal cortex and cerebellum are being prepared in our laboratory to further investigate the therapeutic efficacy of rTMS and the neural mechanisms underlying the pathophysiology of depression.
In the final section of this paper, we would like to postulate a hypothetical model for rTMS research in the domain of depression that builds on the specific involvement of the PFC, right parietal cortex and cerebellum. The findings presented here suggest that these structures and their reciprocal connectivity are part of a neurocircuitry that is dysfunctional in depression. Figure 1 depicts this heuristic working framework for rTMS applications in the treatment of depression, which includes, besides the PFC, the right parietal cortex and the cerebellum. It should be noted that the framework is limited, because it focuses primarily on regions directly reachable by rTMS and on the involvement of the neurotransmitter dopamine. The connections outlined in Figure 1 are based on neuroimaging and rTMS electrophysiologic studies.
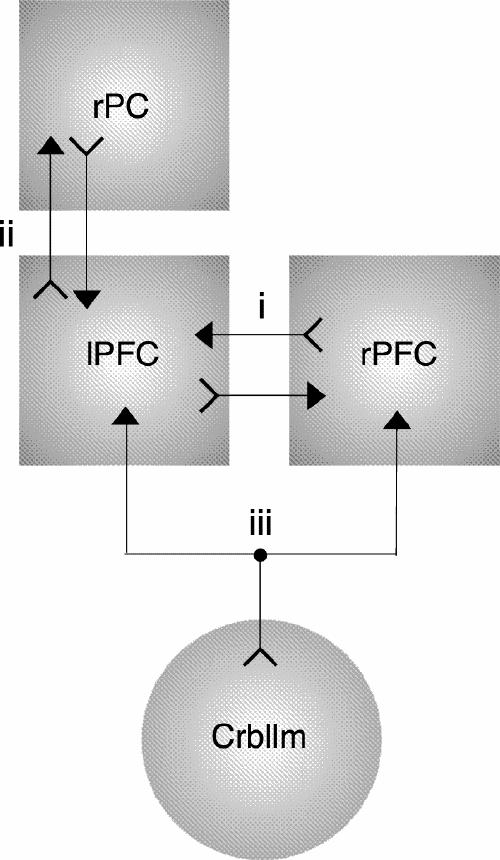
Fig. 1: A repetitive transcranial magnetic stimulation-oriented theoretical framework of depression involving the left prefrontal cortex (lPFC), the right prefrontal cortex (rPFC), the right parietal cortex (rPC) and the cerebellum (Crbllm). The arrows between the left and right PFC (i) depict the transcallosal inhibitory mechanism, whereas the arrows labelled (ii) represent the functional connectivity between the left PFC and the right parietal cortex. The arrows labelled (iii) denote the cerebellar projections to the PFC, directly via the thalamus and indirectly through dopaminergic activation. Note that the connections represent anatomical interdependencies based on the various neuroscientific methodologies and do not reflect inhibitory or excitatory links per se.
This rTMS-oriented theoretical framework enables us to distill several hypotheses regarding potential efficient antidepressant stimulation parameters. Moreover, the model might actually predict antidepressant efficacy for different subtypes of depression when applying rTMS with specific stimulation parameters over selected brain regions. A hyperactive right parietal cortex has, for instance, been implicated in comorbid depression and anxiety.28 On the basis of the premise that 2-Hz rTMS over the right parietal cortex is capable of dampening cortical arousal and enhancing functional corticocortical connectivity with the left PFC, slow rTMS might be especially effective in the treatment of this subtype of depression. Furthermore, elevations in mood and alertness after fast cerebellar rTMS25 are arguably the result of increased dopaminergic activity, hence, apathetically depressed patients may benefit from this kind of high-frequency stimulation. On the other hand, bipolar disorder could be treated by both slow and fast rTMS depending on the state of illness. Using carefully selected stimulation parameter settings, dopamine could be partly enhanced or blocked, in bipolar disorder, in an attempt to set the brain properly and thus achieve euthymic stabilization. Although these hypotheses are speculative and need further verification, our aim was to develop a progressive theoretical approach for clinical rTMS research that is capable of linking subtypes of depression to different rTMS treatment protocols.
After a decade of research, rTMS over the PFC has proven to be superior to sham stimulation in reducing depressive psychopathology, but the clinical efficacy remains inconclusive as of yet.60 In the present overview, a framework is postulated for investigating alternative brain regions by rTMS in clinical depression. A possible antidepressant efficacy is hypothesized for both slow rTMS over the right parietal cortex and fast rTMS over the cerebellum.
Acknowledgments
Dr. Schutter was supported by an Innovational Research Grant (#451-04-070) and Dr. van Honk was supported by an Innovational Research Grant (# 016-005-060) from the Netherlands Organization for Scientific Research (NWO).
Footnotes
Competing interests: None declared.
Correspondence to: Dr. Dennis J.L.G. Schutter, Affective Neuroscience Section, Department of Psychonomics, Helmholtz Research Institute, Utrecht University, Heidelberglaan 2, 3584 CS Utrecht, The Netherlands; fax 31 30 2534511; d.schutter@fss.uu.nl
Submitted Aug. 31, 2003; Revised Feb. 6, 2004; Accepted Mar. 17, 2004
References
- 1.Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, et al. Cross-national epidemiology of major depression and bipolar disorder. JAMA 2001;276:293-9. [PubMed]
- 2.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: the Association; 2000.
- 3.Hoflich G, Kasper S, Hufnagel A, Ruhrmann S, Moller HJ. Application of transcranial magnetic stimulation in treatment of drug-resistant major depression: a report of two cases. Hum Psychopharmacol 1993;8:361-5.
- 4.Wassermann EM, Lisanby SH. Therapeutic application of repetitive transcranial magnetic stimulation: a review. Clin Neurophysiol 2001;112:1367-77. [DOI] [PubMed]
- 5.Burt T, Lisanby SH, Sackeim HA. Neuropsychiatric applications of transcranial magnetic stimulation: a meta analysis. Int J Neuropsychopharmacol 2002;5:73-103. [DOI] [PubMed]
- 6.Heller W, Nitschke JB, Etienne MA, Miller GA. Patterns of regional brain activity differentiate types of anxiety. J Abnorm Psychol 1997;106:376-85. [DOI] [PubMed]
- 7.Schmahmann JD. Dysmetria of thought: clinical consequences of cerebellar dysfunction on cognition and affect. Trends Cogn Sci 1998;2:362-71. [DOI] [PubMed]
- 8.Hallet M. Transcranial magnetic stimulation and the human brain. Nature 2000;406:147-50. [DOI] [PubMed]
- 9.Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 1997;48:1398-403. [DOI] [PubMed]
- 10.Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain 1994;117:847-58. [DOI] [PubMed]
- 11.Speer AM, Kimbrell TA, Wassermann EM, Repella JD, Willis MW, Herscovitch P, et al. Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol Psychiatry 2000;48:1133-41. [DOI] [PubMed]
- 12.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5-7, 1996. Electroencephalogr Clin Neurophysiol 1998;108:1-16. [DOI] [PubMed]
- 13.Nahas Z, Lomarev M, Roberts DR, Shastri A, Lorberbaum JP, Teneback C, et al. Unilateral left prefrontal transcranial magnetic stimulation (TMS) produces intensity-dependent bilateral effects as measured by interleaved BOLD fMRI. Biol Psychiatry 2001; 50:712-20. [DOI] [PubMed]
- 14.Fox P, Ingham R, George MS, Mayberg H, Ingham J, Roby J, et al. Imaging human intra-cerebral connectivity by PET during TMS. Neuroreport 1997;8:2787-91. [DOI] [PubMed]
- 15.Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans AC. Transcranial magnetic stimulation during positron emission tomography: a new method for studying connectivity of the human cerebral cortex. J Neurosci 1997;17:3178-84. [DOI] [PMC free article] [PubMed]
- 16.Drevets WC, Price JL, Simpson JR Jr, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature 1997;386:824-7. [DOI] [PubMed]
- 17.Pizzagalli DA, Nitschke JB, Oakes TR, Hendrick AM, Horras KA, Larson CL, et al. Brain electrical tomography in depression: the importance of symptom severity, anxiety, and melancholic features. Biol Psychiatry 2002;52:73-85. [DOI] [PubMed]
- 18.Kozel FA, George MS. Meta-analysis of left prefrontal repetitive transcranial magnetic stimulation (rTMS) to treat depression. J Psychiatr Pract 2002;8:270-5. [DOI] [PubMed]
- 19.Klein E, Kreinin I, Chistyakov A, Koren D, Mecz L, Marmur S, et al. Therapeutic efficacy of right prefrontal slow repetitive transcranial magnetic stimulation in major depression: a double blind controlled study. Arch Gen Psychiatry 1999;56:315-20. [DOI] [PubMed]
- 20.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56-62. [DOI] [PMC free article] [PubMed]
- 21.McNamara B, Ray JL, Arthurs OJ, Boniface S. Transcranial magnetic stimulation for depression and other psychiatric disorders. Psychol Med 2001;31:1141-6. [DOI] [PubMed]
- 22.Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res 2000;133:425-30. [DOI] [PubMed]
- 23.Gershon AA, Dannon PN, Grunhaus L. Transcranial magnetic stimulation in the treatment of depression. Am J Psychiatry 2003; 160:835-45. [DOI] [PubMed]
- 24.George MS, Nahas Z, Lomarev M, Bohning DE, Kellner CH. How knowledge of regional brain dysfunction in depression will enable new somatic treatments in the next millenium. CNS Spectr 1999; 4:53-66. [DOI] [PubMed]
- 25.Schutter DJLG, Van Honk J, D'Alfonso AAL, Peper JS, Panksepp J. High frequency rTMS over the medial cerebellum induces a shift in the prefrontal electroencephalography gamma spectrum: a pilot study in humans. Neurosci Lett 2003;336:73-6. [DOI] [PubMed]
- 26.Van Honk J, Schutter DJLG, Putman P, De Haan EHF, D'Alfonso AAL. Reductions in phenomenological, physiological and attentional indices of depression after 2Hz rTMS over the right parietal cortex. Psychiatry Res 2003;120:95-101. [DOI] [PubMed]
- 27.Uytdenhoef P, Portelange P, Jacquy J, Charles G, Linkowski P, Mendlewicz J. Regional cerebral blood flow and lateralized hemisphere dysfunction in depression. Br J Psychiatry 1983;143:128-32. [DOI] [PubMed]
- 28.Heller W, Nitschke JB. The puzzle of regional brain activity in depression and anxiety: the importance of subtypes and comorbidity. Cogn Emotion 1998;12:421-47.
- 29.Zimmerman M, Chelminski I, McDermut WJ. Major depressive disorder and axis I diagnostic comorbidity. Clin Psychiatry 2002; 63:187-93. [DOI] [PubMed]
- 30.Gold PW, Drevets WC, Charney DS. New insights into the role of cortisol and the glucocorticoid receptor in severe depression. Biol Psychiatry 2002;52:381-5. [DOI] [PubMed]
- 31.Belanoff JK, Rothschild AJ, Cassidy F, DeBattista C, Baulieu EE, Schold C, et al. An open-label trial of C-1073 (mifepristone) for psychotic major depression. Biol Psychiatry 2002;52:386-92. [DOI] [PubMed]
- 32.Schutter DJLG, Van Honk J, Koppeschaar H, Kahn RS. Cortisol and reduced interhemispheric coupling between the left prefrontal and the right parietal cortex. J Neuropsychiatry Clin Neurosci 2002;14:89-90. [DOI] [PubMed]
- 33.Nunez PL, Srinivasan R, Westdorp AF, Wijesinghe RS, Tucker DM, Silberstein RB, et al. EEG coherency. I: Statistics, reference electrode, volume conduction, Laplacians, cortical imaging, and interpretation at multiple scales. Electroencephalogr Clin Neurophysiol 1997;103:499-515. [DOI] [PubMed]
- 34.Leuchter AF, Cook IA, Uijtdehaage SH, Dunkin J, Lufkin RB, Anderson-Hanley C, et al. Brain structure and function and the outcomes of treatment for depression. J Clin Psychiatry 1997;58(16 Suppl):22-31. [PubMed]
- 35.Cook IA, Leuchter AF, Morgan ML, Conlee EW, David S, Lufkin R, et al. Cognitive and physiologic correlates of subclinical structural brain disease in elderly healthy control subjects. Arch Neurol 2000;59:1612-20. [DOI] [PubMed]
- 36.Schweiger U, Deuschle M, Weber B, Korner A, Lammers CH, Schmider J, et al. Testosterone, gonadotropin, and cortisol secretion in male patients with major depression. Psychosom Med 1999;61:292-6. [DOI] [PubMed]
- 37.Schutter DJLG, Peper JS, Koppeschaar HPF, Kahn RS, van Honk J. Administration of testosterone increases functional connectivity in a cortico-cortical depression circuit. J Neuropsychiatry Clin Neurosci. In press. [DOI] [PubMed]
- 38.Jing H, Takigawa M. Observation of EEG coherence after repetitive transcranial magnetic stimulation. Clin Neurophysiol 2000; 111:1620-31. [DOI] [PubMed]
- 39.Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry 1999;156:675-82. [DOI] [PubMed]
- 40.Schutter DJLG, D'Alfonso AAL, Van Honk J. Counterintuitive antidepressant properties of slow rTMS over the left frontal cortex: a possible mechanism. J Neuropsychiatry Clin Neurosci 2003;15:243-4. [DOI] [PubMed]
- 41.Heath RG. Modulation of emotion with a brain pacemaker. J Nerv Ment Dis 1977;165:300-17. [PubMed]
- 42.Heath RG, Rouchell AM, Goethe JW. Cerebellar stimulation in treating intractable behavior disorders. Curr Psychiatr Ther 1981; 20:329-36. [PubMed]
- 43.Schmahmann JD, Sherman JD. The cerebellar cognitive affective syndrome. Brain 1998;121:561-79. [DOI] [PubMed]
- 44.Schmahmann JD. The role of the cerebellum on affect and psychosis. J Neurolinguist 2000;13:189-214.
- 45.Soares JC, Mann JJ. The anatomy of mood disorders-review of structural neuroimaging studies. Biol Psychiatry 1997;41:86-106. [DOI] [PubMed]
- 46.Leroi I, O'Hearn E, Marsh L, Lyketsos CG, Rosenblatt A, Ross CA, et al. Psychopathology in patients with degenerative cerebellar diseases: a comparison to Huntington's disease. Am J Psychiatry 2002;159:1306-14. [DOI] [PubMed]
- 47.Starkstein SE, Robinson RG, Berthier ML, Price TR. Depressive disorders following posterior circulation as compared with middle cerebral artery infarcts. Brain 1988;111:375-87. [DOI] [PubMed]
- 48.Beyer JL, Krishnan KR. Volumetric brain imaging findings in mood disorders. Bipolar Disord 2002;4:89-104. [DOI] [PubMed]
- 49.Schmahmann JD, Pandya DN. Anatomic organization of the basilar pontine projections from prefrontal cortices in rhesus monkey. J Neurosci 1997;17:438-58. [DOI] [PMC free article] [PubMed]
- 50.Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci 2001;21:700-12. [DOI] [PMC free article] [PubMed]
- 51.Schmahmann JD, Anderson CM, Newton N, Ellis R. The function of the cerebellum in cognition, affect and consciousness: empirical support for the embodied mind. Conscious Emotion 2001;2:273-309.
- 52.Keck ME, Welt T, Müller MB, Erhardt A, Ohl F, Toschi N, et al. Repetitive transcranial magnetic stimulation increases the release of dopamine in the mesolimbic and mesostriatal system. Neuropharmacology 2002;43:101-9. [DOI] [PubMed]
- 53.Albert TJ, Dempsey CW, Sorenson CA. Anterior cerebellar vermal stimulation: effect on behavior and basal forebrain neurochemistry in rat. Biol Psychiatry 1985;20:1267-76. [DOI] [PubMed]
- 54.Sackeim HA. Repetitive transcranial magnetic stimulation: What are the next steps? Biol Psychiatry 2002;48:959-61. [DOI] [PubMed]
- 55.Lisanby SH, Luber B, Finck AD, Schroeder C, Sackeim HA. Deliberate seizure induction with repetitive transcranial magnetic stimulation in nonhuman primates. Arch Gen Psychiatry 2001;58:199-200. [DOI] [PubMed]
- 56.Grunhaus A, Schreiber S, Dolberg OT, Polak D, Dannon PN. Randomized controlled comparison of electroconvulsive therapy and repetitive transcranial magnetic stimulation in severe and resistant nonpsychotic major depression. Biol Psychiatry 2003;54:324-31. [DOI] [PubMed]
- 57.Heimer L, Harlan RE, Alheid GF, Garcia MM, De Olmos J. Substantia innominata: a notion which impedes clinical-anatomical correlations in neuropsychiatric disorders. Neuroscience 1997;76:957-1006. [DOI] [PubMed]
- 58.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron 2002;34:13-25. [DOI] [PubMed]
- 59.Zangen A, Hyodo K. Transcranial magnetic stimulation induces increases in extracellular levels of dopamine and glutamate in nucleus accumbens. Neuroreport 2002;13:2401-5. [DOI] [PubMed]
- 60.Schlaepfer TE, Kosel M, Nemeroff CB. Efficacy of repetitive transcranial magnetic stimulation (rTMS) in the treatment of affective disorders. Neuropsychopharmacology 2003;28:201-5. [DOI] [PubMed]


