Abstract
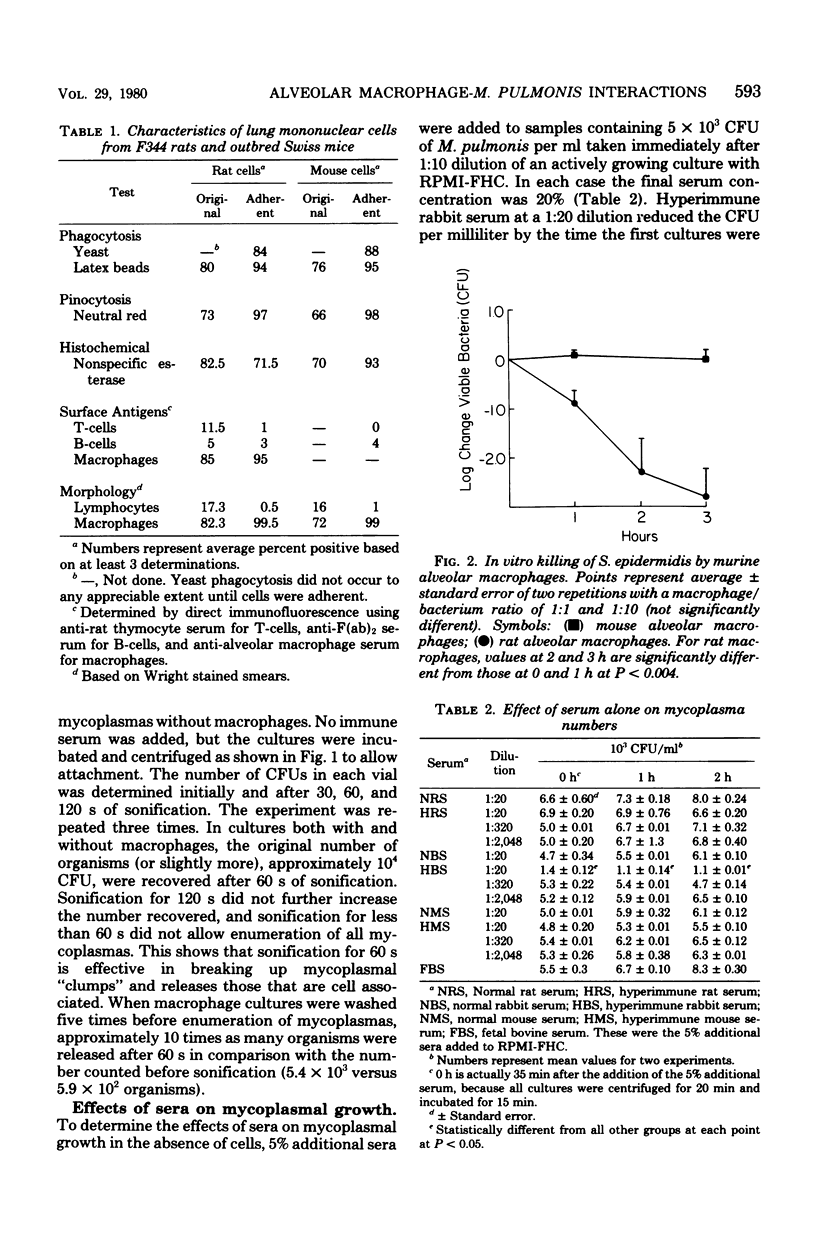
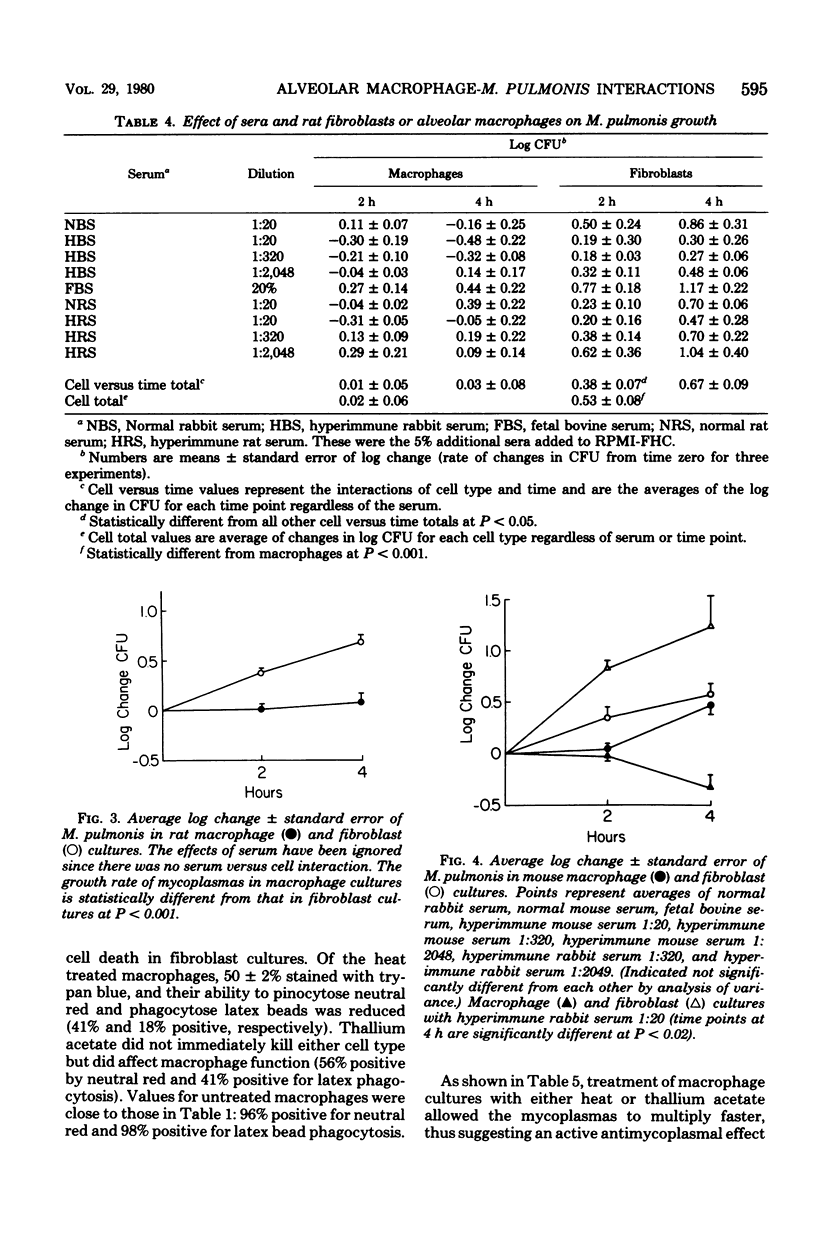
In comparison to syngeneic fibroblasts, alveolar macrophages collected from Fischer 344 rats demonstrated a significant ability to decrease the growth rate of cell-associated Mycoplasma pulmonis, even in the absence of specific actimycoplasmal antibodies. However, when exposed to thallium acetate (a cytotoxic heavy metal), macrophages supported growth of mycoplasmas almost as well as did untreated fibroblasts. This suggests an active antimycoplasmal process operative in untreated macrophages. In contrast, mouse alveolar macrophages were not capable of exerting an antimycoplasmal effect unless rabbit anti-M. pulmonis antibodies were present. Paradoxically, mouse anti-M. pulmonis antibodies did not promote this effect.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch C. M., Feldman J. D. Thymus-dependent (T) lymphocytes in the rat. J Immunol. 1974 Jan;112(1):79–86. [PubMed] [Google Scholar]
- Blakeslee D., Baines M. G. Immunofluorescence using dichlorotriazinylaminofluorescein (DTAF). I. Preparation and fractionation of labelled IgG. J Immunol Methods. 1976;13(3-4):305–320. doi: 10.1016/0022-1759(76)90078-8. [DOI] [PubMed] [Google Scholar]
- Broderson J. R., Lindsey J. R., Crawford J. E. The role of environmental ammonia in respiratory mycoplasmosis of rats. Am J Pathol. 1976 Oct;85(1):115–130. [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., WIENER E. THE PARTICULATE HYDROLASES OF MACROPHAGES. II. BIOCHEMICAL AND MORPHOLOGICAL RESPONSE TO PARTICLE INGESTION. J Exp Med. 1963 Dec 1;118:1009–1020. doi: 10.1084/jem.118.6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell G. H., Lindsey J. R., Baker H. J. Immune response of pathogen-free mice inoculated intranasally with Mycoplasma pulmonis. J Immunol. 1974 Jan;112(1):124–136. [PubMed] [Google Scholar]
- Cohn Z. A. The structure and function of monocytes and macrophages. Adv Immunol. 1968;9:163–214. doi: 10.1016/s0065-2776(08)60443-5. [DOI] [PubMed] [Google Scholar]
- Crum E. D., McGregor D. D. Functional properties of T and B cells isolated by affinity chromatography from rat thoracic duct lymph. Cell Immunol. 1976 May;23(2):211–222. doi: 10.1016/0008-8749(76)90187-8. [DOI] [PubMed] [Google Scholar]
- Del Giudice R. A., Robillard N. F., Carski T. R. Immunofluorescence identification of Mycoplasma on agar by use of incident illumination. J Bacteriol. 1967 Apr;93(4):1205–1209. doi: 10.1128/jb.93.4.1205-1209.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossum S. Non-specific acid esterase activity in rat lymphocytes. Scand J Immunol. 1978;8(4):273–277. doi: 10.1111/j.1365-3083.1978.tb00520.x. [DOI] [PubMed] [Google Scholar]
- Goding J. W. Conjugation of antibodies with fluorochromes: modifications to the standard methods. J Immunol Methods. 1976;13(3-4):215–226. doi: 10.1016/0022-1759(76)90068-5. [DOI] [PubMed] [Google Scholar]
- Hart M. M., Adamson R. H. Antitumor activity and toxicity of salts of inorganic group 3a metals: aluminum, gallium, indium, and thallium. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1623–1626. doi: 10.1073/pnas.68.7.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- Hemsworth G. R., Kochan I. Secretion of antimycobacterial fatty acids by normal and activated macrophages. Infect Immun. 1978 Jan;19(1):170–177. doi: 10.1128/iai.19.1.170-177.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz S. A., Cassell G. H. Detection of antibodies to Mycoplasma pulmonis by an enzyme-linked immunosorbent assay. Infect Immun. 1978 Oct;22(1):161–170. doi: 10.1128/iai.22.1.161-170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard C. J., Taylor G., Collins J., Gourlay R. N. Interaction of Mycoplasma dispar and Mycoplasma agalactiae subsp. bovis with bovine alveolar macrophages and bovine lacteal polymorphonuclear leukocytes. Infect Immun. 1976 Jul;14(1):11–17. doi: 10.1128/iai.14.1.11-17.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Fauci A. S. Immunological reactivity of the lung. I. A guinea pig model for the study of pulmonary mononuclear cell subpopulations. Cell Immunol. 1976 Sep;26(1):89–97. doi: 10.1016/0008-8749(76)90350-6. [DOI] [PubMed] [Google Scholar]
- Jones T. C., Hirsch J. G. The interaction in vitro of Mycoplasma pulmonis with mouse peritoneal macrophages and L-cells. J Exp Med. 1971 Feb 1;133(2):231–259. doi: 10.1084/jem.133.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. C., Yeh S., Hirsch J. G. Studies on attachment and ingestion phases of phagocytosis of Mycoplasma pulmonis by mouse peritoneal macrophages. Proc Soc Exp Biol Med. 1972 Feb;139(2):464–470. doi: 10.3181/00379727-139-36165. [DOI] [PubMed] [Google Scholar]
- Juers J. A., Rogers R. M., McCurdy J. B., Cook W. W. Enhancement of bactericidal capacity of alveolar macrophages by human alveolar lining material. J Clin Invest. 1976 Aug;58(2):271–275. doi: 10.1172/JCI108468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney J. F., Lawton A. R. B lymphocyte differentiation induced by lipopolysaccharide. I. Generation of cells synthesizing four major immunoglobulin classes. J Immunol. 1975 Sep;115(3):671–676. [PubMed] [Google Scholar]
- LEAKE E. S., GONZALEZ-OJEDA D., MYRVIK Q. N. ENZYMATIC DIFFERENCES BETWEEN NORMAL ALVEOLAR MACROPHAGES AND OIL-INDUCED PERITONEAL MACROPHAGES OBTAINED FROM RABBITS. Exp Cell Res. 1964 Feb;33:553–561. doi: 10.1016/0014-4827(64)90020-5. [DOI] [PubMed] [Google Scholar]
- LaForce F. M. Effect of alveolar lining material on phagocytic and bactericidal activity of lung macrophages against Staphylococcus aureau. J Lab Clin Med. 1976 Nov;88(5):691–699. [PubMed] [Google Scholar]
- Lindsey J. R., Baker H. J., Overcash R. G., Cassell G. H., Hunt C. E. Murine chronic respiratory disease. Significance as a research complication and experimental production with Mycoplasma pulmonis. Am J Pathol. 1971 Sep;64(3):675–708. [PMC free article] [PubMed] [Google Scholar]
- Lindsey J. R., Cassell H. Experimental Mycoplasma pulmonis infection in pathogen-free mice. Models for studying mycoplasmosis of the respiratory tract. Am J Pathol. 1973 Jul;72(1):63–90. [PMC free article] [PubMed] [Google Scholar]
- Melnick R. L., Monti L. G., Motzkin S. M. Uncoupling of mitochondrial oxidative phosphorylation by thallium. Biochem Biophys Res Commun. 1976 Mar 8;69(1):68–73. doi: 10.1016/s0006-291x(76)80273-2. [DOI] [PubMed] [Google Scholar]
- Montfort I., Pérez Tamayo R. Two antigenically different types of macrophages. Proc Soc Exp Biol Med. 1971 Oct;138(1):204–207. doi: 10.3181/00379727-138-35862. [DOI] [PubMed] [Google Scholar]
- Morland B., Kaplan G. Macrophage activation in vivo and in vitro. Exp Cell Res. 1977 Sep;108(2):279–288. doi: 10.1016/s0014-4827(77)80035-9. [DOI] [PubMed] [Google Scholar]
- OREN R., FARNHAM A. E., SAITO K., MILOFSKY E., KARNOVSKY M. L. Metabolic patterns in three types of phagocytizing cells. J Cell Biol. 1963 Jun;17:487–501. doi: 10.1083/jcb.17.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAVILLARD E. R. In vitro phagocytic and bactericidal ability of alveolar and peritoneal macrophages of normal rats. Aust J Exp Biol Med Sci. 1963 Jun;41:265–274. doi: 10.1038/icb.1963.26. [DOI] [PubMed] [Google Scholar]
- Portugalov V. V., Durnova G. N., Kaplansky A. S., Kolganova N. S. Cytochemical investigations of the metabolism of alveolar and peritoneal macrophages. Acta Histochem. 1973;46(2):216–226. [PubMed] [Google Scholar]
- Powell D. A., Clyde W. A., Jr Opsonin-reversible resistance of Mycoplasma pneumoniae to in vitro phagocytosis by alveolar macrophages. Infect Immun. 1975 Mar;11(3):540–550. doi: 10.1128/iai.11.3.540-550.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch M., Manejias R. E., Russo M., Abbey E. E. Increased spreading of macrophages from mice treated with interferon inducers. Cell Immunol. 1977 Mar 1;29(1):86–95. doi: 10.1016/0008-8749(77)90277-5. [DOI] [PubMed] [Google Scholar]
- Saito M., Nakagawa M., Muto T., Imaizumi K. Strain difference of mouse in susceptibility to Mycoplasma pulmonis infection. Nihon Juigaku Zasshi. 1978 Dec;40(6):697–705. doi: 10.1292/jvms1939.40.697. [DOI] [PubMed] [Google Scholar]
- Skulskii I. A., Manninen V., Järnefelt J. Interaction of thallous ions with the cation transport mechanism in erythrocytes. Biochim Biophys Acta. 1973 Mar 29;298(3):702–709. doi: 10.1016/0005-2736(73)90086-2. [DOI] [PubMed] [Google Scholar]
- Southern P. M., Jr, Pierce A. K., Sanford J. P. Comparison of the pulmonary bactericidal capacity of mice and rats against strains of Pseudomonas aeruginosa. Appl Microbiol. 1971 Feb;21(2):377–378. [PubMed] [Google Scholar]
- Stewart C. C., Lin H. S., Adles C. Proliferation and colony-forming ability of peritoneal exudate cells in liquid culture. J Exp Med. 1975 May 1;141(5):1114–1132. doi: 10.1084/jem.141.5.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J. S., Watanakunakorn C., Phair J. P. A modified assay of neutrophil function: use of lysostaphin to differentiate defective phagocytosis from impaired intracellular killing. J Lab Clin Med. 1971 Aug;78(2):316–322. [PubMed] [Google Scholar]
- Taylor-Robinson D., Williams M. H., Haig D. A. The isolation and comparative biological and physical characteristics of T-mycoplasmas of cattle. J Gen Microbiol. 1968 Nov;54(1):33–46. doi: 10.1099/00221287-54-1-33. [DOI] [PubMed] [Google Scholar]
- Tucker S. B., Pierre R. V., Jordon R. E. Rapid identification of monocytes in a mixed mononuclear cell preparation. J Immunol Methods. 1977;14(3-4):267–269. doi: 10.1016/0022-1759(77)90137-5. [DOI] [PubMed] [Google Scholar]
- Werb Z., Cohn Z. A. Plasma membrane synthesis in the macrophage following phagocytosis of polystyrene latex particles. J Biol Chem. 1972 Apr 25;247(8):2439–2446. [PubMed] [Google Scholar]
- van oud Alblas A. B., van Furth R. Origin, Kinetics, and characteristics of pulmonary macrophages in the normal steady state. J Exp Med. 1979 Jun 1;149(6):1504–1518. doi: 10.1084/jem.149.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]


