Abstract
Objectives
Genetic and nonshared environmental factors (experienced by 1 family member to the exclusion of the others) have been strongly implicated in the causes of attention-deficit hyperactivity disorder (ADHD). Pregnancy, labour/delivery and neonatal complications (PLDNC) have often been associated with ADHD; however, no investigations aimed at delineating the shared or nonshared nature of these factors have been reported. We aimed to identify those elements of the PLDNC that are more likely to be of a nonshared nature.
Methods
We used an intrafamily study design, comparing the history of PLDNC between children diagnosed with ADHD, according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV), and their unaffected siblings. Children with ADHD were recruited from the outpatient, day-treatment program of the Child Psychiatry Department, Douglas Hospital, Montréal. The unaffected sibling closest in age to the child with ADHD was used as a control. The history of PLDNC was assessed using the Kinney Medical and Gynecological Questionnaire and the McNeil–Sjöstrom Scale for both children with ADHD and their siblings. Seventy children with ADHD along with 50 of their unaffected siblings agreed to participate in the study. Child Behavior Checklist (CBCL), Continuous Performance Test (CPT) and Restricted Academic Situation Scale (RASS) scores were also used as measures of ADHD symptoms in children with ADHD.
Results
The children with ADHD had significantly higher rates of neonatal complications compared with their unaffected siblings (F4,196 = 3.67, p < 0.006). Furthermore, neonatal complications in the children with ADHD were associated with worse CBCL total and externalizing scores and with poorer performance on the CPT.
Conclusions
These results suggest that neonatal complications are probably a nonshared environmental risk factor that may be pathogenic in children with ADHD.
Medical subject headings: attention deficit disorder with hyperactivity, child, perinatal care, pregnancy complications, siblings
Abstract
Objectifs
On a incriminé fortement des facteurs génétiques et des facteurs environnementaux non partagés (vécus par un membre de la famille seulement) dans les causes du trouble d'hyperactivité avec déficit de l'attention (THADA). On a souvent établi un lien entre la grossesse, le travail et l'accouchement et les complications néonatales (GTACN) et le THADA, mais on n'a fait rapport d'aucune étude visant à définir la nature partagée ou non partagée de ces facteurs. Nous voulions déterminer les éléments des facteurs GTACN les plus susceptibles ne pas être partagés.
Méthodes
Nous avons procédé à une étude intrafamiliale et comparé les antécédents de GTACN entre enfants chez lesquels on a diagnostiqué un THADA, en fonction des critères de la quatrième édition du Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), et des frères et sœurs non touchés. On a recruté des enfants atteints du THADA à partir du programme de traitement de jour en service externe de la Division de pédopsychiatrie de l'Hôpital Douglas à Montréal. On a utilisé comme témoin le frère ou la sœur non touché qui avait l'âge le plus proche de celui de l'enfant atteint du THADA. On a évalué les antécédents des facteurs GTACN au moyen du questionnaire médical et gynécologique de Kinney et de l'échelle McNeil-Sjöstrom à la fois chez les enfants atteints du THADA et chez leur frère ou sœur. Soixante-dix enfants atteints du THADA et 50 de leurs frères ou sœurs non atteints ont consenti à participer à l'étude. Pour mesurer les symptômes de THADA chez les enfants atteints, on a utilisé aussi les résultats de la liste de vérification des comportements des enfants (Child Behavior Checklist), du test de rendement continu (Continuous Performance Test) et de l'échelle des situations scolaires restreintes (Restricted Academic Situation Scale).
Résultats
Les enfants atteints du THADA avaient eu beaucoup plus de complications néonatales que leurs sœurs ou frères non atteints (F4,196 = 3,67, p < 0,006). On a de plus établi un lien entre les complications néonatales chez les enfants atteints du THADA et de plus mauvais résultats totaux et externalisants à la liste de vérification ainsi que des résultats plus faibles au test de rendement.
Conclusions
Ces résultats indiquent que les complications néonatales constituent probablement un facteur de risque environnemental non partagé qui peut être pathogène chez les enfants atteints du THADA.
Introduction
Attention-deficit hyperactivity disorder (ADHD) associates inattention, hyperactivity and impulsivity and has a prevalence of 5%–10%.1 Although the cause of ADHD is not known, it has been well established that ADHD has a large genetic component as shown by family,2,3,4 twin5,6 and adoption7 studies. In particular, twin studies have shown that ADHD heritability is high, ranging from 75% to 90%. In addition, these studies indicate that the rest of the variance in the ADHD phenotype (10%–25%) is accounted for mostly by nonshared environmental factors (experienced by 1 member of the family to the exclusion of his or her siblings). In contrast, the involvement of shared environmental factors (shared by all the individuals in the same family) in increasing the risk for ADHD is estimated to be minimal.8
Case–control epidemiologic studies indicate that pregnancy, labour/delivery and neonatal complications (PLDNC) are more frequent environmental factors in children diagnosed with ADHD compared with healthy controls.9,10,11,12 It is believed that such early trauma on the brain during crucial periods of development may have long-lasting effects on cognition and behaviour,13 although the relevant mechanisms mediating the effects of these events remain undetermined. To our knowledge, all studies of PLDNC in ADHD have used case–control designs, comparing children with ADHD with healthy, unrelated controls regarding their history of PLDNC. In addition to the classic limitations of case–control risk studies,14 such experimental designs cannot address the between-relatives differences in exposure to environmental factors. Furthermore, the causal nature of the association between these putative environmental factors and ADHD needs to be clarified, because the same heritable characteristics may increase the risk for these factors (such as smoking and alcohol consumption by mothers) and for ADHD.15,16
Smoking during pregnancy is one of the most studied and consistently identified perinatal factors in ADHD.17 Milberger et al18 compared boys with ADHD with unrelated controls regarding maternal history of smoking during pregnancy. They found that children with ADHD were more likely to have a maternal history of smoking during pregnancy than controls. In a later study, Milberger et al19 reported, in a sample of siblings of children with ADHD and siblings of healthy controls, that the unaffected siblings of children with ADHD had a statistically significantly more frequent history of maternal smoking during pregnancy compared with the siblings of healthy controls. The fact that unaffected siblings of probands also have a statistically significantly more frequent history of maternal smoking compared with siblings of healthy controls suggests that the association between smoking during pregnancy and ADHD is not limited to children affected with ADHD but extends to their unaffected siblings. This may be at least partially compatible with the hypothesis that mothers of children with ADHD are more prone to smoke during all their pregnancies. This maternal lifestyle may be the result of a common familial predisposition to smoking and to ADHD. Consistent with this shared intrafamilial risk hypothesis, children with ADHD and their unaffected siblings were found to be at higher risk for early onset of smoking.20,21
Maternal alcohol consumption has been proposed as a risk factor in ADHD.11 Indeed, ADHD is often diagnosed in children with fetal alcohol syndrome and in the children of alcoholics. Brown et al22 and Aronson et al23 reported that children born to mothers who had abused alcohol throughout pregnancy had more behavioural and attention problems than children born to mothers with no alcohol consumption during pregnancy. There was a correlation between the occurrence and the severity of psychiatric disorders, including attention deficit, and the degree of alcohol exposure of the children in utero.24 However, these dose-dependent effects on neurobehavioural function were not limited to attention but also extended to other disorders such as learning problems. In addition, Hill et al25 found that family history of alcoholism, but not alcohol consumption during pregnancy, is associated with ADHD, suggesting that alcoholism and ADHD may share some of their genetic determinants.
In conclusion, the relation between environmental factors (i.e., maternal smoking and alcohol consumption) and ADHD is complex and points to the difficulty of determining the nature of the environmental risk factors in ADHD and their relation with genetic factors. In the 2 examples cited, the correlation between these risk factors and ADHD may be secondary, at least in part, to a common underlying factor, such as shared genes that increase the risk for these behaviours and ADHD. A study with an intrafamilial design, comparing children with ADHD with their unaffected siblings, would have the advantage of controlling for 50% of the genetic variability. In addition, this design will control for many confounding factors, because all siblings share their socioeconomic status, family environment and history. The within-family design can also help differentiate the critical shared and nonshared nature of these environmental factors.
In this study, we aimed to identify those elements among PLDNC that are potentially nonshared environmental factors by using an intrafamilial case–control design. More specifically, we hypothesized that some of the PLDNC, which are often reported to be associated with ADHD, may represent shared environmental factors, whereas some other PLDNC may represent nonshared (specific to the affected child) environmental factors with different potential roles in increasing the risk for ADHD within families. To distinguish between shared and nonshared PLDNC factors, we first compared children with ADHD with their unaffected siblings with regard to PLDNC. Second, PLDNC that are significantly more common in children with ADHD than in siblings were studied with regard to the severity of ADHD symptoms, on the basis of the assumption that these factors may be correlated with the expression of ADHD.
Methods
This study was part of a larger placebo-controlled clinical trial of methylphenidate in children with ADHD. Clinical data from baseline assessments (before administration of placebo or methylphenidate) were used in this study. Among the 70 children with ADHD, aged 6–12 years, recruited in this study from the Douglas Hospital Child Psychiatry Division, Montréal, 50 had unaffected siblings and 20 children had no unaffected siblings or no siblings at all. Five children agreed to participate in the PLDNC study but not in the methylphenidate clinical trial. The Douglas Hospital Ethics Committee approved the research protocol, and parents gave written informed consent after an explanation of the study purposes and procedures was given. The nature of the study was also explained to the children, who gave their assent to their participation.
Two experienced child psychiatrists made a best-estimate diagnosis of ADHD based on a clinical evaluation according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV),26 and on reports from teachers and parents. Parents also completed a structured interview, the Diagnostic Interview Schedule for Children Version IV (DISC-IV).27 Children with an IQ lower than 70 (Wechsler Intelligence Scale for Children III [WISC-III])28 or psychosis were excluded.
Mothers were interviewed, using DISC-IV, with respect to the history of ADHD in all their children. Siblings who did not meet ADHD criteria were identified, and the child closest in age to and of the same sex as the affected child was included in the study. In addition to the structured interviews, all children were assessed with the Child Behavior Checklist (CBCL).29
The Kinney Medical and Gynecological Questionnaire was used in interviews with the mothers with regard to prenatal, perinatal or postnatal complications that had occurred during all their pregnancies, labours and deliveries and during the neonatal history of all their children.30 This questionnaire was complemented with data from hospital files in 60% of cases. The McNeil–Sjöstrom scale for obstetric complications was used for scoring. The scoring takes into account the severity of complications during the first, second and third trimesters of pregnancy, labour, delivery and the neonatal period (first 8 weeks). It includes 6 severity levels, reflecting the ordinal degree of potential harm to the central nervous system.31 For all the children, scoring was done by the same clinician who was blinded to the subjects' status (ADHD or unaffected sibling). A research assistant blinded to the interview and scoring data selected the siblings to be included as controls.
As part of the clinical trial, performance on the Continuous Performance Test Overall Index (CPT-OI)32 and the Restricted Academic Situation Scale (RASS)33 was assessed. The CPT is a 15-minute computerized test that measures sustained attention ability, and the RASS is a 15-minute test that provides information about motor activity during academic work.
Statistical analysis
For demographic and clinical characteristics, the 2 groups were compared using a t test. Because of the tight matching between cases (children with ADHD) and controls (unaffected siblings), we used a repeated-measures analysis of variance (ANOVA) with a within-subject repeated measure (scores of the subject and of his or her sibling) and a repeat over time for the developmental periods (3 trimesters of pregnancy, labour/delivery and neonatal period). Fisher exact tests were performed to compare the frequency of PLDNC in children with ADHD and their siblings. The effects of PLDNC that differed significantly between subjects and siblings were assessed within the group of children with ADHD. Among the affected children, we compared the ADHD characteristics for those with and those without PLDNC. Statistical significance was set at p < 0.05.
Results
Children with ADHD who had unaffected siblings (n = 50 pairs) were significantly younger than their unaffected siblings (mean age 8.8 [standard deviation {SD} 1.7] yr v. 10.1 [SD 3.7] yr, respectively, p = 0.031) (Table 1). Birth rank was higher in the ADHD group compared with the sibling group (median 2 v. 1 respectively, p = 0.001). As expected, boys were overrepresented in our study group. For children with ADHD and siblings, mean DISC-IV total scores were respectively 13.8 [SD 3.3] and 2.6 [SD 3.0] (the normal score being < 6). Mean CBCL total scores were respectively 72.5 [SD 8.5] and 55.7 [SD 16.2] (the normal score being < 65).
Table 1
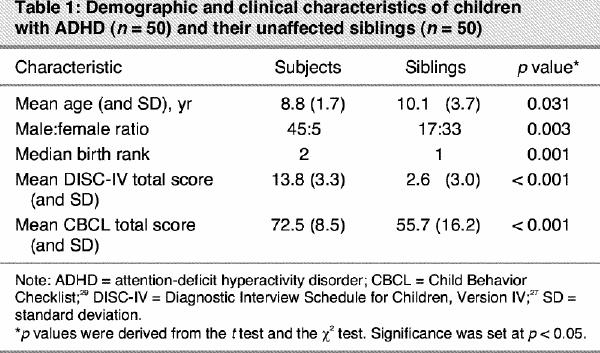
Repeated-measures ANOVA revealed that the within-pair difference in the total score for PLDNC tended to be statistically significant (F1,49 = 3.56, p = 0.06). Patients with ADHD showed a tendency to have higher scores for PLDNC than their unaffected siblings. A significant effect of the period of development (first, second and third trimesters of pregnancy, labour/delivery and neonatal period) was also observed (F4,196 = 3.67, p < 0.006). The main finding of this study is that the 2 profiles of PLDNC over the developmental periods in children with ADHD and their unaffected siblings were not parallel, as indicated by a significant interaction between group and periods of development (F4,196 = 3.89, p < 0.004). Post hoc comparisons using Tukey's Honestly Significant Difference (HSD) showed that this difference stemmed from a significantly greater number of neonatal complications in the children with ADHD compared with the unaffected siblings (p < 0.006) (Table 2).
Table 2
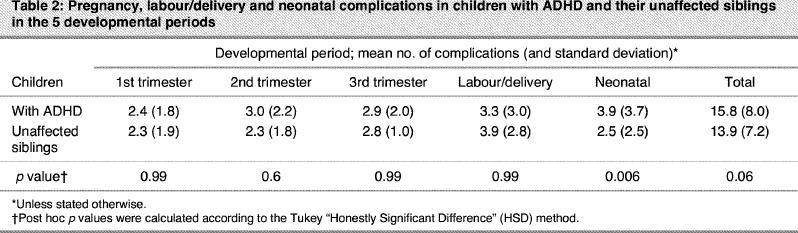
Fisher exact tests were performed to compare the frequency of PLDNC in children with ADHD and their siblings. Children with ADHD had significantly more neonatal admissions to hospital and/or therapeutic interventions (36% v. 16%, p = 0.02). During gestation, children with ADHD had more of a history of early contractions that required medication (14% v. 2%, p = 0.05).
It is noteworthy that the factors often reported to be associated with ADHD in case–control (unrelated) studies, such as maternal smoking and alcoholism, did not show significant differences between patients and their unaffected siblings (58% v. 58%, and 4% v. 8%, respectively, for smoking and alcoholism). Unaffected siblings had a more frequent history of forceps delivery (4% v. 18%, p = 0.05).
Among the initial group of 70 patients, 65 subjects completed the clinical trial in which behavioural and therapeutic responses to methylphenidate were assessed using a 2-week double-blind crossover design (0.5 mg/kg per d). Affected children with neonatal complications (n = 45) and those without neonatal complications (n = 20) did not differ with regard to age, sex ratio and family income. Within the group of children with ADHD, the subgroup of children with a history of neonatal complications showed significantly higher mean scores on the total CBCL (72.0 [SD 6.7] v. 66.3 [SD 14.1], p = 0.031) and tended to have significantly higher mean CBCL externalizing subscores, such as attention and impulsivity (72.7 [SD 7.5] v. 67.4 [SD 14.3], p = 0.05), but not on the CBCL internalizing subscore, such as anxiety (64.7 [SD 10.0] v. 62.2 [SD 14.0], p = 0.40), compared with those without neonatal complications (normal scores < 65). There was no difference between children with and without PLDNC with regard to IQ (95.1 v. 97.1, p = 0.67) (Table 3).
Table 3
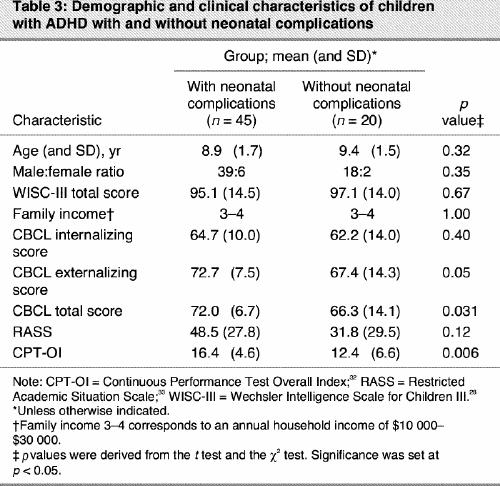
We also compared the average measurements on the CPT-OI and RASS (before medication) in patients with and without neonatal complications. Patients with neonatal complications showed a poorer performance on the CPT-OI (F1,60 = 10.59, p = 0.001), and no differences on motor activity were observed (F1,62 = 2.59, p = 0.12) (Table 3).
Discussion
PLDNC have been the most studied of the environmental factors implicated in the pathogenesis of ADHD and have received some validity from animal studies.34 The literature indicates that many confounding factors (socioeconomic status, maternal IQ, family history) can limit the interpretation of case–control studies. An intrafamilial study comparing children with ADHD with their unaffected siblings may provide excellent-to-good matching between cases and controls for several environmental factors and, to a certain extent, for the genetic background. Genetic epidemiologic studies identify mainly nonshared environmental factors, whereas case–control studies identify factors that are more likely to be common to all children in the same family, such as maternal smoking and alcohol consumption, both of which may share genetic determinants with ADHD. Thus, using intrafamilial case–control studies may help to clarify the complexity of the interaction between genetic and environmental factors that may be implicated in this disorder.
The first main finding of this study is that the profile of PLDNC during developmental periods in children with ADHD and their unaffected siblings was not parallel. This is mainly because of an increased level of neonatal complications in the children with ADHD.
In contrast to pregnancy, labour and delivery, events experienced in the neonatal period, when the child is more independent of the mother, are conceivably more likely to be specific to each individual, that is, a nonshared factor. This result may suggest that neonatal complications may be a risk factor with a putative causal link to the development of ADHD. This result is also consistent with the fact that genetic epidemiologic studies have identified mainly nonshared environmental factors in ADHD. Surprisingly, in the literature, low birth weight (LBW) (≤ 2500 g) as a neonatal risk factor was almost exclusively associated with ADHD.35,36,37,38 In these studies, ADHD was a frequent outcome for LBW children; however, LBW children also experienced greater developmental delay. This association may be the result of the effects of general developmental problems. Moreover, other studies12,39,40 have reported that LBW was associated only with lower IQ scores but not with ADHD. The effect of LBW on attention and motor behaviour remains controversial.13 Apart from LBW, no other abnormal neonatal conditions have been reported to be associated with ADHD.
In the study group, medical conditions that were more frequent in ADHD included several events occurring during the first 2 months of life: neonatal admission to hospital, having been in an incubator, oxygen therapy, general anesthesia and surgery being the most frequent. Although these findings do not point to a single event that may lead to behavioural or cognitive problems, they do support past research indicating that children with ADHD have a higher prevalence of stressful events in early life.12 Moreover, these factors, consistent with previous research, suggest that prolonged chronic rather than acute stresses are more likely to be associated with ADHD. It is interesting that some of these factors are clearly associated with hypoxia (e.g., oxygen therapy). This is consistent with findings from animal models indicating that neonatal hypoxia can result in increased locomotor activity later in life.41,42,43 The relation of these neonatal events to pregnancy and labour/delivery needs to be analyzed in order to assess their specific role in increasing the risk for ADHD.
Surprisingly, smoking and alcohol consumption during pregnancy, which are often reported as environmental risk factors for ADHD, did not differ between patients and their unaffected relatives. Smoking was equally common (58%) in pregnancies leading to affected and unaffected children. Remarkably, this rate is higher than the rate observed in the general population of Quebec (40%).44 This may indicate that smoking in our population is a trait associated not with ADHD but with an intrafamilial nature shared by all the family members. A recent review of the literature17 found that smoking during pregnancy is one of the most frequently reported risk factors but with a small effect. More recently, a large twin study found that maternal smoking during pregnancy explains a small but significant proportion (2%) of the variance in the ADHD phenotype.45 It is, therefore, possible that our study has missed this effect because of the small sample or the fact that intrafamilial studies (including twin studies) tend to obscure the relationship for shared risk factors because of the lack of variability in exposure to some risk factors. A much larger sample with more variability in maternal smoking from one pregnancy to the other is needed to investigate adequately the relationship between genetic factors, smoking and ADHD.
The second main finding of this study is that among the children diagnosed with ADHD, neonatal complication was associated with worse total and externalizing CBCL scores, including hyperactivity and inattention. Neonatal complications were also associated with higher scores on the CPT but not with RASS scores. This interesting result suggests that neonatal complications may have a greater impact on attention deficit but not on motor hyperactivity.
The presence of an association between neonatal complications and the severity of the ADHD (with a worse CBCL score and lower CPT performance), combined with the putative nonshared nature of these complications, suggests that indeed these events may play a role in the pathogenesis of ADHD. Although the mechanism for a positive association between neonatal complications and ADHD has not been established, this finding can be interpreted as consistent with the early dopaminergic disruption hypothesis for this disorder.46 In fact, several studies have linked postnatal insults with alteration in dopaminergic circuits such as the prefrontal cortex and basal ganglia structures that are involved in the development of ADHD. In particular, neuroimaging studies have shown that, in children with ADHD, growth curves of brain structures are parallel to normal growth curves, which suggests that brain abnormalities in ADHD are more likely to be fixed during early development rather than an ongoing process.47
Interestingly, the fact that neonatal complications were associated with worse CBCL scores and poorer CPT performance, but not RASS scores, may suggest that this constellation of personal history events and behavioural characteristics may delineate a specific subgroup within the ADHD syndrome. Kotimaa et al,10 in a recent prospective study, found that maternal smoking during pregnancy is associated only with hyperactivity. This may suggest that other risk factors may be more closely associated with different behavioural dimensions of ADHD. Further investigation of the relationship between neonatal complications and other relevant factors (e.g., genetic and biologic indicators, therapeutic response) is needed to further this hypothesis.48
Several limitations should be kept in mind when interpreting the results of this study. First, our conclusions are based on a sample of 50 children with ADHD and 50 of their unaffected siblings. This is of course a small sample for risk studies. These results need to be confirmed in a larger group in order to ensure their validity. Second, comorbidity is very high in ADHD; it would have been very informative to study PLDNC according to comorbid disorders. However, the small sample precluded this analysis. Another limitation of this study is that boys were overrepresented in the ADHD group. However, given that girls may be more resilient to developing the disorder, it is expected that some girls may have a high level of PLDNC and yet do not express the disease. This bias may therefore be conservative, that is, unlikely to result in false-positive findings. In this study, we also observed that unaffected siblings experienced more forceps interventions. Although this difference may be related to the fact that unaffected siblings were on average more likely to be first-born children compared with their affected siblings, its interpretation is difficult because of the difference in sex between the 2 groups. A further limitation of this study is the difference in age and birth rank. Here again, most of the affected children were younger and resulted from a second pregnancy, which is usually considered to be at lower risk than a first pregnancy. Another limitation of this design is its reliance on a maternal retrospective interview, which is the case for almost all studies of maternal lifestyle during pregnancy.17 In order to have information about the validity of the maternal report, we compared the maternal reports with the information derived from the medical files for 60% of the patients. As reported in the literature,49 we found that mothers tended to underreport PLDNC. Finally, although using siblings as controls reduces a large number of potential biases, it does not control for 50% of the genes that differ between siblings, making this design more robust than case–control studies but less so than twin studies, which are difficult to conduct.
Conclusion
To our knowledge, this is the first study to use an intrafamilial design to address the implication of PLDNC in ADHD. This design has the important advantage of controlling for many confounding factors, including, to a certain extent, the genetic background. It might be a useful intermediate tool between unrelated case–control and twin risk studies. In addition, this type of design may help to delineate the nature of environmental risk factors and to understand the role of the different factors in the expression of ADHD. The results of this study suggest that neonatal complications are more frequent in children with ADHD compared with their unaffected relatives. The severity of these complications, their prolonged character, their association with clinical dimensions and their possible nonshared nature suggest that these factors may play a role in the cause of ADHD and may help to delineate relevant subgroups in this disorder.
Acknowledgments
We would like to thank Julian Doan for his contribution.
Footnotes
We would like to thank the Fonds de la recherche en santé du Québec and the Roaster Foundation for grants to Drs. Joober and Grizenko, and we would like to thank Pfizer Canada for a support grant to Dr. Ben Amor.
Competing interests: None declared.
Correspondence to: Dr. Ridha Joober, Douglas Hospital Research Centre, 6875 LaSalle Blvd., Montréal QC H4H 1R3; fax 514 888-4064; ridha.joober@mcgill.ca
Submitted Sept. 19, 2003; Revised Mar. 16, 2004; Accepted Mar. 29, 2004
References
- 1.Paule MG, Rowland AS, Ferguson SA, Chelonis JJ, Tannock R, Swanson JM, et al. Attention deficit/hyperactivity disorder: characteristics, interventions and models. Neurotoxicol Teratol 2000; 22:631-51. [DOI] [PubMed]
- 2.Faraone SV, Biederman J, Milberger S. An exploratory study of ADHD among second-degree relatives of ADHD children. Biol Psychiatry 1994;35:398-402. [DOI] [PubMed]
- 3.Hechtman L. Families of children with attention deficit hyperactivity disorder: a review. Can J Psychiatry 1996;41:350-60. [DOI] [PubMed]
- 4.Samuel VJ, George P, Thornell A, Curtis S, Taylor A, Brome D, et al. A pilot controlled family study of DSM-III-R and DSM-IV ADHD in African-American children. J Am Acad Child Adolesc Psychiatry 1999;38:34-9. [DOI] [PubMed]
- 5.Eaves LJ, Silberg JL, Meyer JM, Maes HH, Simonoff E, Pickles A, et al. Genetics and developmental psychopathology: 2. The main effects of genes and environment on behavioral problems in the Virginia Twin Study of Adolescent Behavioral Development. J Child Psychol Psychiatry 1997;38:965-80. [DOI] [PubMed]
- 6.Martin N, Scourfield J, McGuffin P. Observer effects and heritability of childhood attention-deficit hyperactivity disorder symptoms. Br J Psychiatry 1997;180:260-5. [DOI] [PubMed]
- 7.Cadoret RJ, Stewart MA. An adoption study of attention deficit/hyperactivity/aggression and their relationship to adult antisocial personality. Compr Psychiatry 1991;32:73-82. [DOI] [PubMed]
- 8.McGuffin P, Martin N. Science, medicine, and the future. Behaviour and genes. BMJ 1999;319:37-40. [DOI] [PMC free article] [PubMed]
- 9.Astbury J, Orgill A, Bajuk B, Relationship between two-year behaviour and neuro-developmental outcome at five years of very low-birthweight survivors. Dev Med Child Neurol 1987;29:370-9. [DOI] [PubMed]
- 10.Kotimaa AJ, Moilanen I, Taanila A, Ebeling H, Smalley SL, McGough JJ, et al. Maternal smoking and hyperactivity in 8-year-old children. J Am Acad Child Adolesc Psychiatry 2003;42:826-33. [DOI] [PubMed]
- 11.Mick E, Biederman J, Faraone SV, Sayer J, Kleinman S. Case- control study of attention-deficit hyperactivity disorder and maternal smoking, alcohol use, and drug use during pregnancy. J Am Acad Child Adolesc Psychiatry 2002;41:378-85. [DOI] [PubMed]
- 12.Milberger S, Biederman J, Faraone SV, Guite J, Tsuang MT. Pregnancy, delivery and infancy complications and attention deficit hyperactivity disorder: issues of gene-environment interaction. Biol Psychiatry 1997;41:65-75. [DOI] [PubMed]
- 13.McGrath MM, Sullivan MC, Lester BM, Oh W. Longitudinal neurologic follow-up in neonatal intensive care unit survivors with various neonatal morbidities, Pediatrics 2000;106:1397-405. [DOI] [PubMed]
- 14.Clayton D, McKeigue PM Epidemiological methods for studying genes and environmental factors in complex diseases. Lancet 2001;358:1356-60. [DOI] [PubMed]
- 15.Swanson JM, Flodman P, Kennedy J, Spence MA, Moyzis R, Schuck S, et al. Dopamine genes and ADHD. Neurosci Biobehav Rev 2000;24:21-5. [DOI] [PubMed]
- 16.Faraone SV, Doyle AE, Mick E, Biederman J. Meta-analysis of the association between the 7-repeat allele of the dopamine D (4) receptor gene and attention deficit hyperactivity disorder. Am J Psychiatry 2001;158:1052-7. [DOI] [PubMed]
- 17.Linnet KM, Dalsgaard S, Obel C, Wisborg K, Henriksen TB, Rodriguez A, et al. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. Am J Psychiatry 2003;160:1028-40. [DOI] [PubMed]
- 18.Milberger S, Biederman J, Faraone SV, Chen L, Jones J. Is maternal smoking during pregnancy a risk factor for attention deficit hyperactivity disorder in children? Am J Psychiatry 1996;153:1138-42. [DOI] [PubMed]
- 19.Milberger S, Biederman J, Faraone SV, Jones J. Further evidence of an association between maternal smoking during pregnancy and attention deficit hyperactivity disorder: findings from a high-risk sample of siblings. J Clin Child Psychol 1998;27:352-8. [DOI] [PubMed]
- 20.Milberger S, Biederman J, Faraone SV, Chen L, Jones J. ADHD is associated with early initiation of cigarette smoking in children and adolescents. J Am Acad Child Adolesc Psychiatry 1997;36:37-44. [DOI] [PubMed]
- 21.Milberger S, Biederman J, Faraone SV, Chen L, Jones J. Further evidence of an association between attention-deficit/hyperactivity disorder and cigarette smoking. Findings from a high-risk sample of siblings. Am J Addict 1997;6:205-17. [PubMed]
- 22.Brown RT, Coles CD, Smith IE, Platzman KA, Silverstein J, Erickson S, et al. Effects of prenatal alcohol exposure at school age. II. Attention and behavior. Neurotoxicol Teratol 1991;13:369-76. [DOI] [PubMed]
- 23.Aronson M, Hagberg B, Gillberg C. Attention deficits and autistic spectrum problems in children exposed to alcohol during gestation: a follow-up study. Dev Med Child Neurol 1997;39:583-7. [DOI] [PubMed]
- 24.Streissguth AP, Barr HM, Sampson PD, Bookstein FL. Prenatal alcohol and offspring development: the first fourteen years. Drug Alcohol Depend 1994;36:89-99. [DOI] [PubMed]
- 25.Hill SY, Lowers L, Locke-Wellman J, Shen SA. Maternal smoking and drinking and the risk for child and adolescent psychiatric disorders. J Stud Alcohol 2000;61:661-8. [DOI] [PMC free article] [PubMed]
- 26.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: the Association; 1994.
- 27.Shaffer D, Fisher P, Lucas CP, Dulcan M, Schwab-Stone M. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry 2000;39(1):28-38. [DOI] [PubMed]
- 28.Wechsler D. Manual for the Wechsler Intelligence Scale for Children. 3rd ed. Oxford (UK): the Psychological Corporation; 1991.
- 29.Achenbach TM. The child behavior checklist/4-18. Burlington (VT): University of Vermont; 1991.
- 30.Kinney DK, Yurgelun-Todd DA, Tohen M, Tramer S. Pre- and perinatal complications and risk for bipolar disorder: a retrospective study. J Affect Disord 1998;50(2-3):117-24. [DOI] [PubMed]
- 31.McNeil TF, Sjöstrom K. McNeil-Sjöstrom scale for obstetric complications. Malmo (Sweden): Department of Psychiatry, Lund University; 1995.
- 32.Conners CK. The computerized continuous performance test. Psychopharmacol Bull 1985;21:891-2. [PubMed]
- 33.Barkley RA. Tests and observational scales. In: Attention deficit hyperactivity disorder: a handbook for diagnosis and treatment. New York: The Guilford Press; 1990. p. 327-53.
- 34.Boksa P, El-Khodor BF. Birth insult interacts with stress at adulthood to alter dopaminergic function in animal models: possible implications for schizophrenia and other disorders. Neurosci Biobehav Rev 2003;27(1-2):91-101. [DOI] [PubMed]
- 35.Botting N, Powls A, Cooke RW, Marlow N. Attention deficit hyperactivity disorders and other psychiatric outcomes in very low birth weight children at 12 years. J Child Psychol Psychiatry 1997; 38:931-41. [DOI] [PubMed]
- 36.Breslau N, Brown GG, DelDotto JE, Kumar S, Ezhuthachan S, Andreski P, et al. Psychiatric sequelae of low birth weight at 6 years of age. J Abnorm Child Psychol 1996;24:385-400. [DOI] [PubMed]
- 37.Breslau N, Chilcoat HD. Psychiatric sequelae of low birth weight at 11 years of age. Biol Psychiatry 2000;47:1005-11. [DOI] [PubMed]
- 38.Szatmari P, Saigal S, Rosenbaum P, Campbell D, King S. Psychiatric disorders at five years among children with birth weights less than 1000g: a regional perspective. Dev Med Child Neurol 1990; 32:954-62. [PubMed]
- 39.Hartsough CS, Lambert NM. Medical factors in hyperactive and normal children: prenatal, developmental, and health history findings. Am J Orthopsychiatry 1985;55:190-201. [DOI] [PubMed]
- 40.O'Callaghan MJ, Harvey JM. Biological predictors and co- morbidity of attention deficit and hyperactivity disorder in extremely low birth weight infants at school. J Paediatr Child Health 1997;33 491-6. [DOI] [PubMed]
- 41.Brake WG, Sullivan RM, Gratton A. Perinatal distress leads to lateralized medial prefrontal cortical dopamine hypofunction in adult rats. J Neurosci 2000;20:5538-43. [DOI] [PMC free article] [PubMed]
- 42.Davis JN, Giron LT Jr, Stanton E, Maury W. The effect of hypoxia on brain neurotransmitter systems. Adv Neurol 1979;26:219-23. [PubMed]
- 43.Speiser Z, Korczyn AD, Teplitzky I, Gitter S. Hyperactivity in rats following postnatal anoxia. Behav Brain Res 1983;7:379-82. [DOI] [PubMed]
- 44.Badlissi D, Guillemette A, Fadin A. Prematurity and low birth weight: effects of active and passive smoking during pregnancy. Can J Public Health 2001;92:272-5. [DOI] [PMC free article] [PubMed]
- 45.Thapar A, Fowler T, Rice F, Scourfield J, van den Bree M, Thomas H, et al. Maternal smoking during pregnancy and attention deficit hyperactivity disorder symptoms in offspring. Am J Psychiatry 2003;160:1985-9. [DOI] [PubMed]
- 46.Biederman J, Faraone SV. Current concepts on the neurobiology of attention-deficit/hyperactivity disorder. J Atten Disord 2002;6 (Suppl 1):S7-16. [DOI] [PubMed]
- 47.Castellanos FX, Giedd JN, Berquin PC, Walter JM, Sharp W, Tran T, et al. Quantitative brain magnetic resonance imaging in girls with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 2001;58:289-95. [DOI] [PubMed]
- 48.Hyman SE. Neuroscience, genetics, and the future of psychiatric diagnosis. Psychopathology 2002;35:139-44. [DOI] [PubMed]
- 49.Buka SL, Goldstein JM, Seidman LJ, Tsuang MT. Maternal recall of pregnancy history: accuracy and bias in schizophrenia research. Schizophr Bull 2000;26:335-50. [DOI] [PubMed]


