Introduction
Urinary tract infections (UTIs) in infants are common. UTIs may be the sentinel event for underlying renal abnormality, although normal anatomy is most common. Prompt diagnosis and initiation of treatment is important in preventing long-term renal scarring. However, increasing antibiotic resistance may delay initiation of appropriate therapy. Antibiotic prophylaxis remains controversial.
Epidemiology and Risk Factors for Urinary Tract Infections in Infants
Occurrence of Urinary Tract Infections in the First 3 Days of Life Is Exceedingly Rare
The true incidence of UTI in the first days of life is difficult to assess, as most large studies have included such cases in the broader age categories (7%–9%).1–4 Small studies indicate the incidence in the febrile infant is between 10.7% and 15.4%.5,6 Occurrence of UTIs in the first 3 days of life is reportedly rare (0%–1%) in the United States7,8 and up to 1.8% in developing countries.9,10 Even in premature infants, virtually no cases are detected in the first 24 hours of life.8
Escherichia coli Is the Most Common Cause of Neonatal Urinary Tract Infection
The most common bacterial etiology for neonatal UTIs, similar to other age groups, is Escherichia coli.3,11–14 However, some studies found that the overall burden of disease by E. coli was lower in this age group (about 50% of all positive cultures) compared with older age groups in which E. coli is responsible for up to 80% of UTIs.10,15 In particular, male infants with vesicoureteral reflux (VUR) were more likely to present with UTIs caused by other pathogens.3,5,12,15 These pathogens include other gram-negative organisms: Klebsiella pneumoniae, Klebsiella oxytoca, Proteus mirabilis, Proteus vulgaris, Enterobacter aerogenes, Pseudomonas aeruginosa, and Morganella morganii.5,12 Neonatal UTI with gram-positive organisms is rare, but cases of Enterococcus faecalis, Staphylococcus aureus, Group B streptococcus, and Streptococcus pneumonia have been reported.6,16–18 Coagulase-negative staphylococci may be causative agents in premature infants, with isolation of the organism in 14% of catheterized urine culture samples from infants with suspected infection and 18% concordance with positive blood cultures.19 However, this finding remains controversial; one study, which included mostly premature infants, showed a less than 1% incidence of coagulase-negative staphylococci UTI.20 Candida UTIs occur more commonly in extremely premature infants. One study reported that 42% of UTIs in a neonatal intensive care unit were caused by Candidia spp, with Enterobacter cloacae being the second most common.21 Table 1 lists the most common pathogens associated with neonatal UTI.
Table 1. Common pathogens isolated in neonatal UTI.
| Organism | Incidence (%) | References |
|---|---|---|
| Gram-negative rods | ||
| E. coli | 40–72 | 5,12,42 |
| Klebsiella spp | 7–40 | 5,35 |
| Enterobacter cloacae | 3–8 | 5,42 |
| Proteus vulgaris | 3 | 5 |
| Serratia marcescens | 1–7 | 5,35 |
| Pseudomonas aeruginosa | 1 | 5 |
| Gram-positive cocci | ||
| Enterococcus spp | 10–16 | 5,42 |
| Staphylococcus aureus | 1–5 | 5,42 |
| Group B streptococcus | 1–3 | 5,70 |
| Staphylococcus, coagulase negative | 1 | 5 |
| Viridans streptococcus | 1 | 5 |
| Yeast | ||
| Candida spp | 25–42 | 21,71 |
Uncircumcised Male Infants Have the Highest Risk of Urinary Tract Infection
A clear male predominance has been associated with neonatal UTI, with boys making up approximately 70% to 90% of all cases.5,11,12 This finding is also true in premature infants.22,23 To evaluate the effect of circumcision on male risk for infantile UTI, Zorc and colleagues3 conducted a prospective multicenter trial, which included approximately 1000 febrile infants less than 60 days of age whose evaluation for sepsis included a urine culture and urinalysis. Infants who had growth of a single organism in the urine culture were included in the subsequent evaluation. Uncircumcised boys had the highest incidence of UTIs (21%), whereas circumcised boys (2%) and girls (5%) has similar incidences. These results were similar to those in a meta-analysis,1 in which 20% of the uncircumcised boys less than 90 days of age with fever had a UTI compared with 2% of circumcised boys and 8% of girls. Phimosis, limited retraction of the foreskin, is significantly associated with an increase in UTIs in male infants.24 A recent study in older children that examined the periurethral flora in boys between the ages of 6 weeks and 96 months before and after circumcision found that the presence of the prepuce results in a significantly higher burden of uropathogens.25
Underlying Renal Abnormalities Increase the Risk of Neonatal Urinary Tract Infection
VUR is associated with approximately 20% of neonatal cases of UTI,5,26 although the incidence of VUR is not significantly different between genders, birth weight, gestational age, or mode of delivery.26,27 A study of infants less than 2 months of age in a neonatal intensive care unit with a median gestation age of 28 weeks reported a less than 5% rate of anatomic abnormalities in patients with UTI. VUR was, however, associated with a younger age at UTI presentation and was 4-fold higher in infants with Klebsiella UTI compared with E coli UTI.5,26 A study of 45 male infants with a first UTI renal ultrasound scan (RUS) and voiding cystourethrogram (VCUG) found a renal abnormality in half of the infants, the most common being VUR and other abnormalities, including duplicated collecting system, posterior urethral valves, ureteropelvic junction stricture, and renal atrophy and scarring.28 The dimercaptosuccinic acid (DMSA) scan was abnormal almost exclusively in those with grade 3 or higher VUR. These results are similar to those in a recent study, in which 47% of febrile infants less than 30 days of age with a UTI had renal abnormalities, most of them hydronephrosis (27%) and pelviectasis (20%).5 However, even in the absence of any abnormalities on the RUS or VCUG, infants with UTI can have an abnormal DMSA scan, indicating renal cortical damage, although that may be an effect rather than a cause of a UTI.29 Representative images for RUS and VCUG in infants are shown in Figs. 1 and 2.
Fig. 1.
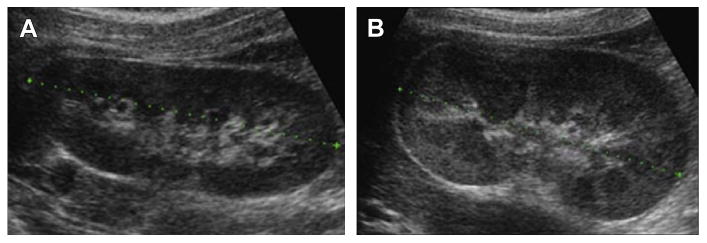
Ultrasound appearance of neonatal hydronephrosis. (A) Right kidney in an infant showing normal structures. (B) Left kidney in the same infant with edematous swollen slightly hyperechoic right kidney. (Courtesy of Dr T.S.A. Geertsma, Ziekenhuis Gelderse Vallei, Ede, The Netherlands.)
Fig. 2.
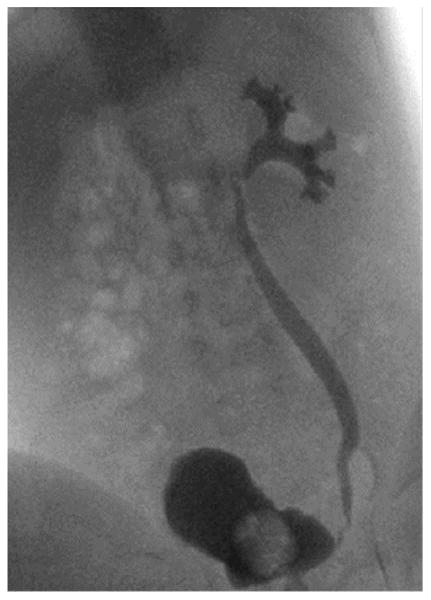
Grade III vesicoureteric reflux during micturition: reflux into the ureter and the calices with mild dilatation. (Courtesy of Dr Adriana Dubbeldam, Belgium. Available at: www.radiopedia.org.)
Maternal History of Urinary Tract Infection Is Associated with a Higher Risk of Urinary Tract Infection in the Infant
A history of maternal UTI during pregnancy has been associated with up to a 5.9-fold higher risk of UTI in infants.30,31 Milas and colleagues31 also observed a higher incidence of UTIs in febrile infants born after premature rupture of membranes. This incidence may be because these mothers are more likely to harbor uropathogens transmitted to the infant that then result in an ascending UTI.
Clinical Correlations
Full-term infants with UTIs often present with fever (≥38°C), poor feeding, vomiting, diarrhea, and lethargy (Table 2).12,32 The clinical manifestations in premature infants are similar. In addition, greater than 50% of premature infants with UTI present with respiratory symptoms such as apnea, hypoxia, or tachypnea.33 A fever greater than 39°C is more likely among infants with a serious bacterial illness such as a UTI compared with infants with a viral illness.3,12,34
Table 2. Common symptoms and signs associated with neonatal UTI.
Neonatal UTIs have been associated with jaundice12,31; 6% to 18% of full-term or preterm infants presenting with prolonged or worsening jaundice were found to have UTIs.35–38 Onset of jaundice after 8 days of life in particular has been associated with UTI.37,39 Twenty-eight of 30 infants with UTI-induced jaundice had indirect hyperbilirubinemia, and about half of them had renal cortical changes on DMSA scan.39 In another cohort,37 most of the infants presenting with a UTI after 8 days of life had direct hyperbilirubinemia. The American Academy of Pediatrics recommends that infants with elevated direct bilirubin levels be screened for UTIs.40 However, those with elevated unconjugated bilirubin levels should not be excluded, especially if other concerning clinical features are present. A urinalysis may not be sufficient to exclude UTI in infants with jaundice, and a urine culture should be obtained.41 E coli is the most commonly isolated pathogen in UTIs associated with jaundice.37,38,41,42
Infants with Urinary Tract Infection Are at Risk for Concomitant Bacteremia and Meningitis
Several studies have examined the concordance between UTI, bacteremia, and cerebrospinal fluid (CSF) pleocytosis/culture positivity. In a cohort of 163 infants less than 1 month of age with a UTI only 2 had meningitis.43 Another study from the United States reported that 44 of 100 patients had concomitant positive blood and urine culture; E coli was isolated in all cases.5 A study from India reported a 6.3% concordance between blood and urine cultures.10 Concordance between urine, blood, and CSF cultures is higher in infants less than 26 weeks' gestation and those with candiduria.19,21
Diagnosis
Blood Cell Indices and Inflammatory Markers Are not Specific Indicators of Urinary Tract Infections
Laboratory values such as white blood cell (WBC) count, erythrocyte sedimentation rate, and C-reactive protein are not significantly different among infants with and without UTIs.5,44
Urethral Catheterization is the Preferred Method for Sample Collection
Urine culture is typically obtained through 3 different methods in infants: urinary catheterization, suprapubic aspiration, or sterile bag collection. The sterile bag collection method has a contamination rate as high as 46% compared with about 9% to 12% for the other methods and, when possible, should be avoided.45,46 Although contamination rates for suprapubic aspiration are slightly lower than those for urethral catheterization,45 it does require a more advanced skill set and has lower parental acceptance rate, making the latter the preferred method by most providers. Although definitions vary, some investigators have defined a positive urine culture as growth of a known bacterial pathogen from a catheterized specimen at a level of (1) ≥50,000 colony-forming units (cfu)/mL or (2) ≥10,000 cfu/mL in association with a positive dipstick test or urinalysis.3,5,47
Pyuria Is Defined as White Blood Cell Count of ≥10/mm3
The standard method of detecting pyuria, defined as at least 5 WBCs per high-powered field, is useful in predicting less than half of the UTIs in infants.48 However, a method (often termed the enhanced method) initially described by Dukes,49 in which WBCs are counted using a hemocytometer in uncentrifuged urine and reported as cells per cubic millimeter has been shown to be reproducible and more closely related to a positive urine culture. A pediatric study including young infants showed that a WBC count of ≥10/mm3 had a sensitivity of 91% and a specificity of 96% for predicting a positive culture of ≥50,000 cfu/mL.50 A more recent study, also including young infants, compared the enhanced method with automated urinalysis and found similar sensitivity and specificity for detecting pyuria associated with a bacterial culture of ≥50,000 cfu/mL.51 For bacteriuria, the same study found that the enhanced method using a manual Gram stain for organisms was about 10% more sensitive and specific than the automated analysis.
Urine Nitrites and Leukocyte Esterase Are Unreliable Parameters in Infants
Other commonly examined urinalysis parameters include nitrites and leukocyte esterase. The nitrite test indicates the presence of nitrate reductase, produced by some but not all uropathogens, which converts endogenous nitrates to nitrites. Leukocyte esterase is released by WBCs and indicates the presence of pyuria. In a systematic review of several studies, nitrites and leukocyte esterase were shown to have good sensitivity and specificity for detection of UTI in older children but were less reliable in infants.52 This is likely related to frequent micturition in infants which does not allow for sufficient concentrations of these substrates to develop. However, a more recent study of infants between 1 and 90 days of age showed that when microscopy is added to the urine dipstick the negative predictive value is 99.2%, but would result on average in 8 false positives for every missed episode of true UTI.53
Treatment
Local Patterns of Antibiotic Resistance Should Determine Choice of Empiric Therapy
Empiric therapy for neonatal UTI and sepsis are similar because of common etiology. Traditionally parental antibiotics such as ampicillin and gentamicin are started once appropriate cultures are obtained. Within the US, the incidence of ampicillin resistance in neonatal E. coli isolates has been reported to be as high as 75% and gentamicin resistance as high as 12%–17%.54,55 In spite of approximately 90% resistance against ampicillin among the E. coli isolates from a neonatal ward, Taheri and colleagues56 reported that clinical response was obtained in 50% of the patients, suggesting that that there is a discordance between in vitro and in vivo activity of these drugs. This may be because the urinary concentration of ampicillin is much higher than the plasma level since it is excreted through the kidneys which may allow it to overcome the minimum inhibitory concentration of certain pathogens.57
Peripartum Use of Maternal Antibiotics Increases the Risk of Resistant Clones in Infants
Use of maternal peripartum exposure antibiotics increases the risk of neonatal UTI and bloodstream infections with β-lactamase producing E coli: 82% versus 36% in infants of treated and untreated mothers, respectively.13 Common scenarios for maternal antibiotic exposure include preterm premature rupture of membranes (PPROM)58,59 and intrapartum prophylaxis.13,60
Treatment of Candiduria
The presence of candiduria in a neonate indicates hematogenous spread and systemic disease. Treatment of systemic candidiasis is reviewed elsewhere in this issue by Kelly and colleagues and Wade and colleagues.
Neonatal Urinary Tract Infection can be Treated with a Combination of Parental and Oral Therapy
Data regarding the length of duration of treatment and the transition from parenteral to oral therapy in the infant are lacking. In the extremely premature infant, bioavailability of most antibiotics is not known; therefore, intravenous therapy is typically preferred. Documentation of negative blood and CSF cultures in both extremely premature and older infants provides optimal care. In older infants, Benador and colleagues61 found that the risk of renal scarring was no different between infants that received 3 days of parental therapy followed by 7 days of oral therapy compared with 10 days of oral therapy. In older and more mature infants with negative blood and CSF cultures, 3 to 4 days of parental therapy followed by transition to oral medications to complete a 7- to 14-day course of treatment can be used.62
Renal Imaging Usually Includes a Renal Ultrasound Scan During the Acute Infection and a Voiding Cystourethrogram to Identify Vesicoureteral Reflux Approximately 2 to 4 Weeks After Resolution of Infection
Most practitioners recommend a RUS after an episode of UTI in the neonatal period to rule out congenital abnormalities. A VCUG is usually delayed for 2 to 4 weeks after successful treatment to assess for vesicoureteral reflux. Grade III or higher VUR is significantly associated with a higher risk of renal cortical damage; a DMSA scan should be considered to assess for renal scarring.28
In a study of 100 infants with UTI, 47% had an abnormal RUS.5 However, the incidence of abnormalities was much lower in premature infants (4%).63 Siomou and colleagues64 prospectively evaluated 72 infants with an RUS and DMSA scan within 72 hours of diagnosis. Approximately 71% of the kidneys with grade III or more VUR on RUS were found to have normal early DMSA scans, 7% had evidence of permanent renal damage at the 6-month follow-up, all of which had an abnormal early DMSA scan. Therefore, an acute DMSA scan may be helpful in identifying the risk of renal scarring, but it does not reliably diagnose VUR.
The Risk of Recurrence Is Highest in First 6 Months After an Episode of Urinary Tract Infection
A long-term follow-up of 71 infants with UTI showed a recurrence rate of 28%.65 Recurrence in premature infants was slightly more common than in full-term infants, but the difference was not significant. Most of the episodes of recurrence (65%) occur in the first 6 months after the initial UTI, and 75% occur in patients without any renal abnormalities.
Prophylactic Antibiotics Are Not Effective in Reducing Renal Scarring, but Do Increase the Risk of Recurrence with a Resistant Strain
Evidence regarding the efficacy of prophylactic therapy to prevent recurrences after the first episode of UTI is lacking for the neonatal population. Even for older infants, several small trials have found that antimicrobial prophylaxis may not be effective in preventing renal scarring.66,67 Other more recent studies found that although the prophylaxis may decrease the risk of recurrence, its effect on renal scarring is not significant, and recurrent episodes are more likely to be caused by a more resistant strain.68,69
Summary
UTI is common in infants. It may indicate an underlying renal disorder, but most cases occur in the absence of any abnormalities. UTIs are rare in the first 3 days of life. Uncircumcised boys are at the highest risk for neonatal UTI. Diagnosis is by a urine culture in association with a positive dipstick test or urinalysis. Ampicillin and gentamicin are the traditional empiric therapies; however, local antibiotic resistance patterns and maternal use of antibiotics before delivery should be considered. The risk of recurrence is highest in the first 6 months after an episode of UTI. Use of prophylaxis is controversial because, although it may reduce the risk of recurrence, it is unclear if there is any effect on renal scarring, and use of prophylaxis increases the risk of infection with a resistant strain if recurrence occurs.
Key Points.
Uncircumcised boys have the highest risk of urinary tract infection.
Escherichia coli is the most common pathogen.
Premature infants are at increased risk for Candida urinary tract infections.
Infants with urinary tract infection are at risk for concomitant bacteremia and meningitis.
Prophylaxis may increase the risk of antibiotic resistance for recurrent urinary tract infection.
Best practices box.
What is the current practice?
Urinary Tract Infections in Neonate
Best Practice/Guideline/Care Path Objective(s)
Early recognition of UTIs in neonates
Initiation of appropriate empiric therapy
Reduction in long-term renal sequelae
What changes in current practice are likely to improve outcomes?
Recognition of enhanced risk for bacteremia and meningitis in neonates with UTI
Empiric treatment of UTI based on prior maternal use of antibiotics during pregnancy and local antibiotic susceptibility profiles among uropathogens
Cautious use of prophylactic antibiotics to prevent recurrent UTI and knowledge of the risk for emergence of resistant organisms
Major Recommendations
Obtain urine specimen for bacterial culture by urethral catheterization; other urine parameters can be misleading in infants (grade 1A).
-
Empiric therapy should include coverage against common uropathogens such as E coli and Klebsiella spp
Ampicillin and gentamicin are the most commonly used empiric regimen (grade 1A).
Infants with prolonged or late-onset jaundice should be evaluated for a UTI (grade 1B).
Infants with UTI should be evaluated for concomitant bacteremia and meningitis (grade 1B).
Renal ultrasound scan should be done immediately after an episode of neonatal UTI, followed by a VCUG 2 to 4 weeks later to rule out anatomic abnormalities (grade 1B).
Prophylactic antibiotics do not reduce the risk of scarring and can increase the risk of recurrent UTI with resistant organisms (grade 1B).
Summary statement
UTI in infants may indicate an underlying renal disorder; therefore, appropriate diagnosis and prompt initiation of therapy are essential to reduce long-term renal scarring.
Footnotes
Disclosures: None.
References
- 1.Shaikh N, Morone NE, Bost JE, et al. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J. 2008;27:302–8. doi: 10.1097/INF.0b013e31815e4122. [DOI] [PubMed] [Google Scholar]
- 2.Ismaili K, Lolin K, Damry N, et al. Febrile urinary tract infections in 0- to 3-month-old infants: a prospective follow-up study. J Pediatr. 2011;158:91–4. doi: 10.1016/j.jpeds.2010.06.053. [DOI] [PubMed] [Google Scholar]
- 3.Zorc JJ, Levine DA, Platt SL, et al. Clinical and demographic factors associated with urinary tract infection in young febrile infants. Pediatrics. 2005;116:644–8. doi: 10.1542/peds.2004-1825. [DOI] [PubMed] [Google Scholar]
- 4.Lin DS, Huang SH, Lin CC, et al. Urinary tract infection in febrile infants younger than eight weeks of Age. Pediatrics. 2000;105:E20. doi: 10.1542/peds.105.2.e20. [DOI] [PubMed] [Google Scholar]
- 5.Bonadio W, Maida G. Urinary tract infection in outpatient febrile infants younger than 30 days of age: a 10-year evaluation. Pediatr Infect Dis J. 2014;33:342–4. doi: 10.1097/INF.0000000000000110. [DOI] [PubMed] [Google Scholar]
- 6.Morley EJ, Lapoint JM, Roy LW, et al. Rates of positive blood, urine, and cerebrospinal fluid cultures in children younger than 60 days during the vaccination era. Pediatr Emerg Care. 2012;28:125–30. doi: 10.1097/PEC.0b013e318243fa50. [DOI] [PubMed] [Google Scholar]
- 7.Visser VE, Hall RT. Urine culture in the evaluation of suspected neonatal sepsis. J Pediatr. 1979;94:635–8. doi: 10.1016/s0022-3476(79)80040-2. [DOI] [PubMed] [Google Scholar]
- 8.Tamim MM, Alesseh H, Aziz H. Analysis of the efficacy of urine culture as part of sepsis evaluation in the premature infant. Pediatr Infect Dis J. 2003;22:805–8. doi: 10.1097/01.inf.0000083822.31857.43. [DOI] [PubMed] [Google Scholar]
- 9.Riskin A, Toropine A, Bader D, et al. Is it justified to include urine cultures in early (<72 hours) neonatal sepsis evaluations of term and late preterm infants? Am J Perinatol. 2013;30:499–504. doi: 10.1055/s-0032-1329180. [DOI] [PubMed] [Google Scholar]
- 10.Samayam P, Ravi Chander B. Study of urinary tract infection and bacteriuria in neonatal sepsis. Indian J Pediatr. 2012;79:1033–6. doi: 10.1007/s12098-012-0727-7. [DOI] [PubMed] [Google Scholar]
- 11.Wang SF, Huang FY, Chiu NC, et al. Urinary tract infection in infants less than 2 months of age. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 1994;35:294–300. [PubMed] [Google Scholar]
- 12.Kanellopoulos TA, Salakos C, Spiliopoulou I, et al. First urinary tract infection in neonates, infants and young children: a comparative study. Pediatr Nephrol. 2006;21:1131–7. doi: 10.1007/s00467-006-0158-7. [DOI] [PubMed] [Google Scholar]
- 13.Didier C, Streicher MP, Chognot D, et al. Late-onset neonatal infections: incidences and pathogens in the era of antenatal antibiotics. Eur J Pediatr. 2012;171:681–7. doi: 10.1007/s00431-011-1639-7. [DOI] [PubMed] [Google Scholar]
- 14.Watt K, Waddle E, Jhaveri R. Changing epidemiology of serious bacterial infections in febrile infants without localizing signs. PLoS One. 2010;5:e12448. doi: 10.1371/journal.pone.0012448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo DS, Shieh HH, Ragazzi SL, et al. Community-acquired urinary tract infection: age and gender-dependent etiology. J Bras Nefrol. 2013;35:93–8. doi: 10.5935/0101-2800.20130016. [DOI] [PubMed] [Google Scholar]
- 16.Bitsori M, Maraki S, Raissaki M, et al. Community-acquired enterococcal urinary tract infections. Pediatr Nephrol. 2005;20:1583–6. doi: 10.1007/s00467-005-1976-8. [DOI] [PubMed] [Google Scholar]
- 17.Zurina Z, Rohani A, Neela V, et al. Late onset group b beta-hemolytic streptococcus infection in a neonate manifesting as a urinary tract infection: a rare clinical presentation. Southeast Asian J Trop Med Public Health. 2012;43:1470–3. [PubMed] [Google Scholar]
- 18.Hassoun A, Stankovic C, Rogers A, et al. Listeria and enterococcal infections in neonates 28 days of age and younger: is empiric parenteral ampicillin still indicated? Pediatr Emerg Care. 2014;30:240–3. doi: 10.1097/PEC.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 19.Downey LC, Benjamin DK, Jr, Clark RH, et al. Urinary tract infection concordance with positive blood and cerebrospinal fluid cultures in the neonatal intensive care unit. J Perinatol. 2013;33:302–6. doi: 10.1038/jp.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jean-Baptiste N, Benjamin DK, Jr, Cohen-Wolkowiez M, et al. Coagulase-negative staphylococcal infections in the neonatal intensive care unit. Infect Control Hosp Epidemiol. 2011;32:679–86. doi: 10.1086/660361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips JR, Karlowicz MG. Prevalence of Candida species in hospital-acquired urinary tract infections in a neonatal intensive care unit. Pediatr Infect Dis J. 1997;16:190–4. doi: 10.1097/00006454-199702000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Airede AI. Urinary-tract infections in African neonates. J Infect. 1992;25:55. doi: 10.1016/0163-4453(92)93513-p. [DOI] [PubMed] [Google Scholar]
- 23.Eliakim A, Dolfin T, Korzets Z, et al. Urinary tract infection in premature infants: the role of imaging studies and prophylactic therapy. J Perinatol. 1997;17:304. [PubMed] [Google Scholar]
- 24.Shim YH, Lee JW, Lee SJ. The risk factors of recurrent urinary tract infection in infants with normal urinary systems. Pediatr Nephrol. 2009;24:309–12. doi: 10.1007/s00467-008-1001-0. [DOI] [PubMed] [Google Scholar]
- 25.Laway MA, Wani ML, Patnaik R, et al. Does circumcision alter the periurethral uropathogenic bacterial flora. Afr J Paediatr Surg. 2012;9:109–12. doi: 10.4103/0189-6725.99394. [DOI] [PubMed] [Google Scholar]
- 26.Cleper R, Krause I, Eisenstein B, et al. Prevalence of vesicoureteral reflux in neonatal urinary tract infection. Clin Pediatr (Phila) 2004;43:619–25. doi: 10.1177/000992280404300706. [DOI] [PubMed] [Google Scholar]
- 27.Jantunen ME, Siitonen A, Ala-Houhala M, et al. Predictive factors associated with significant urinary tract abnormalities in infants with pyelonephritis. Pediatr Infect Dis J. 2001;20:597–601. doi: 10.1097/00006454-200106000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Goldman M, Lahat E, Strauss S, et al. Imaging after urinary tract infection in male neonates. Pediatrics. 2000;105:1232–5. doi: 10.1542/peds.105.6.1232. [DOI] [PubMed] [Google Scholar]
- 29.Sastre JB, Aparicio AR, Cotallo GD, et al. Urinary tract infection in the newborn: clinical and radio imaging studies. Pediatr Nephrol. 2007;22:1735–41. doi: 10.1007/s00467-007-0556-5. [DOI] [PubMed] [Google Scholar]
- 30.Khalesi N, Khosravi N, Jalali A, et al. Evaluation of maternal urinary tract infection as a potential risk factor for neonatal urinary tract infection. J Family Reprod Health. 2014;8:59–62. [PMC free article] [PubMed] [Google Scholar]
- 31.Milas V, Puseljić S, Stimac M, et al. Urinary tract infection (UTI) in newborns: risk factors, identification and prevention of consequences. Coll Antropol. 2013;37:871–6. [PubMed] [Google Scholar]
- 32.Littlewood JM. 66 infants with urinary tract infection in first month of life. Arch Dis Child. 1972;47:218–26. doi: 10.1136/adc.47.252.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levy I, Comarsca J, Davidovits M, et al. Urinary tract infection in preterm infants: the protective role of breastfeeding. Pediatr Nephrol. 2009;24:527–31. doi: 10.1007/s00467-008-1007-7. [DOI] [PubMed] [Google Scholar]
- 34.Levine DA, Platt SL, Dayan PS, et al. Risk of serious bacterial infection in young febrile infants with respiratory syncytial virus infections. Pediatrics. 2004;113:1728–34. doi: 10.1542/peds.113.6.1728. [DOI] [PubMed] [Google Scholar]
- 35.Shahian M, Rashtian P, Kalani M. Unexplained neonatal jaundice as an early diagnostic sign of urinary tract infection. Int J Infect Dis. 2012;16:e487–90. doi: 10.1016/j.ijid.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Pashapour N, Nikibahksh AA, Golmohammadlou S. Urinary tract infection in term neonates with prolonged jaundice. Urol J. 2007;4:91–4. [PubMed] [Google Scholar]
- 37.Garcia FJ, Nager AL. Jaundice as an early diagnostic sign of urinary tract infection in infancy. Pediatrics. 2002;109:846–51. doi: 10.1542/peds.109.5.846. [DOI] [PubMed] [Google Scholar]
- 38.Mutlu M, Cayir Y, Asian Y. Urinary tract infections in neonates with jaundice in their first two weeks of life. World J Pediatr. 2014;10:164–7. doi: 10.1007/s12519-013-0433-1. [DOI] [PubMed] [Google Scholar]
- 39.Xinias I, Demertzidou V, Mavroudi A, et al. Bilirubin levels predict renal cortical changes in jaundiced neonates with urinary tract infection. World J Pediatr. 2009;5:42–5. doi: 10.1007/s12519-009-0007-4. [DOI] [PubMed] [Google Scholar]
- 40.American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297–316. doi: 10.1542/peds.114.1.297. [DOI] [PubMed] [Google Scholar]
- 41.Fang SB, Lee HC, Yeung CY, et al. Urinary tract infections in young infants with prolonged jaundice. Acta Paediatr Taiwan. 2005;46:356–60. [PubMed] [Google Scholar]
- 42.Chen HT, Jeng MJ, Soong WJ, et al. Hyperbilirubinemia with urinary tract infection in infants younger than eight weeks old. J Chin Med Assoc. 2011;74:159–63. doi: 10.1016/j.jcma.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 43.Tebruegge M, Pantazidou A, Clifford V, et al. The age-related risk of co-existing meningitis in children with urinary tract infection. PLoS One. 2011;6:e26576. doi: 10.1371/journal.pone.0026576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foglia EE, Lorch SA. Clinical predictors of urinary tract infection in the neonatal intensive care unit. J Neonatal Perinatal Med. 2012;5:327–33. doi: 10.3233/NPM-1262812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karacan C, Erkek N, Senel S, et al. Evaluation of urine collection methods for the diagnosis of urinary tract infection in children. Med Princ Pract. 2010;19:188–91. doi: 10.1159/000273068. [DOI] [PubMed] [Google Scholar]
- 46.Tosif S, Baker A, Oakley E, et al. Contamination rates of different urine collection methods for the diagnosis of urinary tract infections in young children: an observational cohort study. J Paediatr Child Health. 2012;48:659–64. doi: 10.1111/j.1440-1754.2012.02449.x. [DOI] [PubMed] [Google Scholar]
- 47.Hoberman A, Wald ER. Urinary tract infections in young febrile children. Pediatr Infect Dis J. 1997;16:11–7. doi: 10.1097/00006454-199701000-00004. [DOI] [PubMed] [Google Scholar]
- 48.Crain EF, Gershel JC. Urinary tract infections in febrile infants younger than 8 weeks of age. Pediatrics. 1990;86:363–7. [PubMed] [Google Scholar]
- 49.Dukes C. The examination of urine for pus. Br Med J. 1928;1:391–3. doi: 10.1136/bmj.1.3505.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoberman A, Wald ER, Reynolds EA, et al. Pyuria and bacteriuria in urine specimens obtained by catheter from young children with fever. J Pediatr. 1994;124:513–9. doi: 10.1016/s0022-3476(05)83127-0. [DOI] [PubMed] [Google Scholar]
- 51.Shah AP, Cobb BT, Lower DR, et al. Enhanced versus automated urinalysis for screening of urinary tract infections in children in the emergency department. Pediatr Infect Dis J. 2014;33:272–5. doi: 10.1097/INF.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 52.Mori R, Yonemoto N, Fitzgerald A, et al. Diagnostic performance of urine dipstick testing in children with suspected UTI: a systematic review of relationship with age and comparison with microscopy. Acta Paediatr. 2010;99:581–4. doi: 10.1111/j.1651-2227.2009.01644.x. [DOI] [PubMed] [Google Scholar]
- 53.Glissmeyer EW, Korgenski EK, Wilkes J, et al. Dipstick screening for urinary tract infection in febrile infants. Pediatrics. 2014;133(5):e1121–7. doi: 10.1542/peds.2013-3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hasvold J, Bradford L, Nelson C, et al. Gentamicin resistance among Escherichia coli strains isolated in neonatal sepsis. J Neonatal Perinatal Med. 2013;6:173–7. doi: 10.3233/NPM-1365512. [DOI] [PubMed] [Google Scholar]
- 55.Shakir SM, Goldbeck JM, Robison D, et al. Genotypic and Phenotypic Characterization of Invasive Neonatal Escherichia coli Clinical Isolates. Am J Perinatol. 2014;31:975–82. doi: 10.1055/s-0034-1370341. [DOI] [PubMed] [Google Scholar]
- 56.Taheri PA, Navabi B, Shariat M. Neonatal urinary tract infection: clinical response to empirical therapy versus in vitro susceptibility at Bahrami Children's Hospital-Neonatal Ward: 2001–2010. Acta Med Iran. 2012;50:348–52. [PubMed] [Google Scholar]
- 57.Williamson JC, Craft DW, Butts JD, et al. In vitro assessment of urinary isolates of ampicillin-resistant enterococci. Ann Pharmacother. 2002;36:246–50. doi: 10.1345/aph.1A085. [DOI] [PubMed] [Google Scholar]
- 58.Laugel V, Kuhn R, Beladdale J, et al. Effects of antenatal antibiotics on the incidence and bacteriological profile of early-onset neonatal sepsis. A retrospective study over five years Biol Neonate. 2003;84:24–30. doi: 10.1159/000071439. [DOI] [PubMed] [Google Scholar]
- 59.Kuhn P, Dheu C, Bolender C, et al. Incidence and distribution of pathogens in early-onset neonatal sepsis in the era of antenatal antibiotics. Paediatr Perinat Epidemiol. 2010;24:479–87. doi: 10.1111/j.1365-3016.2010.01132.x. [DOI] [PubMed] [Google Scholar]
- 60.Glasgow TS, Young PC, Wallin J, et al. Association of intrapartum antibiotic exposure and late-onset serious bacterial infections in infants. Pediatrics. 2005;116:696–702. doi: 10.1542/peds.2004-2421. [DOI] [PubMed] [Google Scholar]
- 61.Benador D, Neuhaus TJ, Papazyan JP, et al. Randomised controlled trial of three day versus 10 day intravenous antibiotics in acute pyelonephritis: effect on renal scarring. Arch Dis Child. 2001;84:241–6. doi: 10.1136/adc.84.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cherry J, Demmler-Harrison GJ, Kaplan SL, et al. Feigin and Cherry's textbook of pediatric infectious diseases. Philadelphia: Elsevier Saunders; 2013. [Google Scholar]
- 63.Nowell L, Moran C, Smith PB, et al. Prevalence of renal anomalies after urinary tract infections in hospitalized infants less than 2 months of age. J Perinatol. 2010;30:281–5. doi: 10.1038/jp.2009.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Siomou E, Giapros V, Fotopoulos A, et al. Implications of 99mTc-DMSA scintigraphy performed during urinary tract infection in neonates. Pediatrics. 2009;124:881–7. doi: 10.1542/peds.2008-1963. [DOI] [PubMed] [Google Scholar]
- 65.Biyikli NK, Alpay H, Ozek E, et al. Neonatal urinary tract infections: analysis of the patients and recurrences. Pediatr Int. 2004;46:21–5. doi: 10.1111/j.1442-200X.2004.01837.x. [DOI] [PubMed] [Google Scholar]
- 66.Garin EH, Olavarria F, Garcia Nieto V, et al. Clinical significance of primary vesicoureteral reflux and urinary antibiotic prophylaxis after acute pyelonephritis: a multicenter, randomized, controlled study. Pediatrics. 2006;117:626–32. doi: 10.1542/peds.2005-1362. [DOI] [PubMed] [Google Scholar]
- 67.Hayashi Y, Kojima Y, Kamisawa H, et al. Is antibiotic prophylaxis effective in preventing urinary tract infections in patients with vesicoureteral reflux? Expert Rev Anti Infect Ther. 2010;8:51–8. doi: 10.1586/eri.09.111. [DOI] [PubMed] [Google Scholar]
- 68.Williams GJ, Wei L, Lee A, et al. Long-term antibiotics for preventing recurrent urinary tract infection in children. Cochrane Database Syst Rev. 2006;(19) doi: 10.1002/14651858.CD001534.pub2. CD001534. [DOI] [PubMed] [Google Scholar]
- 69.RIVUR Trial Investigators. Hoberman A, Greenfield SP, et al. Antimicrobial prophylaxis for children with vesicoureteral reflux. N Engl J Med. 2014;370:2367–76. doi: 10.1056/NEJMoa1401811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harris MC, Deuber C, Polin RA, et al. Investigation of apparent false-positive urine latex particle agglutination tests for the detection of group B streptococcus antigen. J Clin Microbiol. 1989;27:2214–7. doi: 10.1128/jcm.27.10.2214-2217.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Benjamin DK, Jr, Stoll BJ, Gantz MG, et al. Neonatal candidiasis: epidemiology, risk factors, and clinical judgment. Pediatrics. 2010;26:e865–73. doi: 10.1542/peds.2009-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cantey JB, Wozniak PS, Sánchez PJ. Prospective surveillance of antibiotic use in the neonatal intensive care unit: results from the SCOUT study. Pediatr Infect Dis J. 2014 doi: 10.1097/INF.0000000000000542. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


