Abstract
Obesity is an ongoing pandemic and serves as a causal factor of a wide spectrum of metabolic diseases including diabetes, fatty liver disease, and cardiovascular disease. Much evidence has demonstrated that nutrient overload/overnutrition initiates or exacerbates inflammatory responses in tissues/organs involved in the regulation of systemic metabolic homeostasis. This obesity-associated inflammation is usually at a low-grade and viewed as metabolic inflammation. When it exists continuously, inflammation inappropriately alters metabolic pathways and impairs insulin signaling cascades in peripheral tissues/organs such as adipose tissue, the liver and skeletal muscle, resulting in local fat deposition and insulin resistance and systemic metabolic dysregulation. In addition, inflammatory mediators, e.g., proinflammatory cytokines, and excessive nutrients, e.g., glucose and fatty acids, act together to aggravate local insulin resistance and form a vicious cycle to further disturb local metabolic pathways and exacerbate systemic metabolic dysregulation. Owing to the critical role of nutrient metabolism in the control of the initiation and progression of inflammation and insulin resistance, nutritional approaches have been implicated as effective tools for managing obesity and obesity-associated metabolic diseases. Based on the mounting evidence generated from both basic and clinical research, nutritional approaches are commonly used for suppressing inflammation, improving insulin sensitivity, and/or decreasing fat deposition. Consequently, the combined effects are responsible for improvement of systemic insulin sensitivity and metabolic homeostasis.
Keywords: Metabolism, nutrients, inflammation, obesity, metabolic diseases
1. Introduction
With the increasing prevalence of obesity worldwide, there is a drastic rise in the incidence of metabolic diseases. Metabolic syndrome, type 2 diabetes mellitus (T2DM)/insulin resistance, and cardiovascular disease are only a few of the obesity-associated diseases that can cause serious health problems (Bhaskaran, et al. ; Wilson, et al. 2002). While disturbing physiological metabolism and exacerbating other metabolic problems, these obesity-associated diseases account for upwards of 1.4 billion dollars spent per year in healthcare costs, in the US alone, for the treatment and management of such diseases (Cawley and Meyerhoefer 2012). Numerous pharmaceuticals are available for treating such disorders; however, it is important for patients/consumers to remember the value and health benefits of food, including both macro- and micronutrients. Nutrients are essential in all aspects of health, including the absorption and digestion of food, immune function, brain activity, and exercise ability. Not to mention, proper nutrition for maintaining health and staving off metabolic disease could greatly contribute to lower healthcare costs. Therefore, the purpose of this review is to emphasize the importance of nutrients in metabolism and more importantly, summarize how nutritional approaches can be used to support proper health and treat and/or prevent the onset of metabolic disorders, especially those associated with obesity.
2. Nutrients and the Regulation of Metabolic Homeostasis
All nutrients play various yet vital roles in metabolism. This section briefly highlights the physiological significance of macro- and micro-nutrients. Discussions include current dietary recommendations, primary metabolic pathways and/or functions in which each nutrient is involved, and examples of major foods that are known to contribute to obesity and/or metabolic diseases or shown to benefit metabolism and support health. Although not a nutrient, gut microbiota is discussed given its importance in regulating metabolic homeostasis.
2.1. Carbohydrates
Carbohydrates (CHO) are the primary energy source throughout the body, with multiple tissues and cell types solely depending on CHOs for metabolic needs. For this reason the current acceptable macronutrient distribution range (AMDR) and recommended daily allowance (RDA) for CHOs are 45–65% of total daily calories and 130 g/d, respectively (Dietary Reference Intakes (DRIs), United States Department of Agriculture (USDA)). These values are suggested for males and females of all age groups.
Following digestion of dietary CHOs, the resulting monosaccharides (mainly glucose) are converted to energy using three major metabolic pathways: glycolysis, the Krebs cycle, and the electron transport chain. From start to finish, this process accounts for the most efficient breakdown/usage of any macronutrient. Therefore, proper CHO intake not only ensures that cells’ energy requirements are met, but promotes overall metabolic efficiency. On the other hand, inadequate intake and/or overconsumption of CHOs can result in metabolic complications. For example, long-term CHO deficiency in healthy/normal weight adults can result in hypoglycemia, muscle atrophy, or ketoacidosis, while overconsumption, in addition to causing weight gain and/or obesity if chronically overconsumed, can lead to disruptions in sleep-wake cycles, increased risk for non-alcoholic fatty liver disease (NAFLD) and insulin resistance (Flowers and Ntambi 2009; He, et al. ; López-Alarcón, et al. 2014; Neuschwander-Tetri 2013).
While over- or under-consumption of CHOs can be detrimental to metabolism, the type of CHO ingested can also differentially affect health. Complex CHO intake (i.e. starches such as amylose, and fibers) is associated with a reduced-risk for metabolic diseases including heart disease, T2DM/insulin resistance, and colon cancer compared to consumption of primarily simple CHOs (Argiana, et al. 2015; Domínguez Coello, et al. 2010; Foster-Powell, et al. 2002; Jenkins, et al. 2002; Mirrahimi, et al. 2013; Sieri, et al. 2015; Turati, et al.). While still a controversial topic, more research is necessary to further define the relationship between simple CHOs and insulin resistance, and/or the onset of metabolic diseases in normal-weight vs. overweight/obese populations. Overall however, results appear to support that reducing simple CHO intake, fructose and sucrose for example, and increasing intake of complex CHOs, especially in the long-term, is more supportive of and beneficial to health.
2.2. Fats
Unfortunately, when it comes to health and body weight, or more accurately, body image, dietary fats commonly receive a disparaging view. However, fats serve multiple beneficial purposes for health and metabolism. For example, fats provide insulation, drive the absorption of fat-soluble vitamins, serve as primary components in phospholipid bilayers and lipoprotein particles, and provide a long-term energy source when needed. Thus, adequate fat intake is vital for metabolism. No RDA for dietary fats exists but the current AMDR for males and females 19 years of age and older is 20–35% of daily caloric intake (DRIs, USDA). Also included with the adult AMDR is a suggested intake of linoleic acid at 12–17 g/d and α-linolenic acid at 1.1–1.6 g/d, both of which are essential fatty acids and thus, cannot be synthesized innately but only obtained through the diet.
High-fat diets (HFD; daily caloric intake which exceeds body requirements) are indeed detrimental to health as they can be a potent contributor to lipid accumulation throughout the body and thus, can lead to weight gain relatively quickly (Roseno, et al. 2015; Sampey, et al. 2011; Senthil Kumar, et al. 2014). However, more imperative to health and metabolism is the well-documented association between long-term HFD and the generation of inflammation. For example, it is well established that feeding a saturated fatty acid-enriched HFD to mice causes inflammation in various tissues and organs including adipose tissue (Huo, et al. 2010; Xu, et al. 2003), the liver (Cai, et al. 2005; Guo, et al. 2016; Woo, et al. 2014), small intestine (Botchlett, et al. 2016; Guo, et al. 2013), and even the brain (Tang, et al. 2015; Zhang, et al. 2008). Significantly, chronic HFD-induced inflammation is now considered as a major factor in the onset of many metabolic disorders including obesity, T2DM, cardiovascular disease and some forms of cancer (Egger 2012; Hotamisligil 2006; Terzić, et al.). Furthermore, HFD is known to alter the expression of genes involved in metabolic signaling and thus, impair normal metabolic function throughout the body (Banin, et al. 2014; Choi, et al. 2013).
While both overconsumption of total calories and dietary fat can be major driving forces in weight gain/obesity, the type of fat consumed can have significant effects on metabolism and/or the generation of inflammation as well. Numerous studies have indeed provided evidence to support the pro-inflammatory role of saturated fats, compared to unsaturated fats (Milanski, et al. 2009; Teng, et al. 2014), and have demonstrated mechanisms of how saturated fats are linked to metabolic diseases. Although intake of unsaturated fats is recommended over saturated (DRIs, USDA), overconsumption of unsaturated fats can also negatively affect health (Azrad, et al. 2013). Nonetheless, adequate intake of unsaturated fats, i.e. within the AMDR, is shown to be less inflammatory and more beneficial to health compared to saturated fats.
2.3. Proteins
Dietary proteins/amino acids are only metabolized for energetic needs under extreme conditions, so they primarily benefit health/metabolism in other ways. They provide structure and rigidity to cells, are necessary for the growth of muscle, bone, and skin, participate in secondary messenger signaling cascades, drive major metabolic pathways such as the Krebs cycle, and play important roles in the metabolism of other macronutrients. As such the current AMDR and RDA for dietary proteins for average adults are 10–35% and 46–56 g/d, respectively (DRIs, USDA).
Of the twenty essential amino acids, arginine, citrulline, and leucine are among the most vital for preventing obesity and/or obesity-related metabolic diseases. For example, beneficial impacts of L-arginine supplementation include increasing energy expenditure favoring reduced growth of white adipose tissue (WAT) (McKnight, et al. 2010) whereas dietary L-leucine and L-alanine supplementation reveal similar acute effects on preventing HFD-induced obesity likely by acutely influencing satiety (Freudenberg, et al. 2013). Citrulline is shown to play an important role as a marker for the onset of diet-induced obesity (Sailer, et al. 2013) and more recently, exerts a counteractive effect on aging- and excess weight-induced metabolic and inflammatory effects in WAT (Joffin, et al. 2015). Increased intake of dietary leucine shows significant effects on preventing diet-induced obesity and modulating insulin signaling and glucose homeostasis within muscle (Layman and Walker 2006; Zhang, et al. 2007). Interestingly however, leucine supplementation has the opposite effect when given to already obese rats. Furthermore, obese mice display additional fat accumulation within WAT following leucine supplementation (Zampieri, et al. 2014). Consistently, White et al demonstrate that obese rats display higher levels of circulating branched-chained amino acids (BCAA; leucine, isoleucine, and valine) compared with lean control rats. Furthermore, feeding obese rats an isonitrogenous and isocaloric diet in which all three BCAA were reduced by 45% lowers circulating BCAA to levels observed in control diet-fed lean rats and improves skeletal muscle insulin sensitivity. These results suggest a detrimental role of BCAA in obesity and insulin resistance (White, et al. 2016). A recent study by Zhenyukh et al. also suggest that high levels of BCAA could contribute to the pro-inflammatory and oxidative status (Zhenyukh, et al. 2017), thereby exerting a deleterious effect. However, there is also evidence suggesting that higher BCAA levels do not have a causal effect on insulin resistance whereas increased insulin resistance appears to increase the circulating fasting BCAA levels (Mahendran, et al. 2017). Interestingly, a recent prospective study demonstrates that putting individuals with type 2 diabetes and NAFLD on diets high in protein (either animal or plant) increases postprandial levels of BCAA and significantly reduces liver fat independently of body weight, and reduces markers of insulin resistance and hepatic necroinflammation. These results validate the beneficial effects of BCAA on diabetes complicated with NAFLD, which is opposite to a detrimental role of BCAA in insulin resistance. Regardless of the controversy, proper intake of amino acids, in particular arginine, citrulline, and leucine, can greatly maintain health and seemingly prevent the onset of obesity and obesity-related metabolic diseases.
2.4. Micronutrients
Although only needed in trace amounts compared to macronutrients, micronutrients are nonetheless vital to metabolism and crucial in maintaining health. The overall group of micronutrients includes all vitamins and minerals, and can therefore be characterized by the varying functions associated with each subgroup. For example, there are electrolytes (sodium, potassium, chloride), antioxidant micronutrients (selenium, vitamins A, C and E), enzyme cofactors (calcium, manganese, copper, molybdenum), and micronutrients that aid in nutrient absorption and/or metabolism (chromium, vitamins A, D and E). With such a wide array of abilities, micronutrients are active in relatively all metabolic pathways/processes including insulin signaling, lipid metabolism, cell differentiation, and the immune response.
Given the obesity epidemic, there is a significant body of literature addressing the relationships between obesity and micronutrients. For example, obesity is shown to be associated with reduced absorption of copper, iron, zinc, and significantly lower the levels of circulating potassium and magnesium (J. Suliburska 2013; Lukaski HC 2001; Mariosa, et al. 2008; Sonnweber, et al. 2012; Zaakouk, et al. 2016). Considering this, deficiencies in any one or combination of micronutrients can greatly contribute to impaired metabolism and likely be detrimental to health. As such, the USDA provides a recommend daily intake for most vitamins and minerals (DRIs, USDA). However, there is also research suggesting that high circulating levels of selenium are associated with increased risk of diabetes independent of obesity (Lu, et al. 2016). A similar study also indicates that serum vitamin D concentrations are not associated with the risk of the incidence of insulin resistance in Swiss adults (Marques-Vidal, et al. 2015). In adults with moderate obesity, vitamin D status also appears to be unrelated to insulin resistance (M, et al. 2017). In contrast, in the presence of vitamin D deficiency, supplementation of vitamin D for 6 months generates beneficial effects on glycemic control in type 2 diabetic patients with coronary artery disease (Farrokhian, et al. 2017), suggesting the importance of vitamin D in managing metabolic diseases. Regardless, to date, most randomized intervention trials of micronutrient supplements have failed to show an improvement in meaningful clinical biomarkers, such as improved antioxidant status or benefits to vascular or non-vascular endpoints (Woodside, et al. 2005). These negative findings, however, should not be considered as evidence to downplay the importance of micronutrients in disease prevention and health promotion. Instead, it indicates the complexity of micronutrients in the context of battling obesity (Woodside et al. 2005), and suggests the necessity of conducting more well-designed, placebo-controlled, and randomized studies in various groups with considerations of age, gender, degree of obesity and ethnicity as biological variables (Astrup and Bugel 2010; García, et al. 2009).
2.5. Diet Composition
While energy density is a critical determinant of nutritional consequences, diet composition appears to be more important in terms of generating profound impact on health. Because of this, a significant amount of studies have examined the effects of healthy diets on metabolic health. In an early isocaloric feeding trial addressing the effects of optimal diets after replacing saturated fats with a diet rich in CHO, protein, or MUFA on blood pressure, Appel et al. demonstrated that each of the three diets lowers blood pressure compared with baseline. Interestingly, partial replacement of CHO with either protein or MUFA further lowers blood pressure and reduces cardiovascular risk (Appel, et al. 2005). A similar trial by Furtado et al. also demonstrated that healthy diets that emphasize CHO, protein, or unsaturated fat reduce plasma total and lipoprotein apo B compared with a typical high-saturated fat diet (Furtado, et al. 2008). Interestingly, compared with both the CHO diet and un-saturated fat diet, the protein diet generates the most favorable apo B-containing lipoprotein profile and the lowest plasma total apo B concentrations while reducing plasm levels of triglycerides. A recent trial by Gadgil et al. further demonstrated that a diet that partially replaces CHO with unsaturated fat may improve insulin sensitivity in a population at risk for cardiovascular disease (Gadgil, et al. 2013). Clearly, these randomized trials provide convincing evidence supporting the importance of diet composition in managing metabolic diseases.
2.6. Microbiota
Distinct microbial populations span relatively every part of the human body, including the lungs, nasal passages, vagina, and intestine, and all play their own unique role in maintaining health (Cho and Blaser 2012). The intestinal microbial community is especially vital to metabolic homeostasis as it participates in nutrient absorption and maintaining the health of the intestinal tract. Specifically, intestinal microbiota aid in the production of bioactive compounds and some essential nutrients and metabolites and, arguably their most beneficial role within the intestinal environment, generate short chain fatty acids (SCFA) (Natarajan and Pluznick 2015). Although pathogenic bacteria are also present, within normal/healthy hosts diseased/inflammatory states are efficiently regulated and do not negatively alter metabolism.
However, alterations within the microbial community have been implicated in the onset of diet-induced metabolic disorders (Cani, et al. 2008; Suez, et al. 2014; Turnbaugh, et al. 2008; Turnbaugh and Gordon 2009). Specifically, changes to the normally-occurring gut bacteria are associated with reduced metabolite and/or peptide production, impaired nutrient absorption, diminished SCFA production, and a proinflammatory state (Cani et al. 2008; Ding, et al. 2010; Heinritz, et al. 2016; Ridaura, et al. 2013; Samuel, et al. 2008). Several studies have even indicated that HFD-induced dysbiosis promotes intestinal inflammation that precedes obesity and other metabolic diseases (Bleau C1 2015 ; Ding et al. 2010). Thus, it seems alterations to healthy microbial populations can both contribute to and be a result of diet-induced obesity and/or a variety of metabolic diseases. Maintaining a healthy flora is therefore essential in treating and/or staving off such disease states. Indeed, many studies have now demonstrated positive effects from a variety of therapeutics or interventions to combat obesity, diabetes, and others (He, et al. ; Sáez-Lara, et al. 2016).
2.7. Nutrients, Epigenetics, and Genetics
The interplays among nutrients, epigenetics, and genetics have been increasingly studied by many researchers, and are detailed by numerous reviews. Additionally, nutrients or nutrient metabolism can be significantly influenced by genetic factors; although nutrients, per se, have limited roles in altering genetics. Now, it is convincing that nutrients, e.g., nutrients that alter one carbon metabolism, are capable of altering epigenetics to influence the status of health and/or diseases. Indeed, various nutritional approaches are shown to act through, at least in part, modulating epigenetics to bring about beneficial effects, in particular anti-cancer effects (Bishop and Ferguson 2015). Unlike anti-cancer approaches, there are relatively fewer nutritional approaches that may be used for managing obesity and related metabolic diseases. However, it would be of particular importance in targeting maternal nutrition to reduce the incidence of future offspring obesity.
3. Pathology of Metabolic Dysfunction
Many factors can contribute to impaired local and/or systemic metabolic signaling and in turn, augment the onset of metabolic diseases. This section briefly focuses on the pathology of three primary dysfunctions related to diet-induced obesity. Tissue specific pathologies as well as nutritional interventions to reduce their onset are discussed in subsequent sections. Also, the highlights of nutrition and metabolic dysfunction are summarized in Figure 1 (see text for detail).
Figure 1. Obesity, inflammation, and metabolic dysregulation.
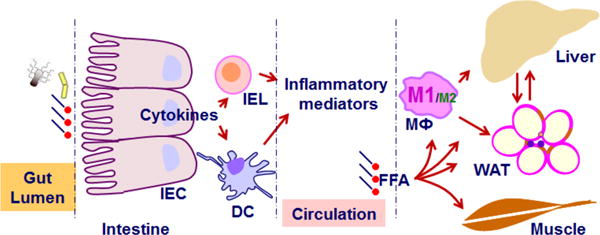
During obesity, nutrient overload initiates or exacerbates inflammation in intestine, adipose tissue, and the liver. Inflammation, in turn, impairs functions of the liver, adipose tissue, and skeletal muscle to critically contribute to the development of insulin resistance and metabolic dysregulation via working with or without excessive free fatty acids (in particular saturated fatty acids). The interactions between nutrients and host cells e.g., intestinal cells and adipocytes, also generate factors that exert profound actions the brain to alter feeding behaviors, the dysregulation of which contributes significantly to positive energy balance (not depicted). IEC, intestine epithelial cells; IEL, intestine epithelial lymphocytes; DC, dendritic cells; FFA, free fatty acids, and M1/M2, macrophage (MФ) polarization.
3.1. Inflammation/Inflammatory Responses
Inflammation is the principal response of the body invoked to deal with injuries and infections. As an acute or short-term adaptive response, inflammation is a crucial component of tissue repair and vital for maintaining cell viability and function. However, long-term consequences of prolonged inflammation are often associated with metabolic disorders, such as insulin resistance, fatty liver disease, T2DM, atherosclerosis and some cancers (Wellen and Hotamisligil 2003; Zimmermann, et al. 2011). Substantial evidence now links obesity with inflammation (Emilsson, et al. 2008; Hardy, et al. 2011). Unlike acute inflammation, which is usually localized to the site of injury (Warren, et al. 2010), obesity-associated inflammation is a low-grade, chronic state (Neels and Olefsky 2006). Furthermore, inflammation in obesity is a systemic progression that involves activation of several inflammatory pathways, and the subsequent generation of proinflammatory cytokines, which can contribute to the dysfunction of multiple organ/organ systems (Lumeng and Saltiel 2011; Ouchi, et al. 2010).
Many factors, including diet, play a role in promoting the inflammatory processes. Excess intake of overall or saturated fat and calories for example can activate inflammatory pathways in cells by nutrient-sensing and cytokine signaling mechanisms (Arkan, et al. 2005), both of which occur via the stimulation of cell surface receptors including toll-like receptors (TLRs), and/or cytokine receptors such as intracellular pathogen sensing NOD-like receptors. Furthermore, these pathways can converge and activate other pro-inflammatory pathways including the mitogen activated protein kinase (MAPK), c-jun N-terminal kinase (JNK), extracellular regulated kinase (ERK), P38 MAPK, the inhibitor κB kinase (IKK), and protein kinase C (PKC) pathways (Mogensen 2009; Saxena and Yeretssian 2014). Following stimulation of such pathways, activated transcription factors including nuclear factor kappa beta (NFκB) can further direct the inflammatory response by binding target gene promoters in the nucleus and in turn lead to the translation of inflammatory cytokine and chemokine genes to activate or enhance the immune response (Fan, et al. 2005).
3.2. Fat Deposition
As discussed above, intake of dietary fats is vital to overall health and normal functioning of many metabolic pathways. Following intake, digestion and absorption of dietary fats, triglycerides are formed within the small intestine, packaged into lipoprotein particles, and transported to the liver. During this process triglycerides can be hydrolyzed and converted into glycerol and free fatty acids (FFAs). This is necessary in order for FFAs to enter adipocytes, be re-esterified into triglycerides, and stored. This overall process is the primary pathway for fat deposition in the adipose tissue and continues as dietary fats are consumed.
When caloric intake chronically exceeds the body’s requirements however, fat deposition can be amplified (Speakman 2004). In some cases, excess intake of any one macronutrient can also result in such consequences (Ambrosini, et al. 2012; Carreiro, et al. 2016; Hörnell, et al. 2013; Moslehi, et al. 2015). It seems multiple mechanisms exist for how overnutrition stimulates this process. For example, many studies have reported increased expressions of genes involved in fat deposition and/or decreased expressions of genes that regulate beta oxidation following overnutrition (Guo et al. 2016; Hudgins, et al. 2000). Overnutrition with an HFD is a particularly common cause of weight gain/obesity as it is shown to contribute to both subcutaneous and visceral WAT deposition (Kubant, et al. 2015). In addition, HFD feeding is associated with morphological changes in the intestine, including an increased number and length of villi (Dailey 2014; Sukhotnik, et al. 2004), which ultimately increases the efficiency of fat absorption and assimilation into the body (Tchernof and Després 2013). The combination of enlarged WAT and increased intestinal fat absorption culminates in HFD-induced obesity, which is correlated with abnormal lipid metabolism and dyslipidemia, increased oxidative stress and inflammation, increased intestinal permeability, and a decrease in systemic insulin sensitivity (Botchlett et al. 2016; Guo et al. 2013; Huo et al. 2010; Kim, et al. 2012).
3.3. Insulin Resistance
Insulin resistance is a pathophysiological condition in which physiological levels of insulin do not adequately produce an insulin response in insulin-sensitive target tissues including adipose, skeletal muscle and liver (Fig. 1). One of the major molecular mechanisms underlying insulin resistance is the defects in the insulin signaling pathway (Morino, et al. 2006). For example, impaired/altered activation of a member of the family of insulin receptor substrates (IRS), specifically IRS-1, can greatly contribute to systemic insulin resistance. Inflammation/proinflammatory cytokines are widely known to interfere with the insulin signaling pathway, including suppression/interference of IRS-1 and IRS-2 (White 2003). Given that diet-induced inflammation can promote the expression and release of multiple inflammatory stimuli, it is now highly implicated in reduced insulin action/insulin resistance (Hirosumi, et al. 2002; Hotamisligil 2006).
HFDs in particular interfere with several sites in the insulin signaling cascade. For example, HFD reduces insulin receptor (IR) and IRS1/2 expression and/or phosphorylation (Liu, et al. 2015), impairs the phosphorylation of PI3K (Zierath, et al. 1997), and decreases the translocation of the glucose transporter GLUT4 to the cell membrane (Zierath et al. 1997). HFD feeding also directly contributes to insulin resistance in adipose tissue through adipocyte hypertrophy. One major consequence when adipocytes become overly enlarged is increased secretion of FFAs secreted. Excess FFAs enter the bloodstream and accumulate in various tissues causing steatosis and systemic insulin resistance. For example, increased FFAs impair the insulin signal in muscle (Kim, et al. 2002) and markedly stimulate hepatic gluconeogenesis (Chu, et al. 2002), which contribute to increased glucose production and an exacerbated hyperglycemic phenotype. Moreover, excess FFAs are toxic to pancreatic beta cells and can cause reduced insulin production (Shimabukuro, et al. 1998). HFD can also directly impair lipid metabolism. Specifically, it leads to reduced lipolysis through inhibition of 5′ AMP-activated protein kinase (Barroso, et al. 2011) and increased lipid accumulation via increased expression of fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC) (Guo et al. 2016), all of which can intensify systemic insulin resistance.
4. Nutritional Approaches for Suppression of Obesity-Association Inflammation
Over recent decades, as the rates of health disparities and metabolic diseases have increased, research has investigated the mechanisms underlying the onset of such conditions. It is now relatively well understood that inflammation is a primary causal factor in many diseases, especially those associated with diet/overnutrition, such as obesity. This section will discuss dietary interventions/nutrients that prevent and/or regulate inflammation specifically within the adipose tissue (Figure 3), liver (Figure 4), and intestine (Figure 5).
Figure 3. Nutritional approaches for managing obesity-associated metabolic impairments in the liver.
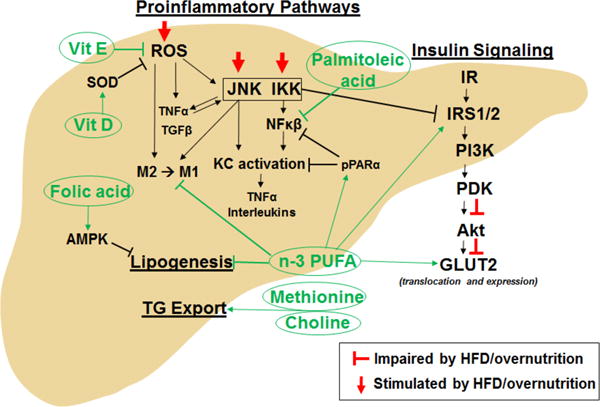
AMPK, AMP-activated protein kinase; GLUT2, glucose transporter 2; IKK, Iκβ kinases; IR, insulin receptor; IRS1/2, insulin receptor substrate 1/2; JNK, c Jun N-terminal kinase; KC, Kupffer cell; n-3 PUFA, omega-3 polyunsaturated fatty acid; NFκβ, nuclear factor kappa beta; M1, proinflammatory macrophage; M2, anti-inflammatory macrophage; PDK, phosphoinositide-dependent kinase; PI3K, phosphoinositide 3-kinase; pPARα, peroxisome proliferator-activated receptor alpha; ROS, reactive oxygen species; SOD, superoxide dismutase; TG, triglyceride; TGFβ, transforming growth factor beta; TNFα, tumor necrosis factor alpha; Vit D, vitain D; Vit E, vitamin E.
Figure 4. Nutritional approaches for managing obesity-associated metabolic impairments in the small intestine.
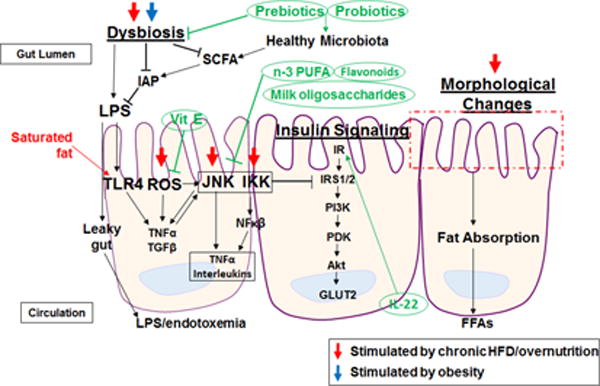
AMPK, AMP-activated protein kinase; FFAs, free fatty acids; GLUT4, glucose transporter 4; IKK, Iκβ kinases; IR, insulin receptor; IRS1/2, insulin receptor substrate 1/2; JNK, c Jun N-terminal kinase; M1, proinflammatory macrophage; M2, anti-inflammatory macrophage; MCP-1, monocyte chemoattractant protein 1; n-3 PUFA, omega-3 polyunsaturated fatty acid; NFκβ, nuclear factor kappa beta; PDK, phosphoinositide-dependent kinase; PI3K, phosphoinositide 3-kinase; pPARα, peroxisome proliferator-activated receptor alpha; ROS, reactive oxygen species; SIRT1, sirtuin 1; SOD, superoxide dismutase; TG, triglyceride; TGFβ, transforming growth factor beta; TNFα, tumor necrosis factor alpha.
Figure 5. Summary of nutritional approaches for managing obesity-associated metabolic dysregulation.
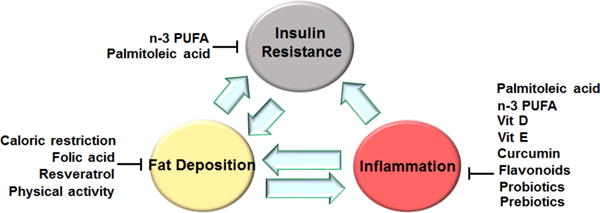
Most of the currently used approaches are shown to be effective at suppressing inflammation, improving insulin sensitivity, and/or decreasing fat deposition. At the integrative level, the combined effects on key metabolic tissues including the intestine, the liver, adipose tissue, and muscle bring about improvement of metabolic dysregulation. In addition, nutritional approaches should be combined with healthy life style, e.g., sufficient physical activity and appropriate circadian clock rhythms of daily life, to maximize the beneficial effects (not depicted). n-3 PUFA, omega-3 polyunsaturated fatty acid; Vit D, vitamin D; Vit E, vitamin E.
4.1. Suppression of Adipose Tissue Inflammation
Under normal conditions, adipose tissue responds to insulin properly, which includes inhibition of lipolysis and stimulation of glucose uptake and fat deposition. Pathological states however, such as diet-induced obesity, cause excessive tissue expansion and dysfunction, leading to insulin resistance and subsequently, increased lipolysis and generation of FFA. Several proinflammatory pathways are triggered in such states including JNK, IKK, NFκB, cyclic AMP-responsive element-binding protein H (CREBH), and ROS are generated (Hotamisligil and Erbay 2008; Huo et al. 2010). Adipocytes in turn secret cytokines in response to enhanced inflammatory signaling, including tumor necrosis factor alpha (TNFα), interleukin (IL)-1, IL-6, resistin and monocyte chemoattractant protein-1 (MCP-1). In addition to adipocytes, adipose tissue also contains numerous immune cells that are activated during proinflammatory/disease states. For example, in obesity there is increased macrophage infiltration into adipose tissue and more importantly, a phenotypic switch from anti-inflammatory, M2, to proinflammatory, M1, macrophages. Newly recruited macrophages, together with adipose tissue proinflammatory resident macrophages, promote further secretion of cytokines and chemokines. This low-degree, chronic inflammation in adipose tissue plays an important role in the development of systemic insulin resistance, and can lead to metabolic and cardiovascular diseases (Berg and Scherer 2005).
One proposed dietary intervention for reducing obesity-associated adipose tissue inflammation is intake of long-chain (LC) omega-3 (n-3) polyunsaturated fatty acids (PUFAs), such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Firstly, they are precursors to resolvins and protectins, endogenous chemical compounds for the withdrawal of proinflammatory cytokines (Bannenberg, et al. 2005). Secondly, LC n-3 PUFAs can bind G protein-coupled receptor 120 (GPR120) and repress macrophage-induced inflammation and promote insulin sensitivity (Oh, et al. 2010). Thirdly, EPA and DHA give rise to series 3 prostaglandins which are less inflammatory compared to series 2, normally derived from arachidonic acid (Calder 2010). In fact, in db/db mice, a rodent model for diet-induced diabetes, mice supplemented with n-3 PUFA showed significantly reduced macrophage infiltration into adipose tissue and reduced JNK phosphorylation compared with HFD alone, or HFD supplemented with n-6 PUFA (Todoric, et al. 2006). In addition to regulating systemic inflammation, n-3 LC PUFAs can also prevent hyperplasia and hypertrophy in adipose tissue, and induce adiponectin secretion and mitochondrial biogenesis in adipocytes (Kopecky, et al. 2009).
While the results from basic nutrition research are promising and encouraging, there is also increasing evidence validating the efficacy of nutritional approaches for suppressing adipose tissue inflammation. As indicated by the results from a human trial by Itariu et al., treatment of severely obese nondiabetic patients with LC n-3 PUFAs for 8 weeks decreased proinflammatory biomarkers in both visceral and subcutaneous adipose tissues, as well as circulating levels of IL-6 (Itariu, et al. 2012). This anti-inflammatory effect of LC n-3 PUFAs, however, was not associated with a decrease in body weight and improvement of insulin sensitivity. A similar trial showed that treatment of insulin-resistant, nondiabetic subjects with n-3 fatty acids for 12 weeks decreased adipose tissue macrophages, increased capillaries, and reduced MCP-1 expression (Spencer, et al. 2013). Consistently, treatment with n-3 fatty acids did not significantly alter body weight and systemic insulin sensitivity. Although the effects of supplementation of n-3 fatty acids on reducing body weight remained controversial (see section 5.1), there appears to be no doubt that n-3 fatty acids are capable of reducing inflammation, including that in adipose tissue. Additional to n-3 fatty acids, monounsaturated fatty acids (MUFAs) also exhibit anti-inflammatory effects. As supporting evidence, a parallel controlled-feeding trial in abdominally overweight subjects revealed that consumption of a MUFA diet for 8 weeks caused a more anti-inflammatory profile in adipose tissue comparing to consumption of a saturated fatty acid diet (van Dijk, et al. 2009). Unlike PUFA, MUFA diets appear to be more promising in improving insulin sensitivity (see Section 6).
Another dietary component with anti-inflammatory actions is polyphenolic compounds which inhibit NFκB and MAPK inflammatory pathways, and activate the AMPK pathway in adipose tissue. Curcumin, derived from thizomes of the plant Curcuma longa (tumeric), is a major polyphenol investigated for this purpose. In fact, in vitro studies have demonstrated that curcumin inhibits hydrogen peroxide (H2O2)-induced oxidative damage and reduces apoptotic death in human bone marrow-derived mesenchymal stem cells (Yagi, et al. 2013). Specifically, it appears that curcumin inhibits the generation of ROS via alteration of the PI3K/AKT/mTOR signaling pathway and subsequent inhibition of FOXO1 translocation to the nucleus (Han, et al. 2012). In endothelial cells, curcumin protects from oxidative injury through blocking Notch signaling which in turn reduces cell apoptosis (Yang, et al. 2013). Further, within macrophages curcumin is reported to decrease expressions of NFκB and IL-1β (Du, et al. 2013). Systemically, in vivo studies using rats have shown significantly decreased TNF-α and IL-6 levels after treatment with 50 mg/kg per day of curcumin (Alghasham, et al. 2013). Similarly, Kant et al. showed that 0.3% (400 μL) curcumin treatment not only reduced inflammatory cytokines, but promoted wound contraction and increased expressions of anti-inflammatory molecules such as IL-10 and anti-oxidant enzymes such as superoxide dismutase, catalase and GPx (Kant, et al. 2014). Given the premise of anti-oxidants in suppressing proinflammatory responses, a significant amount of human trials have been performed to examine the effects of mixed fruit and vegetable supplements on inflammatory biomarkers. As summarized by the review article by Esfahan et al., most studies have validated the beneficial effects of daily consumption of fruits and vegetables on suppressing inflammation (Esfahani, et al. 2011). However, most studies also have limitations related to the diversity of studies and study population.
4.2. Suppression of Liver Inflammation
The liver is a complex organ composed of various cell populations including hepatocytes and Kupffer cells (KCs). During both acute and chronic liver inflammation, activated KCs promote the generation of proinflammatory cytokines via activation of JNK and NFκB signaling pathways. This manifestation of inflammation is especially true with obesity as increased circulating levels of FFAs are well known activators of KCs. Chronic overnutrition/HFD intake, and subsequent chronic activation of proinflammatory pathways, could also trigger a fibrotic response as the generation of myofibroblasts is triggered to replace damaged/dead hepatocytes. This irreversible damage signifies the onset of declining hepatic function in disease states including obesity.
Much research has investigated dietary factors to combat and/or regulate inflammation within the liver. In particular, n-3 PUFAs, palmitoleic acid, and vitamins E and D have known beneficial effects. Systemically, n-3 PUFA intake is associated with reduced circulating proinflammatory markers including CRP, cytokines, proinflammatory eicosanoids, and others (Schwab and Serhan 2006). Studies using experimental models of liver injury have specifically shown that n-3 PUFAs are able to prevent liver necroinflammatory injury through decreasing liver oxidative stress and DNA damage (González-Périz, et al. 2006). In addition, n-3 PUFAs are also known agonists of peroxisome proliferator activated receptor α (PPARα) (Zúñiga, et al. 2011), which is known to inhibit NFκB activity (Huang, et al. 2016). Recently, it has been reported that the activation of liver PPARα by n-3 PUFAs is able to increase the PPARα/NFκB interaction, thereby reducing the expression of proinflammatory cytokines (Zúñiga et al. 2011). It seems that n-3 PUFAs exert this effect in KCs through activation of GPR120, a putative receptor for n-3 fatty acids (Oh et al. 2010). These effects in turn decrease activation/phosphorylation of JNK and NFκB, as well as stimulate the phenotypic shift from M1 to M2 macrophages, to concomitantly reduce the generation of inflammation.
Palmitoleate, a MUFA, has recently been discovered to exert anti-inflammatory effects in the liver (Guo, et al. 2012). For example, palmitoleate serves to reduce the liver inflammatory status in an animal model of HFD-induced insulin resistance seemingly by reducing NFκB signaling in macrophages (Guo et al. 2012). Similar results have also recently been reported by Souza et al. Specifically, palmitoleic acid supplementation in mice resulted in reduced liver proinflammatory cytokine production of IL-1β, IL-12, and TNFα, all of which was associated with reduced NFκB signaling and decreased TLR4 expression (Souza, et al. 2014).
Vitamin E is well known for its anti-inflammatory ability throughout the body. In the liver, Vitamin E acts at the propagation phase of lipid peroxidation to donate a hydrogen group, affectively neutralizing the free radical. The vitamin E itself is then converted to tocopheroxyl radical, which reacts with other peroxyl radicals to form non-radical products. This ability of vitamin E is especially important in non-alcoholic steatohepatitis (NASH), a condition commonly associated with obesity, where increased levels of oxidative stress within hepatocytes trigger the formation of proinflammatory cytokines such as TNFα, TGFβ, and IL-8. In fact, supplementation with vitamin E in an animal model of NASH significantly reduced NFκB signaling and the expression of markers for cell apoptosis such as Bax and Bcl-2 (Nan, et al. 2009). Vitamin D, although most widely known for its role in calcium homeostasis, recently has also been shown to exert anti-inflammatory properties within the liver. For example, vitamin D supplementation in mice resulted in increased expression of antioxidant molecules including glutathione peroxidase and superoxide dismutase (George, et al. 2012). Further, vitamin D supplementation in a rodent model for NASH lead to a decrease in the progression of liver inflammation, as shown by an improvement in liver histology (Nakano, et al. 2011). This study also reported that phototherapy elevated levels of the active form of vitamin D which contributed to a reduction in hepatocyte apoptosis and reduced gene expressions of several proinflammatory cytokines. Interestingly, vitamin D deficiency is associated with increased TLR4 expression in the liver. Roth et al., suggested this effect is due to a shift in microbiota following vitamin D deficiency, which causes an increase in bacterial translocation and thus, increased endotoxin exposure within the liver (Roth, et al. 2012). Unlike animal studies, there are limited human trials studying the effects of dietary interventions on liver inflammation. However, the anti-inflammatory effects generated by nutritional approaches, i.e., PUFAs, MUFAs, and anti-oxidants, may also lead to suppression of liver inflammation in human subjects.
4.3. Suppression of Intestine Inflammation
Several disease states can lead to the development of inflammation in the small intestine. Physical damage, bacterial, viral or parasitic infections, or severe disorders such as Crohn’s disease can all contribute to increased inflammation as the responding immune system involves macrophage recruitment and T-cell activation, both of which stimulate inflammatory cytokine pathways. Much research also implicates obesity in inflammatory cytokine production in the small intestine. Diet-induced models of obesity, particularly HFD feeding studies in rodent models using saturated fats, are especially powerful to induce an obese phenotype relatively quickly. Results from such studies show that chronic HFD feeding directly contributes to the generation of inflammation in the small intestine through increased TLR4 expression (de La Serre, et al. 2010), phosphorylation of JNK (Guo et al. 2013) and IKK (Arkan et al. 2005), and mRNA levels of IL-6 and TNFα (Guo et al. 2013). Interestingly, TLR4 and NFκB expressions are also increased after only short-term HFD (Wang, et al. 2013). In addition, HFD-induced obesity also seems to indirectly contribute to small intestinal inflammation via changes in the gut microbial composition. Specifically, several mouse studies have demonstrated a shift in the ratio of Firmicutes to Bacteriodetes populations (Turnbaugh et al. 2008; Turnbaugh, et al. 2006). This shift leads to increased LPS production, which generates inflammation locally through stimulation of the TLR4 signaling pathway, and can contribute to systemic inflammation and worsen the obese phenotype (Cani et al. 2008). HFD-induced dysbiosis is also associated with reduced levels of intestinal alkaline phosphatase (IAP) (de La Serre et al. 2010) which normally functions to detoxify LPS. Changes in microbiota could also alter SCFA concentrations which, when lacking, may promote small intestinal inflammation as reviewed by de Besten et al. (den Besten, et al. 2013). Collectively, these findings indicate that diet-induced obesity contributes to small intestinal inflammation via both direct and indirect mechanisms.
Many adults suffer from intestinal discomfort, whether from minor conditions or diagnosed diseases. Current dietary interventions to reduce intestine inflammation and thus, lessen the pain associated with such conditions include treatment with both macro- and micronutrients. For example, in rodent models of colitis and/or in vitro studies investigating anti-inflammatory therapeutics milk oligosaccharides, PUFAs, and polyphenolic compounds such as flavonoids are shown to significantly reduce intestinal inflammation (Lara-Villoslada, et al. 2006; Vezza, et al. 2016). Given this, it is a logic extension to use dietary approaches for protecting against obesity-associated intestinal inflammation. Indeed, there is an ongoing clinical trial in obese and non-obese individuals testing the ability of low-fat dairy yogurt to improve gastrointestinal health and reduce chronic inflammation (ClinicalTrials.gov Identifier: NCT01686204). Regardless of the outcomes, targeting intestinal inflammation to maintain metabolic homeostasis or correct metabolic dysregulation via nutritional approaches is increasingly attracting research attention. Among established anti-inflammatory dietary components, n-3 PUFAs are shown to decrease obesity-associated intestinal inflammation, evidenced by its effects on suppressing Th1/Th17 cells (Monk, et al. 2012). In addition to dietary components, pre- and pro-biotics can prevent HFD-induced shifts in the intestinal microbiota (Heo, et al. 2016). In fact, following HFD feeding treatment with probiotics can actually return the microbial community to its original pre-diet distribution (Heo et al. 2016). Given the vast diversity of the human microbiome across subjects/patients, a few interventions will certainly not benefit everyone. Thus, continued research is further investigating additional nutritional therapeutics for HFD-induced/obesity-associated inflammation within the intestinal environment.
Over the past several decades, a number of human trails have focused on the efficacy of various dietary components on intestine inflammation and related diseases, in particular inflammatory bowel disease (IBD). However, to date, there are limited trails examining the effects of nutritional approaches for suppressing obesity-related intestine inflammation. This may be due to the fact that it is a relatively new concept that obesity-related intestine inflammation precedes and correlates with insulin resistance (Ding et al. 2010; Du, et al. 2015). However, it is expected that in the future more trials will specifically address whether and how diets suppress intestine inflammation in the context of preventing or reversing obesity-related metabolic dysregulation.
5. Nutritional Approaches for Reducing Fat Deposition
In non-diseased physiological states, with balanced nutrient and caloric intake, fat deposition is closely regulated and well-managed within the adipose tissue. However, with abnormal metabolism and/or diseases, such as obesity, fat deposition can become uncontrolled, induce inflammation, and occur outside of adipocytes. This section focuses on nutrient/dietary interventions to prevent against the onset of impaired/abnormal lipid metabolism and fat deposition within the adipose tissue, liver and intestine (Figures 2, 3, and 4).
Figure 2. Nutritional approaches for managing obesity-associated metabolic impairments in adipose tissue.
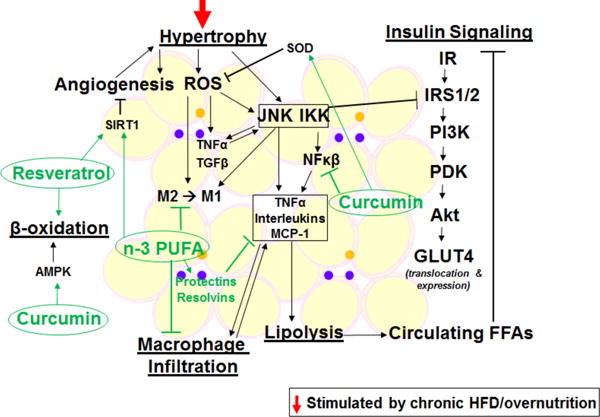
AMPK, AMP-activated protein kinase; FFAs, free fatty acids; GLUT4, glucose transporter 4; IKK, Iκβ kinases; IR, insulin receptor; IRS1/2, insulin receptor substrate 1/2; JNK, c Jun N-terminal kinase; M1, proinflammatory macrophage; M2, anti-inflammatory macrophage; MCP-1, monocyte chemoattractant protein 1; n-3 PUFA, omega-3 polyunsaturated fatty acid; NFκβ, nuclear factor kappa beta; PDK, phosphoinositide-dependent kinase; PI3K, phosphoinositide 3-kinase; pPARα, peroxisome proliferator-activated receptor alpha; ROS, reactive oxygen species; SIRT1, sirtuin 1; SOD, superoxide dismutase; TG, triglyceride; TGFβ, transforming growth factor beta; TNFα, tumor necrosis factor alpha.
5.1. Adipose Tissue and Reduction of Adiposity
To support proper/healthy fat deposition, it is recommended to consume complex carbohydrates and lean protein, and limit saturated fat intake. In addition, several functional foods are thought to contribute to healthy fat deposition. Curcumin for example, increases the levels of activated AMPK which in turn decreases ACC activity, which is a master regulator of lipogenesis. Inactivity of ACC results in elevated carnitine palmitoyl transferase 1 (CPT1) activity and subsequently, increased LC fatty acid β-oxidation. Curcumin also inhibits the expression of PPARγ and C/EBPα, which are the key transcription factors involved in adipogenesis and lipogenesis within adipose tissue. By suppressing the differentiation of pre-adipocytes into adipocytes, curcumin can also suppress the enlargement of adipocytes/adipose tissue and thus, potentially help in staving off weight gain and/or obesity (Aggarwal 2010).
Resveratrol, a non-flavanol polyphenol found in red wine, is another functional food with beneficial abilities in combating diet-induced obesity. Specifically, resveratrol is involved in the downregulation of lipogenesis via increasing levels of SIRT 1 and PPARγ coactivator 1α (PGC1α) (Higashida, et al. 2013). SIRT 1 represses PPARγ and thus, serves to inhibit adipogenesis and trigger lipolysis in mature fat cells (Picard, et al. 2004). PGC1α helps to regulate mitochondrial biogenesis and oxidative metabolism, which increases rates of β-oxidation in adipose tissue and can lead to reduced body fat (Liang and Ward 2006).
It appears that caloric restriction (CR) is a powerful and effective approach for reducing body weight, as well as adiposity (see Section 7.1). Additional to CR, both PUFAs and MUFAs are shown to alter body fat composition or even reduce fat mass. For example, in a small-size human study involving healthy adults, substitution of fish oil for visible fats in a control diet (52% carbohydrates, 16% protein, 32% fat; polyunsaturated: saturated (P: S 0.2)) for three weeks reduced body fat mass (Couet, et al. 1997). Similarly, in a trail examining the effects of dietary oils, supplementation with conjugated linoleic acid (CLA), but not safflower oil (SAF) for 36 weeks significantly reduced body mass index and total adipose mass without altering lean mass (Norris, et al. 2009). In overweight or obese children, supplementation of CLA also reduced body fatness (Racine, et al. 2010). However, there also are many studies that could not support an exact anti-obesity role for n-3 PUFAs in overweight/obese subjects, which suggest the necessity of conducting more large-scale and long-term clinical trials (Du et al. 2015). Compared with PUFAs, MUFAs appear to be more promising in terms of reducing fat mass. As supporting evidence, the results of a 6-month randomized and controlled trial involving nondiabetic overweight or obese men and women revealed that MUFA diets allowed less body fat regain than did the control diet (Due, et al. 2008). More interestingly, consumption of an isocaloric MUFA-rich diet prevented central fat redistribution induced by a CHO-rich diet in insulin-resistant subjects (Paniagua, et al. 2007). In future trials, it may be important to examine the anti-obesity effects of mixed diets.
5.2. Brown Adipose Tissue and Promotion of Thermogenesis
Brown adipose tissue (BAT) is unique from the more abundant WAT primarily in its ability to generate heat via thermogenesis. Within the mitochondria of BAT, uncoupling protein 1 (UCP1) allows hydrogen ion flux directly from the inner membrane to the matrix, without activating ATP synthase. BAT contains significantly more mitochondria compared to WAT which equates to more nutrient energy converted to heat, which then elevates glucose metabolism and increases the production of energy. Therefore, more BAT theoretically means more energy expenditure and/or weight loss. However, humans lose a significant amount of BAT as they mature from infants to adults. Nonetheless, nutrients and foods to increase BAT is a popular research focus for reducing and/or preventing diet-induced obesity.
Some research has indicated that BAT can be activated or stimulated by dietary components, particularly those in spicy foods. For example, non-pungent analogs of capsaicin (capsinoids) are reported to activate BAT in adult humans (Yoneshiro, et al. 2012; Yoneshiro, et al. 2013). One suggested mechanism is that thermogenesis is increased via stimulation of the transient receptor potential vanilloid receptor (TRPV1) (Szallasi, et al. 1999) and upregulation of UCP1 (Yoneshiro et al. 2013). In addition, ingestion of capsinoids increases energy expenditure significantly, even in as short as 30 mins (Saito 2013). Specifically, consumption of 2.56 mg capsaicin per meal can counteract the unfavorable decrease in energy expenditure during negative energy balance and can boost fat oxidation (Janssens, et al. 2013). There are many vanilloids with structures similar to capscin, such as piperine found in pepper, gingerols, shogaol, and 6-paradol found in ginger. All of these compounds are also shown to act as agonists for TRPV1 and recruit BAT for thermogenesis (Saito 2013). Thus, repeated ingestion of these compounds could potentially contribute to repeated stimulation of BAT thermogenesis in humans. Continued research to investigate other activators of BAT is of significant interest.
The discovery of beige adipocytes led to the consideration of promoting the development of beige adipocytes in WAT (browning) as a new avenue for management of metabolic disease (Bartelt and Heeren 2014). In addition, a great number of studies have advanced the understanding of the molecular mechanisms underlying browning. To date, however, there is no effective browning-based therapeutic approach for managing obesity and related metabolic diseases. Consistently, Abdullahi et al. raised the concern that the notion of browning is supposedly beneficial may be inadequate (Abdullahi and Jeschke 2016).
5.3. Liver and Reduction of Steatosis
The liver is a major metabolic organ involved in fat metabolism. Although a major role of the liver under normal/healthy conditions is not to store fat, it is responsible for a significant amount of de novo lipogenesis, including esterification of dietary fatty acids derived from chylomicron remnants, and plasma non-esterified fatty acids (NEFA) derived from adipose tissue lipolysis. Beta-oxidation and triglyceride export in the form of very-low density lipoprotein (VLDL) also occur within the liver. Thus, in pathological states, such as overnutrition and/or diet-induced obesity, dysregulation of any of these processes could lead to abnormal fat deposition in the liver. In fact, excessive fat deposition could pose significant physiological consequences that could lead to metabolic diseases such as alcoholic and non-alcoholic fatty liver diseases.
Several nutrients have been found to protect against excessive hepatic fat deposition. These nutrients include methionine, choline, n-3 PUFAs, and folic acid. Both methionine and choline play important roles in the endogenous synthesis of phosphatidylcholine (Corbin and Zeisel 2012), one of the components of VLDL, and thus, the export of triglycerides from the liver. Indeed, mice fed a methionine and choline-deficient diet (MCD) develop severe hepatic steatosis (Anstee and Goldin 2006). In fact, MCD diet is so potent it is now used to fed mice as a model for in vivo/diet-induced liver damage. As discussed in a previous section, one of the primary benefits of n-3 PUFAs is its anti-inflammatory ability. However, within the liver n-3 PUFAs exert additional effects. As ligands of PPARα, which is a nuclear receptor for genes involved in β-oxidation, n-3 PUFAs have been shown to increase hepatic β-oxidation via upregulation of fatty acid binding protein (FABP) and CPT1 (Bordoni, et al. 2006; Jump 2008). In addition, n-3 PUFAs are shown to decrease hepatic lipogenesis by reducing the expression and maturation of sterol regulatory element binding protein-1c (SREBP-1c), thereby downregulating the transcription of hepatic lipogenic genes such as ACC and FAS (Howell, et al. 2009; Jump 2008). Lastly, because folic acid is a cofactor in the synthesis of 5-aminoimidazole-4-carboxamide 1-β-D-ribofuranoside (AICAR), which is an activator of AMPK, there is recent evidence suggesting that folic acid may be protective against hepatic steatosis. The likely mechanism seems that folic acid decreases lipogenesis via increasing activation of AMPK (Buettner, et al. 2010), which in turn reduces expression and/or activity of ACC. However, further validations are necessary to confirm these effects of folic acid.
Although basic research has generated a huge body of knowledge regarding nutritional regulation of the pathogenesis of steatosis, there are limited trials examining dietary interventions on hepatic fat deposition. Given the beneficial effects of dietary approaches for reducing body weight and adiposity, it is interesting to also examine whether the same approaches result in beneficial effects on hepatic steatosis. Indeed, there is an ongoing randomized controlled trail to examine whether consumption of a Mediterranean diet reduce liver fats (Papamiltiadous, et al. 2016). There is a hope that more this type of trials will be performed in near future.
5.4. Intestine and Improvement of Fat Metabolic Homeostasis
The digestion and absorption of triglycerides in the intestine is a complex progression involving emulsification, hydrolysis of fatty acid ester bonds, and aqueous dispersion of lipolytic products, and mainly occurs in the proximal jejunum and distal parts of the ileum. The major products of triglyceride digestion, monoglycerides and fatty acids, are absorbed by enterocytes through simple diffusion by use of micelles, which deliver the products from within the intestinal lumen to the intestinal microvilli. Once absorbed, products are re-synthesized into triglycerides within the smooth endoplasmic reticulum prior to excretion into the lymphatics system. This process occurs repeatedly, as dietary fats are consumed, and in healthy individuals does not seem to pose any negative metabolic affects. During the digestion and absorption processes, chylomicrons deliver dietary fats, and thus play a prominent role in fat homeostasis. Because of this, increasing evidence from animal studies has indicated that limiting the rate of intestinal triglyceride synthesis and/or altering chylomicron response could modulate gut hormone secretion, lipid metabolism, and systemic energy balance (Yen, et al. 2015).
Following overnutrition however, and particularly overnutrition with fat, research shows altered intestinal morphology including increased height and number of microvilli, jejunal mucosal weight, and total protein content (Dailey 2014). Consequently, these effects culminate not only in increased absorptive surface area but increased efficiency of fatty acid absorption within the intestine. While it is unclear if this effect alone impairs metabolism or stimulates pro-inflammatory mechanisms within the intestine, it does show that overnutrition with HFD significantly impacts the small intestine and subsequently, can contribute to increased circulating fatty acids/triglycerides. As discussed previously, this in turn can lead to increased fat deposition (i.e. weight gain and/or obesity) and the stimulation of pro-inflammatory signaling in distal tissues. Further, it is well documented that HFD directly contributes to intestinal inflammation (de La Serre et al. 2010; Guo et al. 2013), which can in turn impair nutrient assimilation (Semrin, et al. 2006). This review discusses dietary options to combat intestinal inflammation in other sections (see Section 4.3 and Section 6.4); however, at this time it is unclear whether dietary components such as phytoactive chemicals, fibers, and/or antioxidant vitamins/minerals can help protect against HFD-induced excessive fat absorption, alterations in intestinal morphology, and/or reverse impairments to nutrient absorption. Continued research to investigate any benefit of such nutrients in this manner would be greatly beneficial in further understanding the underlying mechanisms of HFD-induced/obesity-associated metabolic impairments and more importantly, combat such disturbances to potentially protect normal/healthy intestine function. Nonetheless, to support intestinal and overall health it is recommended to consume a diet low in fat and high in foods such as bran cereal fiber, vegetables, fruits and tea (Ley, et al. 2014; Via and Mechanick 2016; Weisburger 1997) (DRIs, USDA).
6. Nutritional Approaches for Insulin Sensitization
Alongside obesity, rates of diabetes have also drastically risen over the previous decades. In fact, the CDC currently estimates that 29 million Americans have diabetes, with roughly 95% of these cases being T2DM. As such, much research has investigated how to combat the onset of such a disorder both systemically and in specific tissues. Therefore, this section discusses specific consequences of insulin resistance and provides examples of successful nutrient interventions that help regulate impaired insulin signaling and/or protect against the onset of T2DM systemically. In this section, the mechanistic insights are focused on key tissues/organs that are essential for the regulation of systemic insulin sensitivity (Figures 2, 3, and 4).
6.1. Systemic Effects
T2DM/insulin resistance if not monitored can be very dangerous. Abnormal insulin signaling and thus, insufficient glucose uptake can be disastrous to many cell types and tissues that require glucose as a primary source of energy, such as the brain, red blood cells and kidneys. Further, insulin resistance is known to impair normal metabolic signaling in primary tissues involved in glucose homeostasis. For example, in skeletal muscle, the major organ for glucose utilization, impaired insulin signaling leads to reduced glycogen synthesis (Nikoulina, et al. 2001) which can greatly contribute to hyperglycemia. T2DM, especially when paired with obesity, can also severely interfere with normal nutrient-sensing mechanisms (Breen, et al. 2011) and lipid metabolism (Harmel, et al. 2014). All of these systemic impairments in turn exacerbate T2DM, and can lead to a vicious cycle of insulin resistance and metabolic dysfunction.
Several micronutrients are known to improve systemic insulin signaling in humans, even in the presence of obesity. For example, supplementation with vitamins D and E helps improve homeostasis model assessments of insulin resistance (HOMA-IR) scores (Belenchia, et al. 2013; Manning, et al. 2004; Talaei, et al. 2013), which is indicative of overall improved insulin sensitivity. High doses of thiamin/vitamin B1 (Alaei Shahmiri, et al. 2013; Luong and Nguyen 2012) and several minerals (Jayawardena, et al. 2012; Rodríguez-Moran and Guerrero-Romero 2014) are also suggested to prevent or slow the progression of hyperglycemia toward diabetes. Other dietary components have also recently been implicated in the management of obesity-related diseases in rodent models of obesity. Specifically, polyphenolics such as apple extract and the green tea extract epigallocatechin-3-gallate (EGCG) serve to not only lower HOMA-IR scores but exert anti-obesogenic properties as well (Brown, et al. 2009; Santamarina, et al. 2015).
In a study involving insulin-resistant human subjects, consumption of cod protein diet for 4 weeks was shown to cause a significant increase in insulin sensitivity in relative to consumption of a similar diet containing lean beef, pork, veal, eggs, milk, and milk products (Ouellet, et al. 2007). While encouraging, the study, however, could not completely rule out a contribution of n-3 PUFAs to the improvement in insulin sensitivity; although n-3 PUFAs did not improving insulin sensitivity in several trails (Itariu et al. 2012; Spencer et al. 2013). Because fat mass correlates well with systemic insulin resistance, many trials also analyzed changes in insulin sensitivity while examining dietary effects on weight loss. This was particularly true for trials involving MUFAs as described in Section 5.1 (Due et al. 2008; Paniagua et al. 2007). Overall, the insulin-sensitizing effects of nutritional approaches are highly associated with its effects on suppressing inflammation and/or reducing fat deposition.
6.2. Adipose Tissue and Insulin-sensitization
Under normal conditions, adipose tissue secretes a regulated amount of adipokines, such as leptin and adiponectin, both of which are involved in neuroendocrine control of appetite and behavior of food intake. Dietary fat is stored in adipose tissue as triglycerides, which consequently leads to decreased circulating FFAs. However, in pathological states such as overnutrition and particularly, overnutrition-induced obesity, increased triglyceride deposition in adipocytes leads to abnormal adipocyte metabolism and increased secretion of adipokines. Particularly, there are increased secretions of MCP-1 (Sartipy and Loskutoff 2003) and other cytokines including TNFα and IL-1β, secreted by both adipocytes and recruited macrophages. Increased levels of these cytokines are highly associated with increased lipolysis and decreased triglyceride synthesis. Consequently, elevated circulating FFAs damage pancreatic beta cells and reduce insulin secretion, and exacerbate hyperglycemia of T2DM.
Given that obesity is highly linked with T2DM, research to fully understand the pathology and/or focus on preventative/therapeutic benefits has greatly increased. Therefore, investigating dietary factors that may protect against the development of insulin resistance is paramount. Interestingly, both DHA and resveratrol are known to decrease angiogenesis within adipose tissue and improve insulin sensitivity, primarily through activation of SIRT1 (Luo, et al. 2016), which is a deacetylase that regulates expressions of genes involved in insulin secretion and adipocyte metabolism, among others. Further, Chardonnay grape seed flour, which contains high amounts of flavonoids, is shown to ameliorate obesity and insulin resistance, and improve glucose tolerance in diet-induced obese mice via the modulation of genes within adipose tissue (Seo, et al. 2015). The potential use of additional functional food components for combating insulin resistance in adipose tissue will be interesting to investigate, particularly for any potential combination therapeutic treatments.
6.3. Hepatic Regulation of Insulin Resistance
In the liver, normal insulin signaling transduces a cascade of actions that properly stimulate or inhibit glucose and fat metabolism, including stimulating glycolysis, glycogenesis, and lipogenesis, while inhibiting gluconeogenesis and glycogenolysis. During insulin resistance however, the signaling molecules within these cascades become impaired/inhibited by various factors including inflammatory mediators such as TNF-α, IL-6, and IL-1β and result in a loss of insulin signaling (Gregor and Hotamisligil 2011; Hirosumi et al. 2002).
However, dietary factors again prove to be beneficial in regulating these metabolic pathways. Specifically, fatty acids and micronutrients protect against insulin resistance in the liver both preventing the loss of insulin signaling molecules and reducing the generation of inflammation. For instance, n-3 PUFAs are shown to increase liver expression of IRS-1 and GLUT2 (Bargut, et al. 2014; Hein, et al. 2012), and also decrease liver inflammatory status, via increased production of resolvins, to improve insulin sentivity (González-Périz, et al. 2009). Simiar to n-3 PUFAs, palmitoleate is also shown to improve insulin sensitivty at both hepatic and systemic levels in mice (Guo et al. 2012). This insulin-senstizing effect is attributable to, at least in part, the anti-inflammatory effect of palmitoleate. However, palmitoleate supplementation increases hepatic lipogenic program through enhancing the transcription activity of SREBP1c, leading to an increase in hepatic fat deposition. While processing un-wanted effects, palmitoleate, in combination with other nutrients, may still offer benefits similar to those achieved by midterrean diets (Mashek and Wu 2015). Additional to fatty acids, vitamin D can also improve hepatic insulin resistance likely through improving the inflammatory status of immune cells such as macrophages (Sung, et al. 2012). Some evidence suggests that folic acid may also have this effect (Buettner et al. 2010); however, further investigations to fully understand the mechanisms of folic acid are warranted.
6.4. Intestine and Insulin-sensitization
It is now well established that inflammation can significantly contribute to insulin resistance, both systemically and within specific tissues. This is also true within the small intestine. Overnutrition with saturated fat can be particularly causative (Rivellese, et al. 2002) as saturated fat can stimulate multiple pro-inflammatory mechanisms. For instance, TLR4 expression, which is shown to be a key factor in the development of insulin resistance in many insulin-sensitive tissues, is increased in response to high saturated fat intake in small intestine. The interactions between HFD and bacterial populations with the intestinal tract are also associated with insulin resistance. For example, mice with conventional bacteria fed an HFD express significantly higher levels of TNFα, a well-known inhibitor of insulin signaling, compared to germ-free mice fed the same diet (Ding et al. 2010). Further, it has been shown that short-term HFD feeding leads to the translocation of commensal bacteria from the intestine to adipose tissue, which contributes to the development of inflammation and HFD-induced systemic hyperglycemia (Amar, et al. 2011). Thus, the intestine can both directly and indirectly contribute to the development of systemic insulin resistance within the diet-induced obese model, as it not only exhibits impaired insulin signaling itself, but also serves as a mediator for the onset of insulin resistance in distal tissues.
Regarding interventions to alleviate insulin resistance through targeting the small intestine, it seems the best approach is to reduce/prevent the generation of inflammation. Ingestion of vitamin E for example helps reduce the formation of free radicals within the intestine and thus, attenuates lipid peroxidation and protein oxidation to thereby reduce inflammation (Shirpoor, et al. 2007). Additional dietary treatments to reduce intestine inflammation are discussed above in Section 4.3. However, non-dietary treatments have been suggested as well. For example, direct treatment with anti-inflammatory cytokines can greatly improve the intestinal environment and protect against systemic insulin resistance. Specifically, interleukin 22 (IL-22) not only decreases endotoxemia and inflammation, but also serves to preserve the gut mucosal barrier and improve systemic insulin sensitivity (Wang, et al. 2014). IL-22 expression and/or activity are reduced in the obese phenotype (Wang et al. 2014) so treatment with this cytokine in patients with obesity-associated insulin resistance could drastically improve their impaired metabolic state. In addition, it is recently well documented that an HFD also induces phenotypic shifts within the intestinal microbiome that in turn, contribute to the development of obesity and systemic insulin resistance (Cani et al. 2008; Cani, et al. 2009; Patrice and Nathalie 2009; Turnbaugh et al. 2006). Therefore, interventions to retain commensal bacterial populations can also regulate the generation of inflammation and onset of insulin resistance. In fact, many studies have demonstrated that prebiotics and/or probiotics can counteract the negative metabolic effects and associated metabolic disorders induced by HFD (Everard, et al. 2011; Park, et al. 2013; Toral, et al. 2014; Wang, et al. 2015a).
7. Feeding Behavior and Physical Activity
The most effective way to prevent obesity-related metabolic diseases appears to be to prevent the development of obesity or to promote weight loss. Many pharmaceutical and herbal products exist for weight loss, promising “fast results” or “rapid weight loss”. Unfortunately no “quick fix” for weight loss exists. Healthy weight loss however, although a slower process, is greatly beneficial for overall health and metabolism and more importantly, is more sustainable. The most effective interventions to achieve such weight loss, and prevent weight gain in the already overweight/obese, are caloric restriction and physical activity. This section will discuss each intervention and outline the specific metabolic benefits each has to offer.
7. 1. Caloric Restriction
Caloric restriction (CR) involves negative energy balance short of malnutrition or deficiency of essential nutrients. It is a dietary intervention that can improve health and extend the life span in many species, including rodents, primates, and humans (Colman, et al. 2014; Heilbronn and Ravussin 2003). In addition to inducing weight-loss, CR is shown to improve glycemic control and insulin sensitivity in obese diabetic patients (Markovic TP 1998; Wing RR 1994). Similar results were also obtained in a randomized controlled trial involving obese infertile women (Becker, et al. 2015). Even in non-obese healthy adults, 2-year CR results in 10% weight loss without negatively altering life quality (Martin, et al. 2016). Similarly in healthy adults, Sparks et al. demonstrated that 12-month CR does not improve muscle mitochondrial functions, but appears to significantly decrease plasma levels of insulin and tends to decrease plasma levels of glucose, which likely indicate improvement of insulin sensitivity (Sparks, et al. 2017). Of interest, CR per se is a significant factor in generating metabolic benefits during weight loss in obese and diabetic patients (Wing RR 1994). A similar study has further suggested that the beneficial effects of CR on insulin action and glycemic control are related to changes in individual macronutrients. The latter is different from weight loss, whose beneficial effects are related to changes in abdominal fat (Markovic TP 1998). According to accumulating evidence, it seems that CR is beneficial in this manner for both obese and normal-weight subjects, with and without diabetes. For example, one study found that obese, non-diabetic human adults on an acute CR diet for 5 days had improved skeletal muscle insulin sensitivity via enhanced insulin signaling molecules (Wang, et al.). In addition, studies demonstrate that CR can also protect cardiovascular/endothelial function in obesity. For example, short-term CR of 80% of ad libitum in obese rats ameliorates impaired endothelial function and reduces systolic blood pressure (García-Prieto, et al. 2015). The likely mechanism seems that reduced caloric and/or nutrient intake stimulates endothelial AMPK activity and subsequently leads to activation of the PI3K-Akt-eNOS pathway. CR also improves ventricular hypertrophy, fibrosis, and diastolic dysfunction, and decreases the expression of angiotensin converting enzyme and angiotensin II type 1A receptor genes in the heart of obese rats (Takatsu, et al. 2013).
Given that inflammation is a major underlying factor of obesity and obesity-related diseases, much research has also investigated how CR may regulate the generation of inflammation. For instance, CR is viewed as a way to counter systemic inflammation caused by adipose tissue expansion (Ye and Keller 2010). To date, many studies have investigated this using both human and animal models of HFD-induced obesity, given the association between overnutrition and inflammation and/or the onset of obesity. Widespread results show that CR reduces HFD-induced proinflammatory markers, such as proinflammatory cytokines and NFκB, and the production of oxidative stress (Park, et al. 2012; Wasinski, et al. 2013). These findings are also true in clinical studies on diabetic, morbidly obese patients. For example, acute CR (400 kCal less/day) for 7 days reduced inflammation, improved insulin sensitivity and improved renal function (Giordani, et al. 2014). However, it should be noted that CR could cause adverse effects in some populations, such as the elderly or low BMI, diabetic patients receiving insulin (Darmon, et al.). Nonetheless, careful consideration and proper physician supervision should always be taken for any individual following such a diet plan (Bhardwaj, et al. 2013; Buchowski, et al. 2012; Takatsu et al. 2013; Tomada, et al. 2013).
7.2. Physical Activity and Caloric Restriction
Physical activity significantly increases energy expenditure and therefore is commonly used an intervention for weight loss and/or preventing weight gain. This intervention is especially beneficial for obese individuals (Baillot, et al. 2015; Chin, et al. 2016; Fock and Khoo 2013; Jakicic and Otto 2005), who commonly lead an overall sedentary lifestyle. In fact, physical activity is the most highly recommended intervention to prevent and slow weight gain, and lower BMI in obese children (Alkon, et al. 2014; Bautista-Castaño, et al. 2004; Lee, et al. 2012). Furthermore, physical activity is not only beneficial for weight loss. A vast number of studies have demonstrated that physical activity is greatly beneficial in maintaining and promoting overall health via increased cardiovascular efficiency, insulin signaling in skeletal muscle, and in young individuals, the prevention of osteoporosis (Bassuk and Manson 2005; Borer 2005; Klein, et al. 2004; Roberts, et al. 2006).
Due to the beneficial abilities of CR and physical activity, independently, in preventing and/or combating obesity and obesity-associated disorders, numerous studies have investigated the effect of a combination intervention on such disorders. Unsurprisingly, results of such studies demonstrate positive results. For example, exercise and CR together can help lower the obesity index by reducing visceral fat, reduce levels of serum biomarkers such as LDL and triglycerides, and decrease inflammatory indicators (Ekuni, et al. 2014; Vissers, et al. 2013). Further, a combined intervention can reduce cardiovascular risks in obese individuals diagnosed with metabolic syndrome (Dutheil, et al.). Several studies report that a combination of exercise and CR exerts more beneficial effects than either physical activity or CR alone (Wang, et al. 2015b). Such combination interventions are also useful approaches for preventing the complications of NAFLD, commonly seen in the obese (Nseir, et al. 2014).
8. Childhood Obesity
Obesity remains a global epidemic. Alarmingly, the percentage of children with obesity in the United States has more than tripled since the 1970s, based on Childhood Obesity Facts released by the CDC. The situation is even worse in developing countries including China, where the percentage of childhood obesity increases much greater and faster. Given the difficulty in reducing body weight once obesity is established, it is considered as an urgent need to initiate prevention and treatment of obesity in children (Dehghan, et al. 2005). As demonstrated by numerous studies, total diet and physical activity level of a child critically determine a child’s body weight. Therefore, nutrition approaches are crucial for prevention of childhood obesity. It is equally important to establish and enforce programs to increase physical activities in both pre-school and school-age children. The pertinent details have been described elsewhere, and are not discussed here.
9. Conclusion
The increase in the incidence of obesity makes obesity and its related metabolic diseases a huge problem in public health. Over the past several decades, mounting evidence obtained from both basic and clinical research has much advanced our understanding of the pathogenesis of metabolic diseases and led to the validation and application of nutritional approaches for managing metabolic diseases. As described above, nutritional approaches are commonly used for suppressing inflammation, improving insulin sensitivity, and/or decreasing fat deposition (Figure 5). While promising, the current approaches are also challenged by existing and new issues, e.g., poor long-term efficacy and reproducibility, and increased prevalence of childhood obesity. Another significant challenge is that human studies, in relation to animal studies, often have limitations in methodologies, which prevents performing insightful experiments to establish cause-and-effect relationships. To solve the issues, many efforts are needed to enhance nutrition research. In particular, basic and clinical nutrition research is needed to extensively investigate how various diet compositions differentially alter metabolic homeostasis. There is also a need to conduct long-term randomized- and controlled trials to firmly establish the beneficial effects of nutritional approaches. Further, how such factors may differ within the context of childhood and adolescent obesity is of paramount importance. Lastly, it might be a time now for a philosophic change: to use nutritional approaches, together with healthy life style at all ages, for promoting health and preventing obesity and metabolic diseases, not for treating metabolic diseases.
Acknowledgments
The authors thank the Deputy Editor and editorial board for the invitation to contribute this review article. This work was supported in part by grants from the National Institutes of Health (R01DK095828 and R01DK095862) and American Diabetes Association (1-17-IBS-145) (to C.W.). Also, C.W. is supported by the Hatch Program of the National Institutes of Food and Agriculture (NIFA).
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Footnotes
Author Disclosure
R. Botchlett, S. Woo, M. Liu, Y. Pei, X. Guo, H. Li, and C. Wu have no conflicts of interest.
References
- Abdullahi A, Jeschke MG. White Adipose Tissue Browning: A Double-edged Sword. Trends in Endocrinology & Metabolism. 2016;27:542–552. doi: 10.1016/j.tem.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal BB. Targeting Inflammation-Induced Obesity and Metabolic Diseases by Curcumin and Other Nutraceuticals. Annual Review of Nutrition. 2010;30:173–199. doi: 10.1146/annurev.nutr.012809.104755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaei Shahmiri F, Soares MJ, Zhao Y, Sherriff J. High-dose thiamine supplementation improves glucose tolerance in hyperglycemic individuals: a randomized, double-blind cross-over trial. European Journal of Nutrition. 2013;52:1821–1824. doi: 10.1007/s00394-013-0534-6. [DOI] [PubMed] [Google Scholar]
- Alghasham A, Salem TA, Meki A-RM. Effect of cadmium-polluted water on plasma levels of tumor necrosis factor-α, interleukin-6 and oxidative status biomarkers in rats: Protective effect of curcumin. Food and Chemical Toxicology. 2013;59:160–164. doi: 10.1016/j.fct.2013.05.059. [DOI] [PubMed] [Google Scholar]
- Alkon A, Crowley AA, Neelon SEB, Hill S, Pan Y, Nguyen V, Rose R, Savage E, Forestieri N, Shipman L, et al. Nutrition and physical activity randomized control trial in child care centers improves knowledge, policies, and children’s body mass index. BMC Public Health. 2014;14:215. doi: 10.1186/1471-2458-14-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amar J, Chabo C, Waget A, Klopp P, Vachoux C, Bermúdez-Humarán LG, Smirnova N, Bergé M, Sulpice T, Lahtinen S, et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Molecular Medicine. 2011;3:559–572. doi: 10.1002/emmm.201100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosini GL, Emmett PM, Northstone K, Howe LD, Tilling K, Jebb SA. Identification of a dietary pattern prospectively associated with increased adiposity during childhood and adolescence. International Journal of Obesity (2005) 2012;36:1299–1305. doi: 10.1038/ijo.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstee QM, Goldin RD. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. International Journal of Experimental Pathology. 2006;87:1–16. doi: 10.1111/j.0959-9673.2006.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel LJ, Sacks FM, Carey VJ, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: Results of the omniheart randomized trial. JAMA. 2005;294:2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- Argiana V, Kanellos PΤ, Makrilakis K, Eleftheriadou I, Tsitsinakis G, Kokkinos A, Perrea D, Tentolouris N. The effect of consumption of low-glycemic-index and low-glycemic-load desserts on anthropometric parameters and inflammatory markers in patients with type 2 diabetes mellitus. European Journal of Nutrition. 2015;54:1173–1180. doi: 10.1007/s00394-014-0795-8. [DOI] [PubMed] [Google Scholar]
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li Z-W, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-[beta] links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- Astrup A, Bugel S. Micronutrient deficiency in the aetiology of obesity. Int J Obes. 2010;34:947–948. doi: 10.1038/ijo.2010.81. [DOI] [PubMed] [Google Scholar]
- Azrad M, Turgeon C, Demark-Wahnefried W. Current Evidence Linking Polyunsaturated Fatty Acids with Cancer Risk and Progression. Frontiers in Oncology. 2013;3:224. doi: 10.3389/fonc.2013.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillot A, Romain AJ, Boisvert-Vigneault K, Audet M, Baillargeon JP, Dionne IJ, Valiquette L, Chakra CNA, Avignon A, Langlois M-F. Effects of Lifestyle Interventions That Include a Physical Activity Component in Class II and III Obese Individuals: A Systematic Review and Meta-Analysis. PLoS ONE. 2015;10:e0119017. doi: 10.1371/journal.pone.0119017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banin RM, Hirata BKS, Andrade IS, Zemdegs JCS, Clemente APG, Dornellas APS, Boldarine VT, Estadella D, Albuquerque KT, Oyama LM, et al. Beneficial effects of Ginkgo biloba extract on insulin signaling cascade, dyslipidemia, and body adiposity of diet-induced obese rats. Brazilian Journal of Medical and Biological Research. 2014;47:780–788. doi: 10.1590/1414-431X20142983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, Hong S, Serhan CN. Molecular Circuits of Resolution: Formation and Actions of Resolvins and Protectins. The Journal of Immunology. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- Bargut TCL, Frantz EDC, Mandarim-de-Lacerda CA, Aguila MB. Effects of a Diet Rich in n-3 Polyunsaturated Fatty Acids on Hepatic Lipogenesis and Beta-Oxidation in Mice. Lipids. 2014;49:431–444. doi: 10.1007/s11745-014-3892-9. [DOI] [PubMed] [Google Scholar]
- Barroso E, Rodríguez-Calvo R, Serrano-Marco L, Astudillo AM, Balsinde J, Palomer X, Vázquez-Carrera M. The PPARβ/δ Activator GW501516 Prevents the Down-Regulation of AMPK Caused by a High-Fat Diet in Liver and Amplifies the PGC-1α-Lipin 1-PPARα Pathway Leading to Increased Fatty Acid Oxidation. Endocrinology. 2011;152:1848–1859. doi: 10.1210/en.2010-1468. [DOI] [PubMed] [Google Scholar]
- Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014;10:24–36. doi: 10.1038/nrendo.2013.204. [DOI] [PubMed] [Google Scholar]
- Bassuk SS, Manson JE. Epidemiological evidence for the role of physical activity in reducing risk of type 2 diabetes and cardiovascular disease. Journal of Applied Physiology. 2005;99:1193. doi: 10.1152/japplphysiol.00160.2005. [DOI] [PubMed] [Google Scholar]
- Bautista-Castaño I, Doreste J, Serra-Majem L. Effectiveness of interventions in the prevention of childhood obesity. Eur J Epidemiol. 2004;19:617–622. doi: 10.1023/b:ejep.0000036890.72029.7c. [DOI] [PubMed] [Google Scholar]
- Becker GF, Passos EP, Moulin CC. Short-term effects of a hypocaloric diet with low glycemic index and low glycemic load on body adiposity, metabolic variables, ghrelin, leptin, and pregnancy rate in overweight and obese infertile women: a randomized controlled trial. The American Journal of Clinical Nutrition. 2015;102:1365–1372. doi: 10.3945/ajcn.115.117200. [DOI] [PubMed] [Google Scholar]
- Belenchia AM, Tosh AK, Hillman LS, Peterson CA. Correcting vitamin D insufficiency improves insulin sensitivity in obese adolescents: a randomized controlled trial. The American Journal of Clinical Nutrition. 2013;97:774–781. doi: 10.3945/ajcn.112.050013. [DOI] [PubMed] [Google Scholar]
- Berg AH, Scherer PE. Adipose Tissue, Inflammation, and Cardiovascular Disease. Circulation Research. 2005;96:939. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- Bhardwaj P, Du B, Zhou XK, Sue E, Harbus MD, Falcone DJ, Giri D, Hudis CA, Kopelovich L, Subbaramaiah K, et al. Caloric Restriction Reverses Obesity-Induced Mammary Gland Inflammation in Mice. Cancer Prevention Research. 2013;6:282. doi: 10.1158/1940-6207.CAPR-12-0467. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5·24 million UK adults. The Lancet. 384:755–765. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop KS, Ferguson LR. The Interaction between Epigenetics, Nutrition and the Development of Cancer. Nutrients. 2015;7:922–947. doi: 10.3390/nu7020922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleau C1KA, St-Pierre DH2, Lamontagne L. Crosstalk between intestinal microbiota, adipose tissue and skeletal muscle as an early event in systemic low-grade inflammation and the development of obesity and diabetes. Diabetes Metab Res Rev. 2015;31:545–561. doi: 10.1002/dmrr.2617. [DOI] [PubMed] [Google Scholar]
- Bordoni A, Di Nunzio M, Danesi F, Biagi PL. Polyunsaturated fatty acids: From diet to binding to ppars and other nuclear receptors. Genes & Nutrition. 2006;1:95–106. doi: 10.1007/BF02829951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borer KT. Physical activity in the prevention and amelioration of osteoporosis in women. Sports Medicine. 2005;35:779–830. doi: 10.2165/00007256-200535090-00004. [DOI] [PubMed] [Google Scholar]
- Botchlett R, Li H, Guo X, Qi T, Zhao J, Zheng J, Woo S-L, Pei Y, Liu M, Hu X, et al. Glucose and palmitate differentially regulate PFKFB3/iPFK2 and inflammatory responses in mouse intestinal epithelial cells. Scientific Reports. 2016;6:28963. doi: 10.1038/srep28963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen DM, Yang CS, Lam TKT. Gut–brain signalling: how lipids can trigger the gut. Diabetes/Metabolism Research and Reviews. 2011;27:113–119. doi: 10.1002/dmrr.1160. [DOI] [PubMed] [Google Scholar]
- Brown AL, Lane J, Coverly J, Stocks J, Jackson S, Stephen A, Bluck L, Coward A, Hendrickx H. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: randomized controlled trial. The British journal of nutrition. 2009;101:886–894. doi: 10.1017/S0007114508047727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchowski MS, Hongu N, Acra S, Wang L, Warolin J, Roberts LJ., II Effect of Modest Caloric Restriction on Oxidative Stress in Women, a Randomized Trial. PLoS ONE. 2012;7:e47079. doi: 10.1371/journal.pone.0047079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner R, Bettermann I, Hechtl C, Gäbele E, Hellerbrand C, Schölmerich J, Bollheimer LC. Dietary Folic Acid Activates AMPK and Improves Insulin Resistance and Hepatic Inflammation in Dietary Rodent Models of the Metabolic Syndrome. Horm Metab Res. 2010;42:769–774. doi: 10.1055/s-0030-1263122. [DOI] [PubMed] [Google Scholar]
- Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nature medicine. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC. Omega-3 Fatty Acids and Inflammatory Processes. Nutrients. 2010;2:355–374. doi: 10.3390/nu2030355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet–Induced Obesity and Diabetes in Mice. Diabetes. 2008;57:1470. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreiro AL, Dhillon J, Gordon S, Higgins KA, Jacobs AG, McArthur BM, Redan BW, Rivera RL, Schmidt LR, Mattes RD. The Macronutrients, Appetite, and Energy Intake. Annual Review of Nutrition. 2016;36:73–103. doi: 10.1146/annurev-nutr-121415-112624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawley J, Meyerhoefer C. The medical care costs of obesity: An instrumental variables approach. Journal of Health Economics. 2012;31:219–230. doi: 10.1016/j.jhealeco.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Chin SH, Kahathuduwa CN, Binks M. Physical activity and obesity: what we know and what we need to know. Obesity Reviews. 2016:n/a–n/a. doi: 10.1111/obr.12460. [DOI] [PubMed] [Google Scholar]
- Cho I, Blaser MJ. The Human Microbiome: at the interface of health and disease. Nature reviews Genetics. 2012;13:260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Choi Y, Choi Y, Kim S, Jang J, Park T. Piperine reverses high fat diet-induced hepatic steatosis and insulin resistance in mice. Food Chemistry. 2013;141:3627–3635. doi: 10.1016/j.foodchem.2013.06.028. [DOI] [PubMed] [Google Scholar]
- Chu CA, Sherck SM, Igawa K, Sindelar DK, Neal DW, Emshwiller M, Cherrington AD. Effects of free fatty acids on hepatic glycogenolysis and gluconeogenesis in conscious dogs. American Journal of Physiology - Endocrinology And Metabolism. 2002;282:E402. doi: 10.1152/ajpendo.00136.2001. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nature Communications. 2014;5:3557. doi: 10.1038/ncomms4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin KD, Zeisel SH. Choline Metabolism Provides Novel Insights into Non-alcoholic Fatty Liver Disease and its Progression. Current opinion in gastroenterology. 2012;28:159–165. doi: 10.1097/MOG.0b013e32834e7b4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couet C, Delarue J, Ritz P, Antoine JM, Lamisse F. Effect of dietary fish oil on body fat mass and basal fat oxidation in healthy adults. International Journal of Obesity. 1997;21:637–643. doi: 10.1038/sj.ijo.0800451. [DOI] [PubMed] [Google Scholar]
- Dailey MJ. Nutrient-induced intestinal adaption and its effect in obesity. Physiology & behavior. 2014;0:74–78. doi: 10.1016/j.physbeh.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmon P, Kaiser MJ, Bauer JM, Sieber CC, Pichard C. Restrictive diets in the elderly: Never say never again? Clinical Nutrition. 29:170–174. doi: 10.1016/j.clnu.2009.11.002. [DOI] [PubMed] [Google Scholar]
- de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2010;299:G440–G448. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghan M, Akhtar-Danesh N, Merchant AT. Childhood obesity, prevalence and prevention. Nutrition Journal. 2005;4:24. doi: 10.1186/1475-2891-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud D-J, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. Journal of Lipid Research. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NMJ, Magness S, Jobin C, Lund PK. High-Fat Diet: Bacteria Interactions Promote Intestinal Inflammation Which Precedes and Correlates with Obesity and Insulin Resistance in Mouse. PLoS ONE. 2010;5:e12191. doi: 10.1371/journal.pone.0012191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez Coello S, Cabrera de León A, Rodríguez Pérez MC, Borges Álamo C, Carrillo Fernández L, Almeida González D, García Yanes J, González Hernández A, Brito Díaz B, Aguirre-Jaime A. Association between glycemic index, glycemic load, and fructose with insulin resistance: the CDC of the Canary Islands study. European Journal of Nutrition. 2010;49:505–512. doi: 10.1007/s00394-010-0110-2. [DOI] [PubMed] [Google Scholar]
- Du S, Jin J, Fang W, Su Q. Does Fish Oil Have an Anti-Obesity Effect in Overweight/Obese Adults? A Meta-Analysis of Randomized Controlled Trials. PLoS ONE. 2015;10:e0142652. doi: 10.1371/journal.pone.0142652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z-Y, Wei X, Huang M-T, Zheng X, Liu Y, Conney AH, Zhang K. Anti-proliferative, anti-inflammatory and antioxidant effects of curcumin analogue A2. Archives of Pharmacal Research. 2013;36:1204–1210. doi: 10.1007/s12272-013-0216-1. [DOI] [PubMed] [Google Scholar]
- Due A, Larsen TM, Mu H, Hermansen K, Stender S, Astrup A. Comparison of 3 ad libitum diets for weight-loss maintenance, risk of cardiovascular disease, and diabetes: A 6-mo randomized, controlled trial. American Journal of Clinical Nutrition. 2008;88:1232–1241. doi: 10.3945/ajcn.2007.25695. [DOI] [PubMed] [Google Scholar]
- Dutheil F, Lac G, Lesourd B, Chapier R, Walther G, Vinet A, Sapin V, Verney J, Ouchchane L, Duclos M, et al. Different modalities of exercise to reduce visceral fat mass and cardiovascular risk in metabolic syndrome: the RESOLVE* randomized trial. International Journal of Cardiology. 168:3634–3642. doi: 10.1016/j.ijcard.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Egger G. In Search of a Germ Theory Equivalent for Chronic Disease. Preventing Chronic Disease. 2012;9:E95. doi: 10.5888/pcd9.110301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekuni D, Mizutani S, Kojima A, Tomofuji T, Irie K, Azuma T. Relationship between increases in BMI and changes in periodontal status: a prospective cohort study. J Clin Periodontol. 2014;41 doi: 10.1111/jcpe.12273. [DOI] [PubMed] [Google Scholar]
- Emilsson V, Thorleifsson G, Zhang B, Leonardson AS, Zink F, Zhu J, Carlson S, Helgason A, Walters GB, Gunnarsdottir S, et al. Genetics of gene expression and its effect on disease. Nature. 2008;452:423–428. doi: 10.1038/nature06758. [DOI] [PubMed] [Google Scholar]
- Esfahani A, Wong JMW, Truan J, Villa CR, Mirrahimi A, Srichaikul K, Kendall CWC. Health Effects of Mixed Fruit and Vegetable Concentrates: A Systematic Review of the Clinical Interventions. Journal of the American College of Nutrition. 2011;30:285–294. doi: 10.1080/07315724.2011.10719971. [DOI] [PubMed] [Google Scholar]
- Everard A, Lazarevic V, Derrien M, Girard M, Muccioli GM, Neyrinck AM, Possemiers S, Van Holle A, François P, de Vos WM, et al. Responses of Gut Microbiota and Glucose and Lipid Metabolism to Prebiotics in Genetic Obese and Diet-Induced Leptin-Resistant Mice. Diabetes. 2011;60:2775–2786. doi: 10.2337/db11-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Heller NM, Gorospe M, Atasoy U, Stellato C. The role of post-transcriptional regulation in chemokine gene expression in inflammation and allergy. European Respiratory Journal. 2005;26:933. doi: 10.1183/09031936.05.00120204. [DOI] [PubMed] [Google Scholar]
- Farrokhian A, Raygan F, Bahmani F, Talari HR, Esfandiari R, Esmaillzadeh A, Asemi Z. Long-term vitamin D supplementation affects metabolic status in vitamin D–deficient type 2 diabetic patients with coronary artery disease. The Journal of Nutrition. 2017;147:384–389. doi: 10.3945/jn.116.242008. [DOI] [PubMed] [Google Scholar]
- Flowers MT, Ntambi JM. Stearoyl-CoA desaturase and its relation to high-carbohydrate diets and obesity. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2009;1791:85–91. doi: 10.1016/j.bbalip.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fock KM, Khoo J. Diet and exercise in management of obesity and overweight. Journal of Gastroenterology and Hepatology. 2013;28:59–63. doi: 10.1111/jgh.12407. [DOI] [PubMed] [Google Scholar]
- Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. The American Journal of Clinical Nutrition. 2002;76:5–56. doi: 10.1093/ajcn/76.1.5. [DOI] [PubMed] [Google Scholar]
- Freudenberg A, Petzke KJ, Klaus S. Dietary l-leucine and l-alanine supplementation have similar acute effects in the prevention of high-fat diet-induced obesity. Amino Acids. 2013;44:519–528. doi: 10.1007/s00726-012-1363-2. [DOI] [PubMed] [Google Scholar]
- Furtado JD, Campos H, Appel LJ, Miller ER, Laranjo N, Carey VJ, Sacks FM. Effect of protein, unsaturated fat, and carbohydrate intakes on plasma apolipoprotein B and VLDL and LDL containing apolipoprotein C-III: results from the OmniHeart Trial. The American Journal of Clinical Nutrition. 2008;87:1623–1630. doi: 10.1093/ajcn/87.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadgil MD, Appel LJ, Yeung E, Anderson CAM, Sacks FM, Miller ER. The Effects of Carbohydrate, Unsaturated Fat, and Protein Intake on Measures of Insulin Sensitivity: Results from the OmniHeart Trial. Diabetes Care. 2013;36:1132–1137. doi: 10.2337/dc12-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Prieto CF, Pulido-Olmo H, Ruiz-Hurtado G, Gil-Ortega M, Aranguez I, Rubio MA, Ruiz-Gayo M, Somoza B, Fernández-Alfonso MS. Mild caloric restriction reduces blood pressure and activates endothelial AMPK-PI3KP-Akt-eNOS pathway in obese Zucker rats. Vascular Pharmacology. 2015:65–66. doi: 10.1016/j.vph.2014.12.001. [DOI] [PubMed] [Google Scholar]
- García OP, Long KZ, Rosado JL. Impact of micronutrient deficiencies on obesity. Nutrition Reviews. 2009;67:559. doi: 10.1111/j.1753-4887.2009.00228.x. [DOI] [PubMed] [Google Scholar]
- George PS, Pearson ER, Witham MD. Effect of vitamin D supplementation on glycaemic control and insulin resistance: a systematic review and meta-analysis. Diabetic Medicine. 2012;29:e142–e150. doi: 10.1111/j.1464-5491.2012.03672.x. [DOI] [PubMed] [Google Scholar]
- Giordani I, Malandrucco I, Donno S, Picconi F, Di Giacinto P, Di Flaviani A, Chioma L, Frontoni S. Acute caloric restriction improves glomerular filtration rate in patients with morbid obesity and type 2 diabetes. Diabetes Metab. 2014;40:158–160. doi: 10.1016/j.diabet.2013.12.006. [DOI] [PubMed] [Google Scholar]
- González-Périz A, Horrillo R, Ferré N, Gronert K, Dong B, Morán-Salvador E, Titos E, Martínez-Clemente M, López-Parra M, Arroyo V, et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by ω-3 fatty acids: a role for resolvins and protectins. The FASEB Journal. 2009;23:1946–1957. doi: 10.1096/fj.08-125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Périz A, Planagumà A, Gronert K, Miquel R, López-Parra M, Titos E, Horrillo R, Ferré N, Deulofeu R, Arroyo V, et al. Docosahexaenoic acid (DHA) blunts liver injury by conversion to protective lipid mediators: protectin D1 and 17S-hydroxy-DHA. The FASEB Journal. 2006;20:2537–2539. doi: 10.1096/fj.06-6250fje. [DOI] [PubMed] [Google Scholar]
- Gregor MF, Hotamisligil GS. Inflammatory Mechanisms in Obesity. Annual Review of Immunology. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- Guo T, Woo S-L, Guo X, Li H, Zheng J, Botchlett R, Liu M, Pei Y, Xu H, Cai Y, et al. Berberine ameliorates hepatic steatosis and suppresses liver and adipose tissue inflammation in mice with diet-induced obesity. Scientific Reports. 2016;6:22612. doi: 10.1038/srep22612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Li H, Xu H, Halim V, Thomas LN, Woo S-L, Huo Y, Chen YE, Sturino JM, Wu C. Disruption of inducible 6-phosphofructo-2-kinase impairs the suppressive effect of PPARγ activation on diet-induced intestine inflammatory response. The Journal of Nutritional Biochemistry. 2013;24:770–775. doi: 10.1016/j.jnutbio.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Li H, Xu H, Halim V, Zhang W, Wang H, Ong KT, Woo S-L, Walzem RL, Mashek DG, et al. Palmitoleate induces hepatic steatosis but suppresses liver inflammatory response in mice. PLoS ONE. 2012;7:e39286. doi: 10.1371/journal.pone.0039286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Pan X-Y, Xu Y, Xiao Y, An Y, Tie L, Pan Y, Li X-J. Curcumin induces autophagy to protect vascular endothelial cell survival from oxidative stress damage. Autophagy. 2012;8:812–825. doi: 10.4161/auto.19471. [DOI] [PubMed] [Google Scholar]
- Hardy OT, Perugini RA, Nicoloro SM, Gallagher-Dorval K, Puri V, Straubhaar J, Czech MP. BMI-independent inflammation in omental adipose tissue associated with insulin resistance in morbid obesity. Surgery for obesity and related diseases: official journal of the American Society for Bariatric Surgery. 2011;7:60–67. doi: 10.1016/j.soard.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmel E, Grenier E, Ouadda AB, Chebly ME, Ziv E, Beaulieu JF, Sané A, Spahis S, Laville M, Levy E. AMPK in the Small Intestine in Normal and Pathophysiological Conditions. Endocrinology. 2014;155:873–888. doi: 10.1210/en.2013-1750. [DOI] [PubMed] [Google Scholar]
- He C, Shan Y, Song W. Targeting gut microbiota as a possible therapy for diabetes. Nutrition Research. 2015a;35:361–367. doi: 10.1016/j.nutres.2015.03.002. [DOI] [PubMed] [Google Scholar]
- He F, Bixler EO, Liao J, Berg A, Imamura Kawasawa Y, Fernandez-Mendoza J, Vgontzas AN, Liao D. Habitual sleep variability, mediated by nutrition intake, is associated with abdominal obesity in adolescents. Sleep Medicine. 2015b;16:1489–1494. doi: 10.1016/j.sleep.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. The American Journal of Clinical Nutrition. 2003;78:361–369. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- Hein GJ, Chicco A, Lombardo YB. Fish Oil Normalizes Plasma Glucose Levels and Improves Liver Carbohydrate Metabolism in Rats Fed a Sucrose-Rich Diet. Lipids. 2012;47:141–150. doi: 10.1007/s11745-011-3623-4. [DOI] [PubMed] [Google Scholar]
- Heinritz SN, Weiss E, Eklund M, Aumiller T, Louis S, Rings A, Messner S, Camarinha-Silva A, Seifert J, Bischoff SC, et al. Intestinal Microbiota and Microbial Metabolites Are Changed in a Pig Model Fed a High-Fat/Low-Fiber or a Low-Fat/High-Fiber Diet. PLoS ONE. 2016;11:e0154329. doi: 10.1371/journal.pone.0154329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo J, Seo M, Park H, Lee WK, Guan LL, Yoon J, Caetano-Anolles K, Ahn H, Kim S-Y, Kang Y-M, et al. Gut microbiota modulated by probiotics and Garcinia cambogia extract correlate with weight gain and adipocyte sizes in high fat-fed mice. Scientific Reports. 2016;6:33566. doi: 10.1038/srep33566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashida K, Kim SH, Jung SR, Asaka M, Holloszy JO, Han D-H. Effects of Resveratrol and SIRT1 on PGC-1α Activity and Mitochondrial Biogenesis: A Reevaluation. PLoS Biology. 2013;11:e1001603. doi: 10.1371/journal.pbio.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- Hörnell A, Lagström H, Lande B, Thorsdottir I. Protein intake from 0 to 18 years of age and its relation to health: a systematic literature review for the 5th Nordic Nutrition Recommendations. Food & Nutrition Research. 2013;57 doi: 10.3402/fnr.v3457i3400.21083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nature reviews Immunology. 2008;8:923. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell G, Deng X, Yellaturu C, Park EA, Wilcox HG, Raghow R, Elam MB. N-3 Polyunsaturated Fatty Acids Suppress Insulin-induced SREBP-1c Transcription via Reduced Trans-activating Capacity of LXRα. Biochimica et biophysica acta. 2009;1791:1190–1196. doi: 10.1016/j.bbalip.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Zhao Q, Liu H, Guo Y, Xu H. PPAR-α Agonist WY-14643 Inhibits LPS-Induced Inflammation in Synovial Fibroblasts via NF-kB Pathway. Journal of Molecular Neuroscience. 2016;59:544–553. doi: 10.1007/s12031-016-0775-y. [DOI] [PubMed] [Google Scholar]
- Hudgins LC, Hellerstein MK, Seidman CE, Neese RA, Tremaroli JD, Hirsch J. Relationship between carbohydrate-induced hypertriglyceridemia and fatty acid synthesis in lean and obese subjects. Journal of Lipid Research. 2000;41:595–604. [PubMed] [Google Scholar]
- Huo Y, Guo X, Li H, Wang H, Zhang W, Wang Y, Zhou H, Gao Z, Telang S, Chesney J, et al. Disruption of inducible 6-phosphofructo-2-kinase ameliorates diet-induced adiposity but exacerbates systemic insulin resistance and adipose tissue inflammatory response. Journal of Biological Chemistry. 2010;285:3713–3721. doi: 10.1074/jbc.M109.058446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itariu BK, Zeyda M, Hochbrugger EE, Neuhofer A, Prager G, Schindler K, Bohdjalian A, Mascher D, Vangala S, Schranz M, et al. Long-chain n−3 PUFAs reduce adipose tissue and systemic inflammation in severely obese nondiabetic patients: a randomized controlled trial. The American Journal of Clinical Nutrition. 2012;96:1137–1149. doi: 10.3945/ajcn.112.037432. [DOI] [PubMed] [Google Scholar]
- J Suliburska SC, Gajewska E, Kalmus G, Sobieska M, Samborski W, Krejpcio Z, Drzymala-Czyz S, Bogdanski P. The evaluation of selected serum mineral concentrations and their association with insulin resistance in obese adolescents. Eur Rev Med Pharmacol Sci. 2013;17:2396–2400. [PubMed] [Google Scholar]
- Jakicic JM, Otto AD. Physical activity considerations for the treatment and prevention of obesity. The American Journal of Clinical Nutrition. 2005;82:226S–229S. doi: 10.1093/ajcn/82.1.226S. [DOI] [PubMed] [Google Scholar]
- Janssens PLHR, Hursel R, Martens EAP, Westerterp-Plantenga MS. Acute Effects of Capsaicin on Energy Expenditure and Fat Oxidation in Negative Energy Balance. PLoS ONE. 2013;8:e67786. doi: 10.1371/journal.pone.0067786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayawardena R, Ranasinghe P, Galappatthy P, Malkanthi R, Constantine G, Katulanda P. Effects of zinc supplementation on diabetes mellitus: a systematic review and meta-analysis. Diabetology & Metabolic Syndrome. 2012;4:13. doi: 10.1186/1758-5996-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins DJ, Kendall CW, Augustin LS, Franceschi S, Hamidi M, Marchie A, Jenkins AL, Axelsen M. Glycemic index: overview of implications in health and disease. The American Journal of Clinical Nutrition. 2002;76:266S–273S. doi: 10.1093/ajcn/76/1.266S. [DOI] [PubMed] [Google Scholar]
- Joffin N, Jaubert A-M, Durant S, Barouki R, Forest C, Noirez P. Citrulline counteracts overweight- and aging-related effects on adiponectin and leptin gene expression in rat white adipose tissue. Biochimie Open. 2015;1:1–5. doi: 10.1016/j.biopen.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jump DB. N-3 polyunsaturated fatty acid regulation of hepatic gene transcription. Current Opinion in Lipidology. 2008;19:242–247. doi: 10.1097/MOL.0b013e3282ffaf6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant V, Gopal A, Pathak NN, Kumar P, Tandan SK, Kumar D. Antioxidant and anti-inflammatory potential of curcumin accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats. International Immunopharmacology. 2014;20:322–330. doi: 10.1016/j.intimp.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Kim M-Y, Bae Y-J, Bak Y-K, Kim J, Sung M-K. Effects of high-fat diet on intestinal permeability and inflammation-associated tumorigenesis. The FASEB Journal. 2012;26:376–377. [Google Scholar]
- Kim Y-B, Shulman GI, Kahn BB. Fatty Acid Infusion Selectively Impairs Insulin Action on Akt1 and Protein Kinase C λ/ζ but Not on Glycogen Synthase Kinase-3. Journal of Biological Chemistry. 2002;277:32915–32922. doi: 10.1074/jbc.M204710200. [DOI] [PubMed] [Google Scholar]
- Klein S, Burke LE, Bray GA, Blair S, Allison DB, Pi-Sunyer X, Hong Y, Eckel RH. Clinical implications of obesity with specific focus on cardiovascular disease. Circulation. 2004;110:2952. doi: 10.1161/01.CIR.0000145546.97738.1E. [DOI] [PubMed] [Google Scholar]
- Kopecky J, Rossmeisl M, Flachs P, Kuda O, Brauner P, Jilkova Z, Stankova B, Tvrzicka E, Bryhn M. n-3 PUFA: bioavailability and modulation of adipose tissue function: Symposium on ‘Frontiers in adipose tissue biology’. Proceedings of the Nutrition Society. 2009;68:361–369. doi: 10.1017/S0029665109990231. [DOI] [PubMed] [Google Scholar]
- Kubant R, Poon AN, Sanchez-Hernandez D, Domenichiello AF, Huot PSP, Pannia E, Cho CE, Hunschede S, Bazinet RP, Anderson GH. A comparison of effects of lard and hydrogenated vegetable shortening on the development of high-fat diet-induced obesity in rats. Nutrition & Diabetes. 2015;5:e188. doi: 10.1038/nutd.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Villoslada F, de Haro O, Camuesco D, Comalada M, Velasco J, Zarzuelo A, Xaus J, Galvez J. Short-chain fructooligosaccharides, in spite of being fermented in the upper part of the large intestine, have anti-inflammatory activity in the TNBS model of colitis. European Journal of Nutrition. 2006;45:418–425. doi: 10.1007/s00394-006-0610-2. [DOI] [PubMed] [Google Scholar]
- Layman DK, Walker DA. Potential Importance of Leucine in Treatment of Obesity and the Metabolic Syndrome. The Journal of Nutrition. 2006;136:319S–323S. doi: 10.1093/jn/136.1.319S. [DOI] [PubMed] [Google Scholar]
- Lee S, Bacha F, Hannon T, Kuk JL, Boesch C, Arslanian S. Effects of Aerobic Versus Resistance Exercise Without Caloric Restriction on Abdominal Fat, Intrahepatic Lipid, and Insulin Sensitivity in Obese Adolescent Boys. Diabetes. 2012;61:2787. doi: 10.2337/db12-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and Management of Type 2 Diabetes: Dietary Components and Nutritional Strategies. Lancet (London, England) 2014;383:1999–2007. doi: 10.1016/S0140-6736(14)60613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Ward WF. PGC-1α: a key regulator of energy metabolism. Advances in Physiology Education. 2006;30:145. doi: 10.1152/advan.00052.2006. [DOI] [PubMed] [Google Scholar]
- Liu Z, Patil IY, Jiang T, Sancheti H, Walsh JP, Stiles BL, Yin F, Cadenas E. High-Fat Diet Induces Hepatic Insulin Resistance and Impairment of Synaptic Plasticity. PLoS ONE. 2015;10:e0128274. doi: 10.1371/journal.pone.0128274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Alarcón M, Perichart-Perera O, Flores-Huerta S, Inda-Icaza P, Rodríguez-Cruz M, Armenta-Álvarez A, Bram-Falcón MT, Mayorga-Ochoa M. Excessive Refined Carbohydrates and Scarce Micronutrients Intakes Increase Inflammatory Mediators and Insulin Resistance in Prepubertal and Pubertal Obese Children Independently of Obesity. Mediators of Inflammation. 2014;2014:849031. doi: 10.1155/2014/849031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C-W, Chang H-H, Yang K-C, Kuo C-S, Lee L-T, Huang K-C. High serum selenium levels are associated with increased risk for diabetes mellitus independent of central obesity and insulin resistance. BMJ Open Diabetes Research & Care. 2016;4:e000253. doi: 10.1136/bmjdrc-2016-000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukaski HC BW, Klevay LM, Milne DB, Sandstead HH. Interactions among dietary fat, mineral status, and performance of endurance athletes: a case study. Int J Sport Nutr Exerc Metab. 2001;11:186–198. doi: 10.1123/ijsnem.11.2.186. [DOI] [PubMed] [Google Scholar]
- Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. The Journal of Clinical Investigation. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Jia R, Yao Q, Xu Y, Luo Z, Luo X, Wang N. Docosahexaenoic acid attenuates adipose tissue angiogenesis and insulin resistance in high fat diet-fed middle-aged mice via a sirt1-dependent mechanism. Molecular Nutrition & Food Research. 2016;60:871–885. doi: 10.1002/mnfr.201500714. [DOI] [PubMed] [Google Scholar]
- Luong Kvq, Nguyen LTH. The Impact of Thiamine Treatment in the Diabetes Mellitus. Journal of Clinical Medicine Research. 2012;4:153–160. doi: 10.4021/jocmr890w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M AM, AJ R, C B, L M, D M-G, V A, E L, M P, A A, A S. Vitamin D status is not related to insulin resistance in different phenotypes of moderate obesity. Applied Physiology, Nutrition, and Metabolism. 2017 doi: 10.1139/apnm-2016-0298. [DOI] [PubMed] [Google Scholar]
- Mahendran Y, Jonsson A, Have CT, Allin KH, Witte DR, Jørgensen ME, Grarup N, Pedersen O, Kilpeläinen TO, Hansen T. Genetic evidence of a causal effect of insulin resistance on branched-chain amino acid levels. Diabetologia. 2017:1–6. doi: 10.1007/s00125-017-4222-6. [DOI] [PubMed] [Google Scholar]
- Manning PJ, Sutherland WHF, Walker RJ, Williams SM, de Jong SA, Ryalls AR, Berry EA. Effect of High-Dose Vitamin E on Insulin Resistance and Associated Parameters in Overweight Subjects. Diabetes Care. 2004;27:2166. doi: 10.2337/diacare.27.9.2166. [DOI] [PubMed] [Google Scholar]
- Mariosa LSS, Ribeiro-Filho FF, Batista MC, Hirota AH, Borges RL, Ribeiro AB, Zanella MT. Abdominal Obesity Is Associated With Potassium Depletion and Changes in Glucose Homeostasis During Diuretic Therapy. The Journal of Clinical Hypertension. 2008;10:443–449. doi: 10.1111/j.1751-7176.2008.07817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovic TP JA, Campbell LV, Furler SM, Kraegen EW, Chisholm DJ. The determinants of glycemic responses to diet restriction and weight loss in obesity and NIDDM. Diabetes Care. 1998;21:687–694. doi: 10.2337/diacare.21.5.687. [DOI] [PubMed] [Google Scholar]
- Marques-Vidal P, Vollenweider P, Guessous I, Henry H, Boulat O, Waeber G, Jornayvaz FR. Serum Vitamin D Concentrations Are Not Associated with Insulin Resistance in Swiss Adults. The Journal of Nutrition. 2015;145:2117–2122. doi: 10.3945/jn.115.211763. [DOI] [PubMed] [Google Scholar]
- Martin CK, Bhapkar M, Pittas AG, et al. Effect of calorie restriction on mood, quality of life, sleep, and sexual function in healthy nonobese adults: The calerie 2 randomized clinical trial. JAMA Internal Medicine. 2016;176:743–752. doi: 10.1001/jamainternmed.2016.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashek DG, Wu C. MUFAs. Advances in Nutrition. 2015;6:276–277. doi: 10.3945/an.114.005926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight JR, Satterfield MC, Jobgen WS, Smith SB, Spencer TE, Meininger CJ, McNeal CJ, Wu G. Beneficial effects of l-arginine on reducing obesity: potential mechanisms and important implications for human health. Amino Acids. 2010;39:349–357. doi: 10.1007/s00726-010-0598-z. [DOI] [PubMed] [Google Scholar]
- Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, Tsukumo DML, Anhe G, Amaral ME, Takahashi HK, et al. Saturated Fatty Acids Produce an Inflammatory Response Predominantly through the Activation of TLR4 Signaling in Hypothalamus: Implications for the Pathogenesis of Obesity. The Journal of Neuroscience. 2009;29:359. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirrahimi A, Chiavaroli L, Srichaikul K, Augustin LSA, Sievenpiper JL, Kendall CWC, Jenkins DJA. The Role of Glycemic Index and Glycemic Load In Cardiovascular Disease And Its Risk Factors: A Review of The Recent Literature. Current Atherosclerosis Reports. 2013;16:381. doi: 10.1007/s11883-013-0381-1. [DOI] [PubMed] [Google Scholar]
- Mogensen TH. Pathogen Recognition and Inflammatory Signaling in Innate Immune Defenses. Clinical Microbiology Reviews. 2009;22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk JM, Hou TY, Turk HF, Weeks B, Wu C, McMurray DN, Chapkin RS. Dietary n-3 polyunsaturated fatty acids (PUFA) decrease obesity-associated Th17 cell-mediated inflammation during colitis. PLoS ONE. 2012;7:e49739. doi: 10.1371/journal.pone.0049739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morino K, Petersen KF, Shulman GI. Molecular Mechanisms of Insulin Resistance in Humans and Their Potential Links With Mitochondrial Dysfunction. Diabetes. 2006;55:S9–S15. doi: 10.2337/db06-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moslehi N, Ehsani B, Mirmiran P, Hojjat P, Azizi F. Association of Dietary Proportions of Macronutrients with Visceral Adiposity Index: Non-Substitution and Iso-Energetic Substitution Models in a Prospective Study. Nutrients. 2015;7:8859–8870. doi: 10.3390/nu7105436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Cheng Y-F, Lai C-Y, Hsu L-W, Chang Y-C, Deng J-Y, Huang Y-Z, Honda H, Chen K-D, Wang C-C, et al. Impact of artificial sunlight therapy on the progress of non-alcoholic fatty liver disease in rats. Journal of Hepatology. 2011;55:415–425. doi: 10.1016/j.jhep.2010.11.028. [DOI] [PubMed] [Google Scholar]
- Nan Y-M, Wu W-J, Fu N, Liang B-L, Wang R-Q, Li L-X, Zhao S-X, Zhao J-M, Yu J. Antioxidants vitamin E and 1-aminobenzotriazole prevent experimental non-alcoholic steatohepatitis in mice. Scandinavian Journal of Gastroenterology. 2009;44:1121–1131. doi: 10.1080/00365520903114912. [DOI] [PubMed] [Google Scholar]
- Natarajan N, Pluznick J. Short chain fatty acid metabolites lower blood pressure via endothelial Gpr41. The FASEB Journal. 2015;29 doi: 10.1152/physiolgenomics.00089.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neels JG, Olefsky JM. Inflamed fat: what starts the fire? Journal of Clinical Investigation. 2006;116:33–35. doi: 10.1172/JCI27280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuschwander-Tetri BA. Carbohydrate intake and nonalcoholic fatty liver disease. Current Opinion in Clinical Nutrition & Metabolic Care. 2013;16:446–452. doi: 10.1097/MCO.0b013e328361c4d1. [DOI] [PubMed] [Google Scholar]
- Nikoulina SE, Ciaraldi TP, Carter L, Mudaliar S, Park KS, Henry RR. Impaired Muscle Glycogen Synthase in Type 2 Diabetes Is Associated with Diminished Phosphatidylinositol 3-Kinase Activation. The Journal of Clinical Endocrinology & Metabolism. 2001;86:4307–4314. doi: 10.1210/jcem.86.9.7872. [DOI] [PubMed] [Google Scholar]
- Norris LE, Collene AL, Asp ML, Hsu JC, Liu L-F, Richardson JR, Li D, Bell D, Osei K, Jackson RD, et al. Comparison of dietary conjugated linoleic acid with safflower oil on body composition in obese postmenopausal women with type 2 diabetes mellitus. The American Journal of Clinical Nutrition. 2009;90:468–476. doi: 10.3945/ajcn.2008.27371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nseir W, Hellou E, Assy N. Role of diet and lifestyle changes in nonalcoholic fatty liver disease. World Journal of Gastroenterology: WJG. 2014;20:9338–9344. doi: 10.3748/wjg.v20.i28.9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an Omega-3 Fatty Acid Receptor Mediating Potent Anti-Inflammatory and Insulin Sensitizing Effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi N, Higuchi A, Ohashi K, Oshima Y, Gokce N, Shibata R, Akasaki Y, Shimono A, Walsh K. Sfrp5 Is an Anti-Inflammatory Adipokine That Modulates Metabolic Dysfunction in Obesity. Science (New York, NY) 2010;329:454–457. doi: 10.1126/science.1188280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet V, Marois J, Weisnagel SJ, Jacques H. Dietary Cod Protein Improves Insulin Sensitivity in Insulin-Resistant Men and Women. Diabetes Care. 2007;30:2816. doi: 10.2337/dc07-0273. [DOI] [PubMed] [Google Scholar]
- Paniagua JA, de la Sacristana AG, Romero I, Vidal-Puig A, Latre JM, Sanchez E, Perez-Martinez P, Lopez-Miranda J, Perez-Jimenez F. Monounsaturated Fat–Rich Diet Prevents Central Body Fat Distribution and Decreases Postprandial Adiponectin Expression Induced by a Carbohydrate-Rich Diet in Insulin-Resistant Subjects. Diabetes Care. 2007;30:1717. doi: 10.2337/dc06-2220. [DOI] [PubMed] [Google Scholar]
- Papamiltiadous ES, Roberts SK, Nicoll AJ, Ryan MC, Itsiopoulos C, Salim A, Tierney AC. A randomised controlled trial of a Mediterranean Dietary Intervention for Adults with Non Alcoholic Fatty Liver Disease (MEDINA): study protocol. BMC Gastroenterology. 2016;16:14. doi: 10.1186/s12876-016-0426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D-Y, Ahn Y-T, Park S-H, Huh C-S, Yoo S-R, Yu R, Sung M-K, McGregor RA, Choi M-S. Supplementation of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 in Diet-Induced Obese Mice Is Associated with Gut Microbial Changes and Reduction in Obesity. PLoS ONE. 2013;8:e59470. doi: 10.1371/journal.pone.0059470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Park N-Y, Valacchi G, Lim Y. Calorie restriction with a high-fat diet effectively attenuated inflammatory response and oxidative stress-related markers in obese tissues of the high diet fed rats. Mediators of Inflammation. 2012;2012:11. doi: 10.1155/2012/984643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrice DC, Nathalie MD. The Role of the Gut Microbiota in Energy Metabolism and Metabolic Disease. Current Pharmaceutical Design. 2009;15:1546–1558. doi: 10.2174/138161209788168164. [DOI] [PubMed] [Google Scholar]
- Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado de Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-[gamma] Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine NM, Watras AC, Carrel AL, Allen DB, McVean JJ, Clark RR, O’Brien AR, O’Shea M, Scott CE, Schoeller DA. Effect of conjugated linoleic acid on body fat accretion in overweight or obese children. The American Journal of Clinical Nutrition. 2010;91:1157–1164. doi: 10.3945/ajcn.2009.28404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, et al. Cultured gut microbiota from twins discordant for obesity modulate adiposity and metabolic phenotypes in mice. Science (New York, NY) 2013;341 doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivellese AA, De Natale C, Lilli S. Type of Dietary Fat and Insulin Resistance. Annals of the New York Academy of Sciences. 2002;967:329–335. doi: 10.1111/j.1749-6632.2002.tb04288.x. [DOI] [PubMed] [Google Scholar]
- Roberts CK, Won D, Pruthi S, Kurtovic S, Sindhu RK, Vaziri ND, Barnard RJ. Effect of a short-term diet and exercise intervention on oxidative stress, inflammation, MMP-9, and monocyte chemotactic activity in men with metabolic syndrome factors. Journal of Applied Physiology. 2006;100:1657. doi: 10.1152/japplphysiol.01292.2005. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Moran M, Guerrero-Romero F. Oral Magnesium Supplementation Improves the Metabolic Profile of Metabolically Obese, Normal-weight Individuals: A Randomized Double-blind Placebo-controlled Trial. Archives of Medical Research. 2014;45:388–393. doi: 10.1016/j.arcmed.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Roseno SL, Davis PR, Bollinger LM, Powell JJS, Witczak CA, Brault JJ. Short-term, high-fat diet accelerates disuse atrophy and protein degradation in a muscle-specific manner in mice. Nutrition & Metabolism. 2015;12:39. doi: 10.1186/s12986-015-0037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth CL, Elfers CT, Figlewicz DP, Melhorn SJ, Morton GJ, Hoofnagle A, Yeh MM, Nelson JE, Kowdley KV. Vitamin D deficiency in obese rats exacerbates nonalcoholic fatty liver disease and increases hepatic resistin and toll-like receptor activation. Hepatology. 2012;55:1103–1111. doi: 10.1002/hep.24737. [DOI] [PubMed] [Google Scholar]
- Sáez-Lara M, Robles-Sanchez C, Ruiz-Ojeda F, Plaza-Diaz J, Gil A. Effects of Probiotics and Synbiotics on Obesity, Insulin Resistance Syndrome, Type 2 Diabetes and Non-Alcoholic Fatty Liver Disease: A Review of Human Clinical Trials. International Journal of Molecular Sciences. 2016;17:928. doi: 10.3390/ijms17060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer M, Dahlhoff C, Giesbertz P, Eidens MK, de Wit N, Rubio-Aliaga I, Boekschoten MV, Müller M, Daniel H. Increased Plasma Citrulline in Mice Marks Diet-Induced Obesity and May Predict the Development of the Metabolic Syndrome. PLoS ONE. 2013;8:e63950. doi: 10.1371/journal.pone.0063950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M. Brown Adipose Tissue as a Regulator of Energy Expenditure and Body Fat in Humans. Diabetes & Metabolism Journal. 2013;37:22–29. doi: 10.4093/dmj.2013.37.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampey BP, Vanhoose AM, Winfield HM, Freemerman AJ, Muehlbauer MJ, Fueger PT, Newgard CB, Makowski L. Cafeteria Diet Is a Robust Model of Human Metabolic Syndrome With Liver and Adipose Inflammation: Comparison to High-Fat Diet. Obesity (Silver Spring, Md) 2011;19:1109–1117. doi: 10.1038/oby.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamarina AB, Oliveira JL, Silva FP, Carnier J, Mennitti LV, Santana AA, de Souza GHI, Ribeiro EB, Oller do Nascimento CM, Lira FS, et al. Green Tea Extract Rich in Epigallocatechin-3-Gallate Prevents Fatty Liver by AMPK Activation via LKB1 in Mice Fed a High-Fat Diet. PLoS ONE. 2015;10:e0141227. doi: 10.1371/journal.pone.0141227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:7265–7270. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena M, Yeretssian G. NOD-Like Receptors: Master Regulators of Inflammation and Cancer. Frontiers in Immunology. 2014;5:327. doi: 10.3389/fimmu.2014.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab JM, Serhan CN. Lipoxins and new lipid mediators in the resolution of inflammation. Current Opinion in Pharmacology. 2006;6:414–420. doi: 10.1016/j.coph.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Semrin G, Fishman DS, Bousvaros A, Zholudev A, Saunders AC, Correia CE, Nemeth E, Grand RJ, Weinstein DA. Impaired Intestinal Iron Absorption in Crohn’s Disease Correlates with Disease Activity and Markers of Inflammation. Inflammatory bowel diseases. 2006;12:1101–1106. doi: 10.1097/01.mib.0000235097.86360.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senthil Kumar SPD, Shen M, Spicer EG, Goudjo-Ako AJ, Stumph JD, Zhang J, Shi H. Distinct metabolic effects following short-term exposure of different high-fat diets in male and female mice. Endocrine journal. 2014;61:457–470. doi: 10.1507/endocrj.ej13-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo K-H, Kim H, Chon J-W, Kim D-H, Nah S-Y, Arvik T, Yokoyama W. Flavonoid-rich Chardonnay grape seed flour supplementation ameliorates diet-induced visceral adiposity, insulin resistance, and glucose intolerance via altered adipose tissue gene expression. Journal of Functional Foods. 2015;17:881–891. [Google Scholar]
- Shimabukuro M, Higa M, Zhou Y-T, Wang M-Y, Newgard CB, Unger RH. Lipoapoptosis in Beta-cells of Obese Prediabeticfa/fa Rats: ROLE OF SERINE PALMITOYLTRANSFERASE OVEREXPRESSION. Journal of Biological Chemistry. 1998;273:32487–32490. doi: 10.1074/jbc.273.49.32487. [DOI] [PubMed] [Google Scholar]
- Shirpoor A, Ansari MHK, Salami S, Pakdel FG, Rasmi Y. Effect of vitamin E on oxidative stress status in small intestine of diabetic rat. World Journal of Gastroenterology: WJG. 2007;13:4340–4344. doi: 10.3748/wjg.v13.i32.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieri S, Krogh V, Agnoli C, Ricceri F, Palli D, Masala G, Panico S, Mattiello A, Tumino R, Giurdanella MC, et al. Dietary glycemic index and glycemic load and risk of colorectal cancer: results from the EPIC-Italy study. International Journal of Cancer. 2015;136:2923–2931. doi: 10.1002/ijc.29341. [DOI] [PubMed] [Google Scholar]
- Sonnweber T, Ress C, Nairz M, Theurl I, Schroll A, Murphy AT, Wroblewski V, Witcher DR, Moser P, Ebenbichler CF, et al. High-fat diet causes iron deficiency via hepcidin-independent reduction of duodenal iron absorption. Journal of Nutritional Biochemistry. 2012;23:1600–1608. doi: 10.1016/j.jnutbio.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Souza CO, Teixeira AAS, Lima EA, Batatinha HAP, Gomes LM, Carvalho-Silva M, Mota IT, Streck EL, Hirabara SM, Neto J, et al. Palmitoleic Acid (N-7) Attenuates the Immunometabolic Disturbances Caused by a High-Fat Diet Independently of PPARα. Mediators of Inflammation. 2014;2014:12. doi: 10.1155/2014/582197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks LM, Redman LM, Conley KE, Harper M-E, Yi F, Hodges A, Eroshkin A, Costford SR, Gabriel ME, Shook C, et al. Effects of 12 months of caloric restriction on muscle mitochondrial function in healthy individuals. The Journal of Clinical Endocrinology & Metabolism. 2017;102:111–121. doi: 10.1210/jc.2016-3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR. Obesity: The Integrated Roles of Environment and Genetics. The Journal of Nutrition. 2004;134:2090S–2105S. doi: 10.1093/jn/134.8.2090S. [DOI] [PubMed] [Google Scholar]
- Spencer M, Finlin BS, Unal R, Zhu B, Morris AJ, Shipp LR, Lee J, Walton RG, Adu A, Erfani R, et al. Omega-3 Fatty Acids Reduce Adipose Tissue Macrophages in Human Subjects With Insulin Resistance. Diabetes. 2013;62:1709–1717. doi: 10.2337/db12-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, Israeli D, Zmora N, Gilad S, Weinberger A, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514:181–186. doi: 10.1038/nature13793. [DOI] [PubMed] [Google Scholar]
- Sukhotnik I, Mor-Vaknin N, Drongowski RA, Miselevich I, Coran AG, Harmon CM. Effect of dietary fat on early morphological intestinal adaptation in a rat with short bowel syndrome. Pediatric Surgery International. 2004;20:419–424. doi: 10.1007/s00383-004-1168-9. [DOI] [PubMed] [Google Scholar]
- Sung C-C, Liao M-T, Lu K-C, Wu C-C. Role of Vitamin D in Insulin Resistance. Journal of Biomedicine and Biotechnology. 2012;2012:11. doi: 10.1155/2012/634195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM, Annicelli LL, Krause JE, Cortright DN. The Cloned Rat Vanilloid Receptor VR1 Mediates Both R-Type Binding and C-Type Calcium Response in Dorsal Root Ganglion Neurons. Molecular Pharmacology. 1999;56:581. doi: 10.1124/mol.56.3.581. [DOI] [PubMed] [Google Scholar]
- Takatsu M, Nakashima C, Takahashi K, Murase T, Hattori T, Ito H, Murohara T, Nagata K. Calorie restriction attenuates cardiac remodeling and diastolic dysfunction in a rat model of metabolic syndrome. Hypertension. 2013;62:957. doi: 10.1161/HYPERTENSIONAHA.113.02093. [DOI] [PubMed] [Google Scholar]
- Talaei A, Mohamadi M, Adgi Z. The effect of vitamin D on insulin resistance in patients with type 2 diabetes. Diabetology & Metabolic Syndrome. 2013;5:8–8. doi: 10.1186/1758-5996-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Purkayastha S, Cai D. Hypothalamic micro-inflammation: a common basis of metabolic syndrome and aging. Trends in neurosciences. 2015;38:36–44. doi: 10.1016/j.tins.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernof A, Després J-P. Pathophysiology of Human Visceral Obesity: An Update. Physiological Reviews. 2013;93:359. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- Teng K-T, Chang C-Y, Chang LF, Nesaretnam K. Modulation of obesity-induced inflammation by dietary fats: mechanisms and clinical evidence. Nutrition Journal. 2014;13:12–12. doi: 10.1186/1475-2891-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzić J, Grivennikov S, Karin E, Karin M. Inflammation and Colon Cancer. Gastroenterology. 2010;138:2101–2114.e2105. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- Todoric J, Löffler M, Huber J, Bilban M, Reimers M, Kadl A, Zeyda M, Waldhäusl W, Stulnig TM. Adipose tissue inflammation induced by high-fat diet in obese diabetic mice is prevented by n−3 polyunsaturated fatty acids. Diabetologia. 2006;49:2109–2119. doi: 10.1007/s00125-006-0300-x. [DOI] [PubMed] [Google Scholar]
- Tomada I, Fernandes D, Guimarães JT, Almeida H, Neves D. Energy restriction ameliorates metabolic syndrome-induced cavernous tissue structural modifications in aged rats. AGE. 2013;35:1721–1739. doi: 10.1007/s11357-012-9473-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toral M, Gómez-Guzmán M, Jiménez R, Romero M, Sánchez M, Utrilla María P, Garrido-Mesa N, Rodríguez-Cabezas María E, Olivares M, Gálvez J, et al. The probiotic <em>Lactobacillus coryniformis</em> CECT5711 reduces the vascular pro-oxidant and pro-inflammatory status in obese mice. Clinical Science. 2014;127:33. doi: 10.1042/CS20130339. [DOI] [PubMed] [Google Scholar]
- Turati F, Dilis V, Rossi M, Lagiou P, Benetou V, Katsoulis M, Naska A, Trichopoulos D, La Vecchia C, Trichopoulou A. Glycemic load and coronary heart disease in a Mediterranean population: The EPIC Greek cohort study. Nutrition, Metabolism and Cardiovascular Diseases. 2014;25:336–342. doi: 10.1016/j.numecd.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Marked alterations in the distal gut microbiome linked to diet-induced obesity. Cell host & microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Gordon JI. The core gut microbiome, energy balance and obesity. The Journal of Physiology. 2009;587:4153–4158. doi: 10.1113/jphysiol.2009.174136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1131. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- van Dijk SJ, Feskens EJ, Bos MB, Hoelen DW, Heijligenberg R, Bromhaar MG, de Groot LC, de Vries JH, Müller M, Afman LA. A saturated fatty acid–rich diet induces an obesity-linked proinflammatory gene expression profile in adipose tissue of subjects at risk of metabolic syndrome. The American Journal of Clinical Nutrition. 2009;90:1656–1664. doi: 10.3945/ajcn.2009.27792. [DOI] [PubMed] [Google Scholar]
- Vezza T, Rodríguez-Nogales A, Algieri F, Utrilla MP, Rodriguez-Cabezas ME, Galvez J. Flavonoids in Inflammatory Bowel Disease: A Review. Nutrients. 2016;8:211. doi: 10.3390/nu8040211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via MA, Mechanick JI. Nutrition in Type 2 Diabetes and the Metabolic Syndrome. Medical Clinics of North America. 2016;100:1285–1302. doi: 10.1016/j.mcna.2016.06.009. [DOI] [PubMed] [Google Scholar]
- Vissers D, Hens W, Taeymans J, Baeyens J-P, Poortmans J, Van Gaal L. The effect of exercise on visceral adipose tissue in overweight adults: a systematic review and meta-analysis. PLoS ONE. 2013;8:e56415. doi: 10.1371/journal.pone.0056415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CCL, Adochio RL, Leitner JW, Abeyta IM, Draznin B, Cornier M-A. Acute effects of different diet compositions on skeletal muscle insulin signalling in obese individuals during caloric restriction. Metabolism - Clinical and Experimental. 2012;62:595–603. doi: 10.1016/j.metabol.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Tang H, Zhang C, Zhao Y, Derrien M, Rocher E, van-Hylckama Vlieg JET, Strissel K, Zhao L, Obin M, et al. Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. The ISME Journal. 2015a;9:1–15. doi: 10.1038/ismej.2014.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Wang H, Yao HUA, Wei QIN, Mao X-M, Jiang TAO, Xiang J, Dila NA. Expression and activity of the TLR4/NF-κB signaling pathway in mouse intestine following administration of a short-term high-fat diet. Experimental and Therapeutic Medicine. 2013;6:635–640. doi: 10.3892/etm.2013.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ota N, Manzanillo P, Kates L, Zavala-Solorio J, Eidenschenk C, Zhang J, Lesch J, Lee WP, Ross J, et al. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature. 2014;514:237–241. doi: 10.1038/nature13564. [DOI] [PubMed] [Google Scholar]
- Wang X, You T, Murphy K, Lyles MF, Nicklas BJ. Addition of Exercise Increases Plasma Adiponectin and Release from Adipose Tissue. Medicine and science in sports and exercise. 2015b;47:2450–2455. doi: 10.1249/MSS.0000000000000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren HS, Fitting C, Hoff E, Adib-Conquy M, Beasley-Topliffe L, Tesini B, Liang X, Valentine C, Hellman J, Hayden D, et al. Resilience to bacterial infection: difference between species could be due to proteins in serum. The Journal of infectious diseases. 2010;201:223–232. doi: 10.1086/649557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasinski F, Bacurau RFP, Moraes MR, Haro AS, Moraes-Vieira PMM, Estrela GR, Paredes-Gamero EJ, Barros CC, Almeida SS, et al. Exercise and caloric restriction alter the immune system of mice submitted to a high-fat diet. Mediators of Inflammation. 2013;2013:8. doi: 10.1155/2013/395672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisburger JH. Tea and health: a historical perspective. Cancer Letters. 1997;114:315–317. doi: 10.1016/s0304-3835(97)04691-0. [DOI] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. Journal of Clinical Investigation. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MF. Insulin signaling in health and disease. Science. 2003;302:1710. doi: 10.1126/science.1092952. [DOI] [PubMed] [Google Scholar]
- White PJ, Lapworth AL, An J, Wang L, McGarrah RW, Stevens RD, Ilkayeva O, George T, Muehlbauer MJ, Bain JR, et al. Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Molecular Metabolism. 2016;5:538–551. doi: 10.1016/j.molmet.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson PF, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: The framingham experience. Archives of Internal Medicine. 2002;162:1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- Wing RR BE, Bononi P, Marcus MD, Watanabe R, Bergman RN. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care. 1994;17:30–36. doi: 10.2337/diacare.17.1.30. [DOI] [PubMed] [Google Scholar]
- Woo S-L, Xu H, Li H, Zhao Y, Hu X, Zhao J, Guo X, Guo T, Botchlett R, Qi T, et al. Metformin ameliorates hepatic steatosis and inflammation without altering adipose phenotype in diet-induced obesity. PLoS ONE. 2014;9:e91111. doi: 10.1371/journal.pone.0091111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodside JV, McCall D, McGartland C, Young IS. Micronutrients: dietary intake v. supplement use. Proceedings of the Nutrition Society. 2005;64:543–553. doi: 10.1079/pns2005464. [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. Journal of Clinical Investigation. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi H, Tan J, Tuan RS. Polyphenols suppress hydrogen peroxide-induced oxidative stress in human bone-marrow derived mesenchymal stem cells. Journal of Cellular Biochemistry. 2013;114:1163–1173. doi: 10.1002/jcb.24459. [DOI] [PubMed] [Google Scholar]
- Yang Y, Duan W, Liang Z, Yi W, Yan J, Wang N, Li Y, Chen W, Yu S, Jin Z, et al. Curcumin attenuates endothelial cell oxidative stress injury through Notch signaling inhibition. Cellular Signalling. 2013;25:615–629. doi: 10.1016/j.cellsig.2012.11.025. [DOI] [PubMed] [Google Scholar]
- Ye J, Keller JN. Regulation of energy metabolism by inflammation: A feedback response in obesity and calorie restriction. Aging (Albany NY) 2010;2:361–368. doi: 10.18632/aging.100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen C-LE, Nelson DW, Yen M-I. Intestinal triacylglycerol synthesis in fat absorption and systemic energy metabolism. Journal of Lipid Research. 2015;56:489–501. doi: 10.1194/jlr.R052902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneshiro T, Aita S, Kawai Y, Iwanaga T, Saito M. Nonpungent capsaicin analogs (capsinoids) increase energy expenditure through the activation of brown adipose tissue in humans. The American Journal of Clinical Nutrition. 2012;95:845–850. doi: 10.3945/ajcn.111.018606. [DOI] [PubMed] [Google Scholar]
- Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, Iwanaga T, Saito M. Recruited brown adipose tissue as an antiobesity agent in humans. The Journal of Clinical Investigation. 2013;123:3404–3408. doi: 10.1172/JCI67803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaakouk AM, Hassan MA, Tolba OA. Serum magnesium status among obese children and adolescents. Egyptian Pediatric Association Gazette. 2016;64:32–37. [Google Scholar]
- Zampieri TT, Torres-Leal FL, Campaña AB, Lima FB, Donato J. l-Leucine Supplementation Worsens the Adiposity of Already Obese Rats by Promoting a Hypothalamic Pattern of Gene Expression that Favors Fat Accumulation. Nutrients. 2014;6:1364–1373. doi: 10.3390/nu6041364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKβ/NF-κB and ER Stress Link Overnutrition to Energy Imbalance and Obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, Yu Y-H. Increasing Dietary Leucine Intake Reduces Diet-Induced Obesity and Improves Glucose and Cholesterol Metabolism in Mice via Multimechanisms. Diabetes. 2007;56:1647. doi: 10.2337/db07-0123. [DOI] [PubMed] [Google Scholar]
- Zhenyukh O, Civantos E, Ruiz-Ortega M, Sánchez MS, Vázquez C, Peiró C, Egido J, Mas S. High concentration of branched-chain amino acids promotes oxidative stress, inflammation and migration of human peripheral blood mononuclear cells via mTORC1 activation. Free Radical Biology and Medicine. 2017;104:165–177. doi: 10.1016/j.freeradbiomed.2017.01.009. [DOI] [PubMed] [Google Scholar]
- Zierath JR, Houseknecht KL, Gnudi L, Kahn BB. High-Fat Feeding Impairs Insulin-Stimulated GLUT4 Recruitment via an Early Insulin-Signaling Defect. Diabetes. 1997;46:215. doi: 10.2337/diab.46.2.215. [DOI] [PubMed] [Google Scholar]
- Zimmermann E, Anty R, Tordjman J, Verrijken A, Gual P, Tran A, Iannelli A, Gugenheim J, Bedossa P, Francque S, et al. C-reactive protein levels in relation to various features of non-alcoholic fatty liver disease among obese patients. Journal of Hepatology. 2011;55:660–665. doi: 10.1016/j.jhep.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Zúñiga J, Cancino M, Medina F, Varela P, Vargas R, Tapia G, Videla LA, Fernández V. N-3 PUFA Supplementation Triggers PPAR-α Activation and PPAR-α/NF-κB Interaction: Anti-Inflammatory Implications in Liver Ischemia-Reperfusion Injury. PLoS ONE. 2011;6:e28502. doi: 10.1371/journal.pone.0028502. [DOI] [PMC free article] [PubMed] [Google Scholar]


