Abstract
Reconstructive surgery following skin tumor resection can be challenging. Treatment options after removing the tumor are skin grafting, local pedicled and axial flaps, or microsurgery for complex and extensive wounds correction. Recently, the use of dermal substitutes has been extended to reconstructive surgery in cutaneous oncology. Objectives. To report both a single-center experience using dermal substitutes in reconstructive surgery for skin malignancies and reconstructive surgery's outcomes. Methods and Results. Among thirteen patients, seven (53.8%) were male with mean age of 62.6 years. Regarding diagnosis, there were five cases (38.5%) of basal cell carcinoma (BCC), two (15.4%) of melanoma in situ, two (15.4%) of dermatofibrosarcoma protuberans, one (7.7%) of squamous cell carcinoma (SCC), one (7.7%) of angiosarcoma, and one (7.7%) of eccrine carcinoma (EC). The most common site of injury was scalp (53.8%) and lower limbs (23.1%). Seven (53.8%) patients used NPWT and six (46.2%) patients underwent Brown's dressing. The most frequent complication of the first stage was wound contamination (38.5%). Average time to second-stage skin grafting was 43.9 days. Three (23%) patients developed tumor recurrence and one died. Conclusions. Use of dermal substitutes in oncology can be an option for reconstruction after extended resections, providing good aesthetical and functional results.
1. Background
Reconstructive surgery following skin tumor resection can be particularly challenging due to the nature of disease process, including the extent of resection, need for preserving original surgical margin orientation, possibility of tumor recurrence, and requirement for adjuvant radiation therapy. Moreover, following tumor resection, reconstruction must be tailored to clinical prognosis, treatment plan, patient's age, and desired cosmesis [1].
Treatment options after tumor removal are skin grafting, local pedicled and axial flaps, or microsurgery for complex and extensive wounds correction. Each technique has not only its advantages but also its limitations.
Dermal substitutes such as Integra® and Matriderm® were initially used for treating burns and were later extended for reconstructive surgeries and treatment of chronic wounds [1–5].
Integra (LifeSciences Corporation, NJ, USA) is a bilaminate synthetic construct consisting of an outer silicone layer and an inner collagen-glycosaminoglycan matrix. The first layer is a matrix of bovine collagen and chondroitin-6-sulfate, cross-linked with glutaraldehyde; after grafting, recipient fibroblasts infiltrate the matrix network and synthetize a neodermis, which is histologically very close to normal human dermis. The second layer is a silicone membrane, which acts as a temporary epidermis [6, 7].
Matriderm (Dr. Suwelack Skin & Health Care AG, Billerbeck, Germany) is a single layer composed of bovine collagen and elastin. The collagen promotes rapid cell migration, proliferation, and revascularization and the elastin encourages early neoangiogenesis and elastin synthesis [8, 9].
More recently, Matriderm and Integra use in oncology and reconstructive surgery has also been reported [10–12]. These reports show no difference in oncological outcomes and are technically feasible. In addition, good aesthetical results have been achieved.
2. Objectives
We aim to report a single-center experience using dermal substitutes in reconstructive surgery for skin malignancies and the outcomes of those surgeries.
3. Patients and Methods
We present the report of thirteen patients treated in the Skin Cancer Department of AC Camargo Cancer Center, São Paulo/SP, Brazil, who underwent reconstructive surgery with artificial dermis between December 2012 and November 2016. All patients were informed before surgeries, and an informed written consent was provided.
Clinical data was analyzed regarding age, tumor type and location, tumor size in pathology report, comorbidities, postoperative complications, and recurrence. Reconstruction was carried out in a two-stage process and the time interval between them was also analyzed. One patient underwent only first-stage surgery, due to complete epithelialization. Another patient is still waiting grafting after multiple debridement surgeries of devitalized and necrotic tissue.
First stage involved removal of tumor and application of artificial dermis. Frozen section exam was performed to ensure clear margins, except both in DFSP cases, in which circumferential and deep margin assessment is the standard of care, and in melanoma cases. First stage was followed by Brown's dressing or Negative Pressure Wound Therapy (NPWT) (Figure 1). Our intention was to use NPWT in every patient. However, some of them could not use it due to local pain or in some specific locations such as nail unity. Besides that, some patients had no coverage of their health insurances to this product.
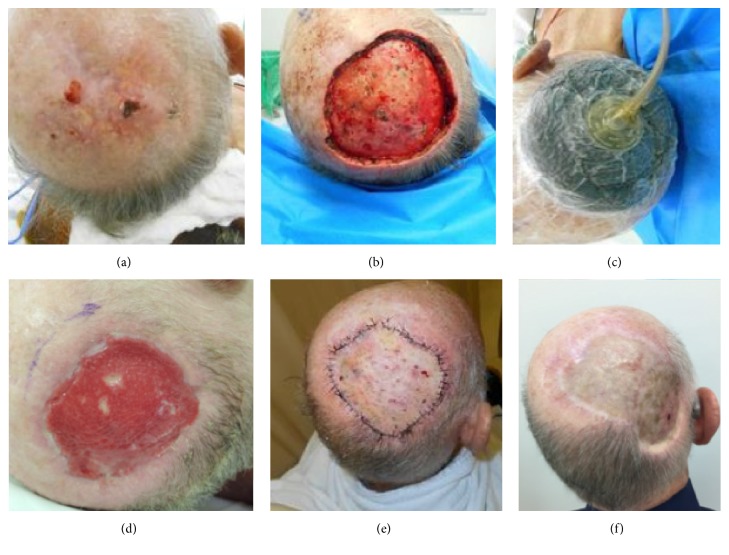
Figure 1.
(a) Basal cell carcinoma in scalp, clinical aspect before surgery; (b) surgical bed with periosteal removal; (c) negative pressure wound therapy was applied after Matriderm to enhance granulation; (d) granulation tissue; (e) skin graft, early postoperative aspect; (f) late postoperative aspect.
Brown's dressing was removed 5 to 7 days after surgery and new dressings were performed three times a week with Mepilex Ag™ (Mölnlycke Health Care, Göteborg, Sweden) or PolyMem Silver™ (Ferris Mfg. Corp., TX, USA) to help control local contamination. Patients with NPWT were evaluated weekly, following the same procedures as mentioned below. Wound was inspected to assess for contamination or local complications until a well-vascularized neodermis was achieved. Second stage was only performed when the wound showed adequate granulation tissue and subsequently covered with a split-thickness skin graft.
Clinical follow-up of patients was done in outpatient clinics periodically to assess treatment plan, tumor recurrence, and durability of the reconstruction.
4. Results
Clinical data is summarized in Table 1. Mean age was 62.6 years (range 39 to 86 years). Cohort comprised seven (53.8%) male and six (46.2%) female patients. Only two patients had no comorbidities. The most common comorbidities were high blood pressure (46.2%), heart disease (30.8%), former smoking (30.8%), hypothyroidism (23.1%), diabetes mellitus (15.4%), and smoking (7.7%).
Table 1.
Clinical features and outcomes summary of the patients who underwent reconstructive surgery with dermal substitutes in AC Camargo Cancer Center, from 2012 to 2016 (M = male, F = female, EC = eccrine carcinoma, DFSP = dermatofibrosarcoma protuberans, BCC = basal cell carcinoma, SCC = squamous cell carcinoma, MMis = malignant melanoma in situ, STSG = split-thickness skin graft, N/A = not available, and ∗ size according to pathology reports, without considering further excisions after frozen section when needed).
| Patient | Age | Sex | Abnormality | Tumor location | Size (cm) | Comorbidity | Dressing | Complications after 1st surgery | Application of STSG (days) | Complications after 2nd surgery | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 64 | M | EC | Scalp | 20,5 × 20 | Hypothyroidism Heart disease Rheumatoid arthritis |
Brown | Wound secretion/debridement | 28 | Partial graft loss | Death |
|
| |||||||||||
| 2 | 56 | M | DFSP | Scalp | 14 × 13 | High blood pressure Obesity Former smoking |
Vacuum | Wound secretion | 50 | — | No recurrence |
|
| |||||||||||
| 3 | 84 | F | BCC sclerodermiform | Lower limbs | 13,8 × 8,6 | Heart disease Former smoking Alzheimer's disease |
Brown | — | 29 | — | No recurrence |
|
| |||||||||||
| 4 | 51 | M | BCC sclerodermiform | Scalp | N/A | — | Vacuum | Wound secretion | 50 | — | Parotid metastasis In transit disease |
|
| |||||||||||
| 5 | 56 | M | BCC sclerodermiform | Nose | 5 × 4,5 | High blood pressure Smoking |
Brown | Wound secretion | 57 | Partial graft loss | Lost |
|
| |||||||||||
| 6 | 65 | M | MMis | Digital | 3,5 × 2,5 | Former smoking Chronic obstructive pulmonary diseases |
Brown | — | Unnecessary | — | Lost |
|
| |||||||||||
| 7 | 75 | M | BCC sclerodermiform | Scalp | 8 × 5 | High blood pressure Former smoking |
Vacuum | — | 41 | — | Recurrence at 20 months |
|
| |||||||||||
| 8 | 50 | F | DFSP | Scalp | 7 × 5,5 | — | Brown | Wound secretion | 35 | — | Lost |
|
| |||||||||||
| 9 | 77 | F | Radionecrosis | Lower limbs | N/A | High blood pressure Heart disease Hypothyroidism |
Vacuum | Slow granulation | Not grafted | — | No recurrence |
|
| |||||||||||
| 10 | 41 | F | MMis | Digital | 3 × 3 | Hypothyroidism | Brown | — | 50 | — | No recurrence |
|
| |||||||||||
| 11 | 39 | F | SCC | Lower limbs | 13 × 6 | High blood pressure, diabetes mellitus, multiple sclerosis | Vacuum | — | 36 | — | No recurrence |
|
| |||||||||||
| 12 | 70 | M | Angiosarcoma | Scalp | 5,2 × 4,5 | Parkinson's disease | Vacuum | — | 43 | — | No recurrence |
|
| |||||||||||
| 13 | 86 | F | BCC sclerodermiform | Scalp | 12 × 12 | High blood pressure Heart disease Diabetes mellitus Renal chronic failure |
Vacuum | — | 64 | — | No recurrence |
M = male, F = female, EC = eccrine carcinoma, DFSP = dermatofibrosarcoma protuberans, BCC = basal cell carcinoma, SCC = squamous cell carcinoma, MMis = malignant melanoma in situ, STSG = split-thickness skin graft, and N/A = not available.
Regarding diagnosis, five (38.5%) patients presented with basal cell carcinoma (BCC), all of them with sclerodermiform subtype, two (15.4%) with melanoma in situ, two (15.4%) with dermatofibrosarcoma protuberans, one (7.7%) with eccrine carcinoma, one (7.7%) with squamous cell carcinoma, and one (7.7%) with angiosarcoma. One patient presented with radionecrosis after treatment for malignant fibrohistiocytoma and another used dermal substitutes in the donor area of a frontal skin flap. The most common site of injury was the scalp (53.8%) followed by lower limbs (23.1%) and fingers (15.4%).
Only one patient used Integra. All the others used Matriderm 2 mm. Seven (46.2%) patients used NPWT after first surgery. However, one patient could not tolerate NPWT and had it removed after first week due to local pain. Six (46.2%) patients underwent Brown's dressing.
Average time to second-stage skin grafting was 43.9 days (range 28 to 64 days). The most frequent complication of first stage was wound contamination (38.5%), which was treated with Mepilex Ag or PolyMem Silver and oral antibiotics. Regarding the second stage, partial loss of the graft occurred twice, treated with the same dressing.
Three (23%) patients developed tumor recurrence. And among patients who underwent skin grafting, two (18.2%) experienced partial loss of the graft. One patient did not require a second-stage reconstructive surgery and the other one is still unable to undergo grafting. One patient died due to disease progression. Three patients were lost to follow-up.
Radionecrosis patient's granulation process in surgical bed was slow, which made grafting unfeasible after first surgery. This patient required other surgeries for debridement of devitalized and necrotic tissue. Currently, granulation tissue presents good aspect and skin grafting is being scheduled.
5. Discussion
Reconstructive surgery plays a very important role in cutaneous oncology. Several skin malignancies may lead to complex defects and, therefore, complex reconstructions.
Skin graft may be the simplest option. It is associated with minimal donor-site morbidity and is cost-effective but can be prone to contraction and suboptimal aesthetic appearance [13–15]. In particular skin graft is unreliable on the previously irradiated wound bed and should be avoided whenever there are bones, tendons, and nerves exposed [6].
Locoregional flaps particularly in the scalp and lower limbs can cover exposed bone or tendons but are limited by the size of the defect. Also, the donor site may require grafting. Previous irradiation of tissues can lead to poor take of the flap or delayed healing. Local pedicled and axial flaps are more tolerant to therapeutic radiation. Moreover, the choice of free flap requires the presence of a long vascular pedicle and an adequate surface area of the flap [1].
Free tissue microvascular reconstruction of oncological defects, particularly in larger defects, remains the gold standard for covering large tissue defects, with successful transplant rates ranging from 95 to 99 percent. However, the application of either free or pedicled vascularized tissue transfer is associated with substantial donor-site morbidity, prolonged operative time, and hospital stay [1]. Also, it requires appropriate equipment and enabled professionals.
Artificial dermis is an alternative for the treatment of complex wounds, as it allows their closure with less morbidity and surgical time. Artificial dermis offers lower wound contraction, improved elasticity, and skin thickness in relation to grafts. It is also a simple procedure when compared to microsurgical flap and can be performed in previously irradiated areas, allowing wound cover in complex resection areas, better local control of the disease, and early detection of recurrence. The need for experienced surgeons, weekly returns to dressing change, high product cost, and inability to use artificial dermis on wounds with exposed dura mater [5, 7, 10] can be pointed out as disadvantages.
In summary, the choice of reconstruction is influenced either by patient factors such as age, coexisting medical conditions, length of procedure, and performance for general anaesthesia, as by surgeon's experience and surgical equipment, and/or material availability. Our experience with dermal substitutes is presented since we believe it is a well-tolerated option for the patient, with no prejudice to oncologic principles (Figure 2). We used it in more aggressive tumors such as angiosarcoma and eccrine carcinoma, and both can be easily followed up either by clinical examination or by radiologic exams.
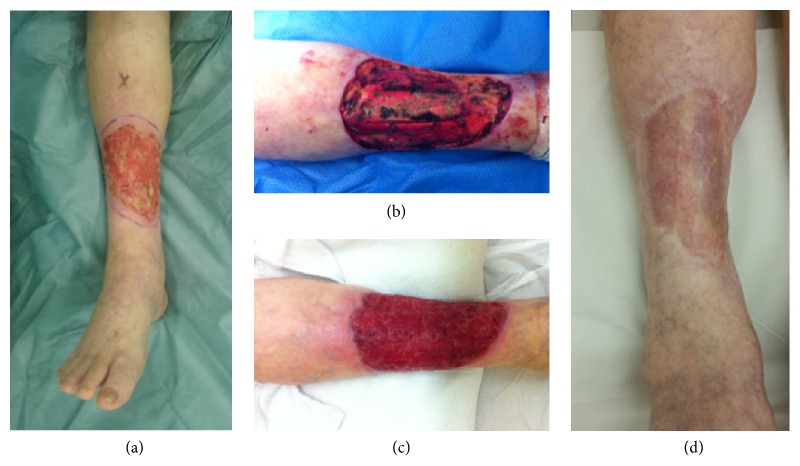
Figure 2.
(a) Basal cell carcinoma in right leg, clinical aspect before surgery; (b) periosteal removal was necessary to ensure deep margins, which would not allow skin graft; (c) granulation tissue 28 days after Matriderm was placed (no negative pressure wound therapy was used in this case); (d) late postoperative aspect.
There were three recurrences in our cohort. One of them, the eccrine carcinoma (patient 1), died due to complications of chemotherapy after disease progression. The second case experienced recurrence in scalp and underwent salvage surgery. The third one relapsed in transit disease and parothid and is currently on Vismodegib. They were both diagnosed with sclerodermiform BCC with perineural invasion.
Regarding clinical associated conditions, smoking and diabetes mellitus are known to have higher rate of surgical complications. In this study, the only smoking patient had no wound contamination, progressing with partial loss of the graft after second surgery. Longer dressing care was needed to this patient without prejudice to the aesthetic and functional results. Patients with diabetes mellitus or former smoking showed no surgical complication. No patient had prohibitive risk for anesthetic procedure.
In our data, there were five cases of wound contamination at the first stage. One patient had purulent discharge and necrotic wound, treated with debridement, washing, and dressing with Mepilex Ag or PolyMem Silver and oral antibiotics. Grafting was undergone successfully, but after five months, the patient developed tumor recurrence (eccrine carcinoma) and once again underwent resection of the tumor and reconstruction with regional flap.
The other four patients had only serohematic/serous secretion and underwent only dressing changes before second-stage surgery. Eleven patients underwent grafting in the second stage and only 2 (18.2%) had partial graft loss, but without aesthetic or functional impairment.
In our cohort, the most frequent skin malignancies were sclerodermiform BCC and DFSP, which are frequently associated with wide excisions due to the risk of local recurrence [16]. We also had the scalp as the most prevalent body region, which may also lead to a more challenging reconstruction.
Scalp defects can also be covered using delayed local flaps and tissue expansion. However, in the oncological patient who requires resection, tissue expansion should be used carefully as a reconstructive option since the tumor resection is not delayed because of use of the expansion [6, 17].
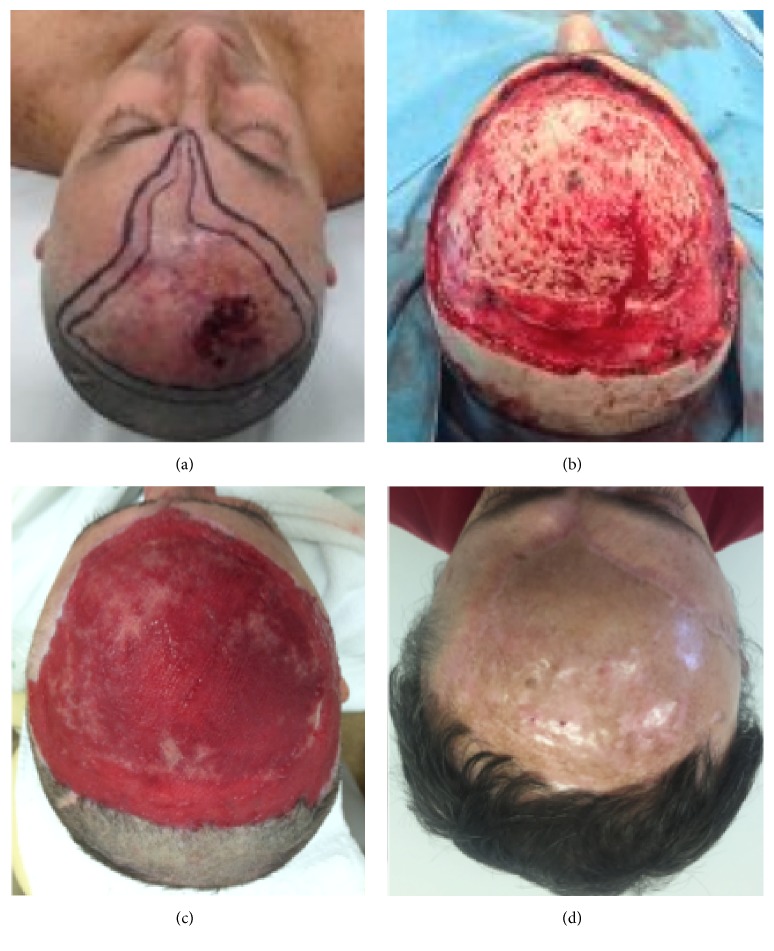
As an example, we can mention patients 4 (Figure 3) and 7 (Figure 1) who underwent resection of BCC on the scalp. In both patients, wide excision of the tumor was performed, which was more extensive in patient 4, who needed to have the bone drilled due to involvement of the periosteum.
Figure 3.
(a) Basal cell carcinoma, clinical aspect before surgery; (b) once again, periosteal removal was necessary; (c) granulation tissue (negative pressure wound therapy was used); (d) late postoperative aspect.
As reported in other studies, it is known that bone drilling is an important tool used in periosteal involvement of cases to ensure safety margin of tumor resection and even in cases of extensive lesions providing vascularized area to the fixing template. In our data, five (71.4%) patients underwent drilling of scalp injury, due to periosteum involvement by the tumor, to hasten granulation process. The two remaining (28.6%) patients required only periosteum excision.
Two patients had in situ acral melanoma. Digital sparing surgery in this situation can be an option. However, in this scenario, reconstruction after wide local excision can be associated with functional loss or chronic pain. In such cases, dermal matrix plays an important role as it provides satisfactory aesthetic and functional outcomes [12]. In both cases, amputation could be avoided and the patients report good quality of life after surgery.
Fixing the artificial dermis in the surgical bed can be done either with Brown's dressing or with NPWT. Brown's dressing promotes intimate contact of template with bloody area, providing adequate fixation thereof to wound with tissue neovascularization and granulation tissue. In our routine, we evaluate granulation tissue 3 times a week and Mepilex Ag or Polymem Silver dressings are changed whenever necessary. NPWT can help remove deleterious substances from the wound, relieve edema, and stimulate cell proliferation, thereby promoting granulation and inhibiting chronic inflammation. Furthermore, NPWT also seems to assist in neovascularization of skin grafts and tissue-engineered skin substitutes and can improve the success rate of skin grafting by strengthening bonding between the skin graft and the recipient area. Additionally, NPWT must be evaluated weekly [18].
NPWT was used on 5 patients with lesions of scalp due to extension of the lesion in order to enhance granulation and subsequent grafting. The average time for grafting in these patients was 49.6 days. Also, we used NPWT in 2 patients with lower limbs injury. One patient did not tolerate the pain and the other patient could undergo grafting in 36 days. One patient who underwent resection of a nail melanoma and used Brown's dressing did not require grafting, due to complete epithelialization after 90 days. Average time for grafting in five patients with Brown's dressing was 39.8 days.
There are evidences in literature that the use of vacuum dressing decreases the time required for formation of granulation tissue and subsequent grafting [19–21]. However, in our cohort, patients who used NPWT took on average 47.3 days to undergo the second surgery compared to patients who used Brown's dressing with an average of 39.8 days.
We believe that this can be explained by the greater extension of the lesions and the exposed bone. Although our sample is insufficient for statistical validation, we also believe that, even in the absence or inability of NPWT use, Brown's dressing is an acceptable option for the use of dermal substitutes in the reconstructive setting, especially in smaller defects.
Two patients did not achieve second surgery. One patient had complete wound epithelialization with artificial dermis. The other one presented with a complex wound secondary to radionecrosis in her leg after radiotherapy for a malignant fibrohistiocytoma treatment.
She underwent three surgical procedures for debridement of devitalized and necrotic tissue, requiring resection of the exposed necrotic tendon in the last procedure. Subsequent placement of dermal matrix was performed in all surgical approaches to enhance the formation of feasible tissue to carry out grafting. After her last surgery, the patient has been developing good but still slow formation of granulation tissue (Figure 4). Skin graft is to be scheduled.
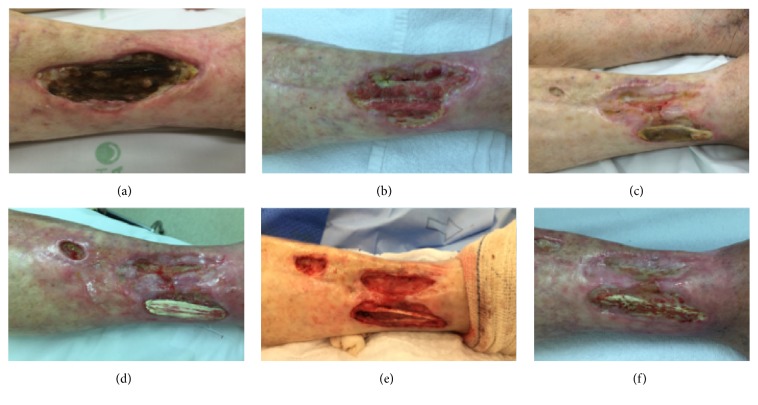
Figure 4.
(a) Radionecrosis in right leg, clinical aspect before first surgery; ((b) and (c)) there was no granulation in the area of exposed and necrotic tendon, which led to ((d) and (e)) debridement and removal of necrotic tendon. This was the third time that Matriderm was placed; (f) clinical aspect, waiting for skin graft.
There is no report in literature of dermal substitutes use in radionecrosis. However, we believe that, in these complex wounds, dermal matrix could be an ally in its treatment. It could enhance granulation tissue formation for subsequent grafting with an adequate aesthetic and functional result.
We present similar data to what is reported in literature. The use of dermal substitutes in oncology can be an option for reconstruction after extended resections, providing good aesthetical and functional results. Therefore, it can be performed within a shorter period of time, which is useful in patients with clinical comorbidities, such as the ones we had in our patients. Further studies are necessary to validate this procedure.
Disclosure
There was no funding or grants of any kind in the elaboration of this paper.
Conflicts of Interest
None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
Authors' Contributions
Mariane Campagnari, Eduardo Bertolli, Eduard R. Brechtbühl, and João P. Duprat Neto were responsible for performing the surgeries mentioned on this paper and for patients' follow-up. Andrea S. Jafelicci and Helio A. Carneiro participated on these procedures and were responsible for collecting the data here presented. All authors were responsible for writing and reviewing this manuscript.
References
- 1.Komorowska-Timek E., Gabriel A., Bennett D. C., et al. Artificial dermis as an alternative for coverage of complex scalp defects following excision of malignant tumors. Plastic and Reconstructive Surgery. 2005;115(4):1010–1017. doi: 10.1097/01.PRS.0000154210.60284.C6. [DOI] [PubMed] [Google Scholar]
- 2.Choi J.-Y., Kim S.-H., Oh G.-J., Roh S.-G., Lee N.-H., Yang K.-M. Management of defects on lower extremities with the use of Matriderm and skin graft. Archives of Plastic Surgery. 2014;41(4):337–343. doi: 10.5999/aps.2014.41.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parren L. J. M. T., Ferdinandus P., Hulst V. D., Frank J., Tuinder S. A novel therapeutic strategy for turban tumor : scalp excision and combined reconstruction with artificial dermis and split skin graft. Dermatologic Surgery. 2013:246–249. doi: 10.1111/ijd.12199. [DOI] [PubMed] [Google Scholar]
- 4.Rehim S. A., Singhal M., Chung K. C. Dermal skin substitutes for upper limb reconstruction: Current status, indications, and contraindications. Hand Clinics. 2014;30(2):239–252. doi: 10.1016/j.hcl.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Unglaub F., Ulrich D., Pallua N. Reconstructive surgery using an artificial dermis (Integra): results with 19 grafts. Zentralblatt für Chirurgie. 2005;130:157–61. doi: 10.1055/s-2005-836367. [DOI] [PubMed] [Google Scholar]
- 6.Chalmers R. L., Smock E., Geh J. L. C. Experience of Integra® in cancer reconstructive surgery. Journal of Plastic, Reconstructive and Aesthetic Surgery. 2010;63:2081–2090. doi: 10.1016/j.bjps.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 7.Dantzer E., Braye F. M. Reconstructive surgery using an artificial dermis (Integra): results with 39 grafts. British Journal of Plastic Surgery. 2001;54:659–664. doi: 10.1054/bjps.2001.3684. [DOI] [PubMed] [Google Scholar]
- 8.De Vries H. J. C., Zeegelaar J. E., Middelkoop E., et al. Reduced wound contraction and scar formation in punch biopsy wounds. Native collagen dermal substitutes. A clinical study. British Journal of Dermatology. 1995;132(5):690–697. doi: 10.1111/j.1365-2133.1995.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 9.Böttcher-Haberzeth S., Biedermann T., Schiestl C., et al. Matriderm® 1 mm versus Integra® Single Layer 1.3 mm for one-step closure of full thickness skin defects: A comparative experimental study in rats. Pediatric Surgery International. 2012;28(2):171–177. doi: 10.1007/s00383-011-2990-5. [DOI] [PubMed] [Google Scholar]
- 10.Bertolli E., Campagnari M., Molina A. S., et al. Artificial dermis (Matriderm®) followed by skin graft as an option in dermatofibrosarcoma protuberans with complete circumferential and peripheral deep margin assessment. International Wound Journal. 2015;12(5):545–547. doi: 10.1111/iwj.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tufaro A. P., Buck D. W., Fischer A. C. The use of artificial dermis in the reconstruction of oncologic surgical defects. Plast. Reconstr. Surg. 2007;120:638–46. doi: 10.1097/01.prs.0000270298.68331.8a. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi K., Uhara H., Koga H., Okuyama R., Saida T. Surgical treatment of nail apparatus melanoma in situ: the use of artificial dermis in reconstruction. Dermatologic Surgery. 2012;38:692–694. doi: 10.1111/j.1524-4725.2011.02217.x. [DOI] [PubMed] [Google Scholar]
- 13.Bloemen M. C. T., Van Leeuwen M. C. E., Van Vucht N. E., Van Zuijlen P. P. M., Middelkoop E. Dermal substitution in acute burns and reconstructive surgery: A 12-year follow-up. Plastic and Reconstructive Surgery. 2010;125(5):1450–1459. doi: 10.1097/PRS.0b013e3181d62b08. [DOI] [PubMed] [Google Scholar]
- 14.Shores J. T., Gabriel A., Gupta S. Skin substitutes and alternatives: a review. Advances in skin & wound care. 2007;20(9):493–510. doi: 10.1097/01.ASW.0000288217.83128.f3. [DOI] [PubMed] [Google Scholar]
- 15.Stekelenburg C. M., Marck R. E., Tuinebreijer W. E., De Vet H. C. W., Ogawa R., Van Zuijlen P. P. M. A Systematic Review on Burn Scar Contracture Treatment: Searching for Evidence. Journal of Burn Care and Research. 2015;36(3):e153–e161. doi: 10.1097/BCR.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 16.Saiag P., Grob J.-J., Lebbe C., et al. Diagnosis and treatment of dermatofibrosarcoma protuberans. European consensus-based interdisciplinary guideline. European Journal of Cancer. 2015;51(17):2604–2608. doi: 10.1016/j.ejca.2015.06.108. [DOI] [PubMed] [Google Scholar]
- 17.Bertolli E., Bretchbuhl E. R., Camarço W. R., et al. Dermatofibrosarcoma protuberans of the vulva: Margins assessment and reconstructive options - A report of two cases. World Journal of Surgical Oncology. 2014;12(1, article no. 399) doi: 10.1186/1477-7819-12-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang C., Liu D., Liang Z., Liu F., Lin H., Guo Z. Repair of refractory wounds through grafting of artificial dermis and autologous epidermis aided by vacuum-assisted closure. Aesthetic Plastic Surgery. 2014;38(4):727–732. doi: 10.1007/s00266-014-0341-3. [DOI] [PubMed] [Google Scholar]
- 19.Molnar J. A., DeFranzo A. J., Hadaegh A., Morykwas M. J., Shen P., Argenta L. C. Acceleration of integra incorporation in complex tissue defects with subatmospheric pressure. Plastic and Reconstructive Surgery. 2004;113(5):1339–1346. doi: 10.1097/01.PRS.0000112746.67050.68. [DOI] [PubMed] [Google Scholar]
- 20.Eo S., Kim Y., Cho S. Vacuum-assisted closure improves the incorporation of artificial dermis in soft tissue defects: Terudermis® and Pelnac®. International Wound Journal. 2011;8(3):261–267. doi: 10.1111/j.1742-481X.2011.00780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinna et al. R. Role of the association artificial dermis and negative pressure therapy: about two cases. Annales de Chirurgie Plastique et Esthétique. 2009;54:582–587. doi: 10.1016/j.anplas.2009.02.003. [DOI] [PubMed] [Google Scholar]