Abstract
Objective
We applied paired transcranial magnetic stimulation (pTMS) to patients with post-traumatic stress disorder (PTSD) secondary to minor accidental head trauma. Our purpose was to determine the potential abnormality of motor cortex excitability in this pathologic condition.
Methods
pTMS stimulation, according to the conditioning–test paradigm employing interstimulus intervals (ISIs) of 1–6 ms, was used to investigate intracortical inhibition in control subjects and patients with PTSD. The study population consisted of 14 patients who had developed PTSD following minor head trauma, 12 healthy volunteers without a clinical history of head trauma and 11 healthy subjects who had reported accidental minor head trauma 1–4 months before the study. This clinical electrophysiologic study was performed at the Department of Neuroscience, University of Rome “Tor Vergata.”
Results
All patients with PTSD exhibited a significantly lower motor evoked potential (MEP) inhibition than controls at 2 ms, 3 ms and 4 ms ISI. The statistical analysis of the pTMS protocol showed a significant effect (F2,36 = 25.63, p < 0.001) of the factor “group,” because patients with PTSD showed a mean conditioned MEP amplitude higher than that observed in both control groups for all 6 ISIs analyzed. The “ISI” factor was also significant (F5,180 = 89.85, Greenhouse–Geisser ε = 0.35; p < 0.001), with the mean conditioned MEP amplitude increasing from 22.5% to 127.8% as the ISI increased from 1 ms to 6 ms. Finally, the interaction of group with ISI was also significant (F10,180 = 8.97, p < 0.001), showing that the condition of PTSD secondary to head trauma was able to affect the MEP amplitude at different ISIs.
Conclusions
Our results demonstrate that PTSD can give rise to abnormalities in intracortical inhibition. Our results provide further evidence that alterations in cortical inhibitory circuits may underlie specific forms of neuroticism in humans.
Medical subject headings: cortical excitability; hyperarousal; stress disorders, post-traumatic; transcranial magnetic stimulation
Abstract
Objectif
Nous avons administré à des patients atteints d'un syndrome de stress post-traumatique (SSPT) apparu suite à un traumatisme crânien accidentel mineur un traitement faisant appel à la magnétostimulation transcrânienne par paires d'impulsions (MSTp). Nous cherchions à établir une éventuelle anomalie de l'excitabilité du cortex moteur chez les personnes atteintes de cette affection pathologique.
Méthodes
On a appliqué un traitement de stimulation par MSTp selon le paradigme d'essai de conditionnement faisant appel à des intervalles inter-stimuli (IIS) de 1 ms à 6 ms pour étudier l'inhibition intracorticale chez des sujets témoins et chez des patients atteints du SSPT. La population visée par l'étude comprenait 14 patients chez lesquels le SSPT était apparu suite à un traumatisme crânien mineur, 12 volontaires en bonne santé n'ayant pas d'antécédents cliniques de traumatisme crânien et 11 sujets en bonne santé ayant signalé un traumatisme crânien mineur accidentel de 1 à 4 mois avant l'étude. Cette étude clinique en électrophysiologie a été effectuée au Département des neurosciences de l'Université Tor Vergata à Rome.
Résultats
Suite à l'application d'IIS de 2 ms, de 3 ms et de 4 ms, l'inhibition du potentiel évoqué moteur (PEM) de tous les patients atteints du SSPT était considérablement plus faible que celle des sujets témoins. L'analyse statistique du protocole de MST par paires d'impulsions a révélé l'effet important (F2,36 = 25,63, p < 0,001) du facteur «groupe», puisque chez les patients atteints du SSPT, la moyenne de l'amplitude du PEM conditionné était supérieure à celle observée dans les deux groupes témoins suite à l'application des 6 IIS analysés. Le facteur «IIS» était aussi important (F5,180 = 89,85, Greenhouse–Geisser ε = 0,35; p < 0,001), étant donné qu'on a observé une augmentation de la moyenne de l'amplitude du PEM conditionné, qui est passée de 22,5 % à 127,8 %, suivant l'accroissement de l'IIS de 1 ms à 6 ms. Enfin, l'interaction du facteur groupe et du facteur IIS était également importante (F10,180 = 8,97, p < 0,001), ce qui démontre que le SSPT consécutif à un traumatisme crânien peut avoir une incidence sur l'amplitude du PEM selon divers IIS.
Conclusions
Les résultats démontrent que le SSPT peut conduire à des anomalies au niveau de l'inhibition intracorticale. Ces résultats produisent d'autres preuves indiquant que des altérations des voies inhibitrices corticales pourraient sous-tendre certaines formes de névrosisme chez l'humain.
Introduction
Aggression or motor vehicle accidents, especially when associated with minor head trauma, are among the most frequent causes of post-traumatic stress disorder (PTSD). PTSD is a condition in which individuals re-experience phenomena and have avoidance behaviours and somatic disturbances coupled with sleep disorders and, typically, hyperarousal. Exclusion of other mental or organic disorders and the presence of symptoms that last for at least 1 month are essential requirements for the diagnosis of PTSD according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV).1
Although the results of functional neuroimaging studies and the hyperarousal typical of this disorder strongly suggest that abnormal brain excitability may play a key role in the genesis of PTSD symptoms,2,3,4,5 a neurophysiologic study aimed at addressing this critical aspect of the disorder is still lacking.
Transcranial magnetic stimulation (TMS) has proved to be capable of evaluating motor cortex excitability by measuring the threshold of motor evoked potentials (MEPs) and intracortical inhibition by ad hoc paired-pulse paradigms.6,7 The application of this technique has substantially contributed to clarification of the pathophysiologic mechanisms underlying other neuropsychiatric conditions, by revealing changes in excitability of the corticospinal tracts.7,8 These changes are generally ascribed to an imbalance between glutamate (the main excitatory neurotransmitter) and the inhibitory transmitter γ-aminobutyric acid (GABA) within the cortex.9,10,11,12 In this respect, stress-induced long-term potentiation of glutamate-mediated transmission might represent a synaptic correlate of the maintenance of traumatic memories that cause PTSD, and a number of studies have shown that PTSD is associated with long-term changes in brain structures and systems that mediate memory and the stress response.13,14 It is, therefore, conceivable that long-lasting alterations of cortical excitability may underlie, at least in part, some of the mental symptoms of PTSD that follow a minor head injury.
In the present study, we applied TMS in an attempt to investigate whether changes in cortical excitability are associated with the development of PTSD disorder after accidental head trauma. In particular, paired stimulation, with different interstimulus intervals (ISIs), was applied in order to gain insights into the functioning of intracortical inhibitory transmission in this pathologic condition.6
Methods
Fourteen patients with PTSD secondary to head trauma (8 women and 6 men, aged between 18 and 47 yr) underwent neurophysiologic examination with TMS at 1–6 months after the trauma. The clinical diagnosis of PTSD was derived according to DSM-IV criteria.
Before TMS investigation, subjects underwent a complete neurologic examination, standard electroencephalography, and brain computed tomography or magnetic resonance imaging (MRI). In addition, they underwent the Minnesota Multiphasic Personality Inventory (MMPI)15 examination and the State–Trait Anxiety Inventory16 (STAI)-based evaluation for anxiety. Eligible patients were considered to be those without brain injury (no loss of consciousness, Glasgow Coma Scale score 15) and normal findings on brain computed tomography or MRI. Patients were excluded from the study if they had a clinical history of epilepsy or neurologic deficits and conditions that could affect nerve conduction along the spinal cord (concomitant cervical injury, treatment with neurotropic drugs, neuropathy with conduction block). Patients were excluded if they had other primary major psychiatric illnesses such as major depressive disorders, obsessive–compulsive disorders, panic disorders, or comorbid disorders including alcohol abuse or dependence, schizophrenia or bipolar disorders.
Patients' neurophysiologic data were age-matched against those of a control population represented by 12 healthy volunteers (7 women and 5 men) who, analogous to the patients, were not familiar with the technique of TMS. We also studied 11 subjects (5 women and 6 men) who reported a minor head trauma 1–4 months before the study but did not develop PTSD or other neurologic or psychiatric sequelae. Anxiety and obsessive–compulsive disorder have been found to be associated with increased motor cortex excitability.7,8 Control subjects, therefore, underwent a complete psychiatric examination with the MMPI and the STAI scale, and subjects who exhibited traits of obsessive–compulsive disorder or anxiety (score > 35 either on the STAI State Anxiety Form or the Trait Anxiety Form) were not included.
Patients and controls were statistically comparable for age (patients: 36.4 [and standard deviation {SD} 8.4] yr, controls without trauma: 38.4 [SD 6.2] yr, controls with trauma: 39 [SD 9.3] yr) and time interval since head trauma (patients: 2.1 [SD 1.4] mo, controls with trauma: 2.4 [SD 1.6] mo). None of the control subjects or patients were being treated with neurotropic drugs. Informed consent for the procedures used was obtained from the whole examined population, and the Ethics Committee of the University of Rome “Tor Vergata” approved the study.
Recording and stimulating procedures
Seizure disorder can be a sequela of all degrees of head trauma.17 Therefore, to limit any risk of inducing seizures by the use of repetitive TMS, in the present study we chose to concentrate on the GABA-mediated intracortical inhibition profile by using a paired TMS (pTMS) technique with ISIs ranging from 1 ms to 6 ms.18 The TMS examination, which was performed with the subject comfortably reclined on a bed in a quiet room, lasted on average 40 minutes. MEPs were recorded with surface electrodes in thenar muscles, during complete muscle relaxation and with open eyes.19 The active electrode was placed over the motor point with the reference on the metacarpophalangeal joint. An acoustic feedback monitored the electromyographic background activity in order to detect and avoid interference possibly induced by voluntary movements; contaminated trials were discarded. A time window of 50 ms preceding stimulation was visualized online in order to monitor the level of muscle relaxation. Recordings were acquired with a filtering bandwidth of 20–2000 Hz and a sampling rate of 10 kHz and were stored for offline analysis.
We used pTMS, according to the conditioning–test paradigm employing short ISIs, to investigate the time course of intracortical excitability.6 This was achieved by using a figure of 8 coil, which was 14 cm in transversal diameter, connected to 2 Magstim 200 stimulators through a Bi-stim module (Magstim, Dyfield, UK). Pairs of stimuli were discharged, each having different intensities, with conditioning pulses delivered from 1 ms to 6 ms before test stimulation. The coil was held manually in an anteroposterior direction, with the handle pointing backward at about 45° toward the occipital pole. The centre of the coil was placed over the left central sulcus, on the scalp region corresponding to the hand motor area, so as to activate pyramidal neurons transsynaptically. The site where MEPs with the lowest intensity were elicited in the contralateral relaxed target muscle was located at around 5–6 cm lateral to Cz along the earlobe line and was marked with a pencil in order to ensure the same coil position during the whole TMS examination. The resting MEP threshold was determined to be the minimum TMS intensity required to produce MEPs greater than or equivalent to 50 μV on 5 or more consecutive trials of stimulation delivered at least 5 s apart. After this, the active MEP threshold was determined according to the same criteria but in the presence of a voluntary contraction (40% of maximum, as determined via a home-made manual transducer), which was monitored with visual and auditory feedback.
pTMS testing was performed as follows: the conditioning pulse intensity was set at 10% below the active MEP threshold. This intensity was chosen to ensure that this pulse would not evoke any physiologically significant activity in the corticospinal tract. The test pulse intensity was set at a level that produced an MEP of about 500 μV, as originally designed.6,20 The ISIs tested were 1 ms, 2 ms, 3 ms, 4 ms, 5 ms and 6 ms and were chosen to sample conditioned MEP amplitude during the inhibited phase of the conditioning effect. Ten trials were delivered at each ISI with isolated test pulses interspersed on every fifth trial. The order of the ISIs was varied randomly. The mean intertrial interval was 7 s and was also varied randomly.7
Neurophysiologic data analysis
The following neurophysiologic parameters were analyzed: (1) the value of the resting motor threshold for test stimuli and the active motor threshold for conditioning ones, expressed as a percentage of the stimulator maximal output; (2) the amplitudes of test and conditioned MEPs measured from peak to peak (negativity upward); (3) MEP mean amplitudes calculated separately for each ISI (from 1 ms to 6 ms) and the differences in amplitude between test and conditioned MEPs, expressed as a percentage of test stimulus alone.
Statistical analysis
Differences in MEP excitability thresholds were evaluated with the Mann–Whitney test. Statistical significance was assumed for values of p < 0.05.
MEP amplitude changes obtained in response to pTMS were expressed as a percentage of MEP control size, that is, resulting from test stimulus alone, in both patients and controls. The statistical evaluation of pTMS data included a 2-way analysis of variance (ANOVA) corrected by the Greenhouse–Geisser method when more than 2 levels were present in a “within” factor. Thus, the electrophysiologic data obtained in the 3 groups studied were analyzed together by means of a between factor “group” with 3 levels (patients with PTSD v. controls without trauma v. controls with trauma) and a within factor “ISI,” with 6 levels, corresponding to the ISI sequence investigated. Whenever a significant interaction between factors was found, the single differences were assessed by means of the post hoc Tukey Honestly Significant Difference (HSD) test. Significance was set at p < 0.05.
An additional evaluation with a 2-way ANOVA was conducted comparing the patients with anxiety (n = 6; 3 women, 3 men; mean age 30.5 [SD 7.2] yr; mean time interval since trauma 2.6 [SD 0.8] mo; score on the STAI State Anxiety Form > 45) and the patients who did not report anxiety (n = 8; 5 women, 3 men; mean age 38.9 [SD 8.3] yr; mean time interval since trauma 1.8 [SD 0.5] mo; score on the STAI State Anxiety Form < 45) because of the possible influence that this symptom can induce on the profile of intracortical inhibition.7
Results
Anxiety was found at STAI evaluation in 6 patients with PTSD. No difference in both resting (TMS intensity for test stimulation) and active (TMS intensity for conditioning stimulation during target muscle contraction) motor thresholds was found between patients and the 2 control groups (test: 72.6% [SD 9.4%] v. 73.4% [SD 6.9%] v. 72.9% [SD 8.2%]; conditioning: 42.5% [SD 7.2%] v. 44.7% [SD 6.2%] v. 43.6% [SD 9.1%] for patients with PTSD, controls without trauma, controls with trauma, respectively). Unconditioned MEPs had similar average amplitudes in the groups under investigation (controls without trauma: 497 [SD 80] μV, controls with trauma: 516 [SD 90] μV, patients with PTSD: 550 [SD 140] μV) (example shown in Fig. 1). In addition, no significant change in MEP threshold was observed when anxious patients with PTSD were compared statistically with those patients who did not exhibit anxiety. These data are in line with a previous report in which anxiety has been reported to correlate with abnormal paired-pulse responses but not with altered MEP threshold.7
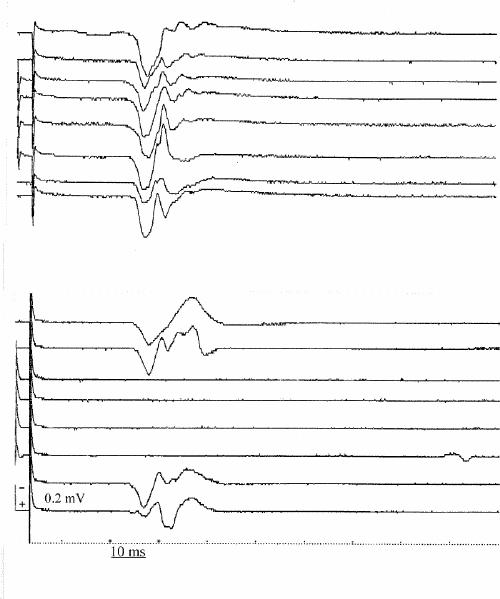
Fig. 1: Right-hand motor evoked potentials (MEPs) from a patient with post-traumatic stress disorder (PTSD) (upper panel) and a control subject (lower panel). In each panel, the sequence of traces displays the same rule of recording: the upper and lower pairs of traces are evoked in response to the test stimulus alone, whereas traces in the middle are the result of paired (conditioning and test) stimulation, with an interstimulus interval of 3 ms. Note the absence of the physiologic MEP suppression in the patient with PTSD compared with the control subject.
A significant loss of MEP inhibition, normally produced by pTMS at first ISI,6 was found in all patients with PTSD in comparison with both control groups at 2 ms, 3 ms and 4 ms ISI (Fig. 1, Fig. 2). In fact, the statistical analysis of pTMS data showed a significant effect (F2,36 = 25.63, p < 0.001) of the factor “group,” because in patients with PTSD the mean conditioned MEP amplitude over the 6 ISI was higher (98.87%) than that observed in the other 2 populations (63.19% and 64.11% for controls without trauma and controls with trauma, respectively). The ISI factor was also significant (F5,180 = 89.85, Greenhouse–Geisser ε = 0.35; p < 0.001), as the mean “conditioned” MEP amplitude increased from 22.5% to 127.8% as the ISI increased from 1 ms to 6 ms. Finally, the group х ISI interaction was also significant (F10,180 = 8.97, p < 0.001), showing that the effect of the conditioning stimulus was different in the 3 studied groups at different ISI. In fact, the Tukey HSD test illustrated a significant loss of MEP inhibition at 2 ms, 3 ms and 4 ms ISI in patients with PTSD in comparison with both controls without trauma (2 ms: p < 0.001, 3 ms: p < 0.001 and 4 ms: p < 0.01) and controls with trauma (2 ms: p < 0.001, 3 ms: p < 0.001 and 4 ms: p < 0.05). It is noticeable that no statistical difference was found between the 2 control groups (Fig. 2).
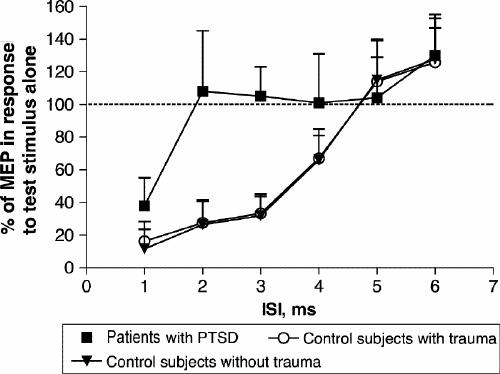
Fig. 2: Average time course of intracortical inhibition in 12 control subjects without trauma, 11 control subjects with trauma and 14 patients with PTSD. At each interstimulus interval (ISI), the size of the conditioned MEP is expressed as a percentage of the size of the MEP in response to the test stimulus alone. Note the large difference between the curves, indicating the lack of inhibition in patients with PTSD, particularly from 2 ms to 4 ms of ISIs. The results are given here as means (and standard deviation).
Discussion
In the present study, we provided evidence that PTSD, when following minor head trauma, is coupled with the loss of physiologic inhibition of cortical stimulation during pTMS. The evidence that subjects who had experienced trauma to the head but no PTSD did not exhibit abnormal intracortical inhibition suggests that the lack of intracortical inhibition is strictly correlated with the presence of PTSD symptoms rather than with the head trauma itself.
The excitability of corticospinal neurons, the activation of which is responsible for motor evoked responses, is finely regulated by both excitatory and inhibitory inputs.21 Although it is generally accepted that MEPs mainly originate from the stimulation of excitatory axons impinging on corticospinal neurons,20,22 the physiologic role of GABAergic inputs in the modulation of cortical excitability can be evaluated by paired-pulse stimulation with short ISIs.6,9,10,18,21,23 Interestingly, GABA-mediated inhibition of cortical pyramidal neurons is essentially intrinsic, arising from local interneurons responsible for a delayed inhibition of corticospinal neurons during TMS. In accordance with this, whereas the amplitude and threshold of motor responses evoked from a single pulse are unaffected by pharmacologic agents that enhance GABA-mediated transmission, these compounds increase MEP inhibition during pTMS.9 The evidence that pTMS-mediated MEP inhibition occurs even when the first, conditioning stimulus is unable to produce an MEP strongly suggests that cortical GABAergic interneurons are particularly prone to excitation.
Our data, therefore, suggest that durable functional and/or structural injury of these highly excitable interneurons can account for the impairment of cortical inhibition after traumatic events and, possibly, for the clinical symptoms of PTSD. In this respect, several neuroimaging findings suggest that after psychological trauma, biologic changes are not restricted to dysregulation of neurochemical systems but also involve alterations in brain function and structure. In particular, a number of structural MRI studies have shown that subjects with PTSD have a hippocampal volume that is smaller than normal,24,25,26,27,28,29 although other groups have failed to show reduced hippocampal volume in PTSD.30,31,32,33,34,35 Brain structures other than the hippocampus have received less attention, although a few studies have reported whole-brain volume reduction,30 reduced total white-matter volume,28 smaller corpus callosum,30 larger superior temporal gyrus grey-matter volume36 and reduction in anterior cingulate grey-matter volume.37 On the other hand, functional neuroimaging has revealed greater activation of the amygdala, anterior paralimbic structures and, importantly, Broca's region and other neocortical regions in response to trauma-related stimuli in individuals with PTSD.38,39,40 Furthermore, a decrease in N-acetyl-aspartate, an indicator of neuronal integrity, has been found by means of proton magnetic resonance spectroscopy in the hippocampus of patients with PTSD.34,41
In the patients with PTSD included in this study, the observed impairment of GABA-mediated MEP inhibition may also reflect a selective vulnerability of cortical GABAergic interneurons to glutamate-mediated excitotoxic events. Accordingly, a significant increase in glutamate concentration occurs transiently in the brain following head trauma,42,43 an effect paralleled by increased sensitivity of cortical neurons to glutamate receptor stimulation.43 It is, therefore, conceivable that the combination of the 2 events may favour excitotoxic events in highly excitable neurons, thereby causing a preferential injury of GABA interneurons.
Our findings are in line with basic experimental data indicating that acute stress affects brain activity and promotes long-term changes of synaptic efficacy. In this respect, long-term potentiation of excitatory synapses is the most extensively accepted form of neuroplasticity, and it is believed to be the substrate for both explicit and implicit learning and memory processes. Interestingly, this form of synaptic plasticity follows massive stimulation of glutamate receptors44,45 and is primed by environmental physical and mental stressful events.46,47,48,49 It can be postulated, therefore, that similar plastic reorganization changes take place in patients who develop PTSD, accounting for the neurophysiologic abnormalities found in our study. According to this hypothesis, it has been reported that slow (< 1 Hz) repetitive TMS, a procedure believed to induce long-term depression of synaptic transmission or depotentiation of pathologic synaptic potentiation,50 exerts beneficial effects in patients with PTSD.51,52
The evaluation of excitatory ISIs, obtained with longer intervals,9,53 in addition to the inhibitory ones, could also have been informative regarding the dysregulation of excitatory/ inhibitory mechanisms at cortical levels. However, to limit any possible seizures induced by cortical stimulation, we decided to select a procedure that should be reasonably short and at the same time eloquent.
Taken together with the recent findings that motor cortex excitability correlates with obsessive–compulsive disorder and an anxiety-related personality trait,7 our data on PTSD support the conclusion that cortical hyperexcitability potentially underlies several psychiatric disturbances. Understanding the neurophysiologic bases of psychiatric disorders is essential for the development of more effective therapeutic strategies.
Footnotes
Competing interests: None declared.
Correspondence to: Dr. Diego Centonze, Clinica Neurologica, Dipartimento di Neuroscienze, Università di Tor Vergata, Via Montpellier 1, 00133 Rome, Italy; fax 39 06 7259 6006; centonze@uniroma2.it
Submitted Apr. 2, 2003; Revised Dec. 11, 2003; May 1, 2004; Accepted May 18, 2004
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: the Association; 1994.
- 2.Rauch SL, van der Kolk BA, Fisler RE, Alpert NM, Orr SP, Savage CR, et al. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Arch Gen Psychiatry 1996;53:380-7. [DOI] [PubMed]
- 3.Shin LM, Kosslyn SM, McNally RJ, Alpert NM, Thompson WL, Rauch SL, et al. Visual imagery and perception in posttraumatic stress disorder: a positron emission tomographic investigation. Arch Gen Psychiatry 1997;54:233-41. [DOI] [PubMed]
- 4.Van der Kolk BA, Burbridge JA, Suzuki J. The psychobiology of traumatic memory: clinical implications of neuroimaging studies. Ann N Y Acad Sci 1997;821:99-113. [DOI] [PubMed]
- 5.Shaw ME, Strother SC, McFarlane AC, Morris P, Anderson J, Clark CR, et al. Abnormal functional connectivity in posttraumatic stress disorder. Neuroimage 2002;15:661-74. [DOI] [PubMed]
- 6.Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, et al. Corticocortical inhibition in human motor cortex. J Physiol 1993;473:501-19. [DOI] [PMC free article] [PubMed]
- 7.Wassermann EM, Greenberg BD, Nguyen MB, Murphy DL. Motor cortex excitability correlates with an anxiety-related personality trait. Biol Psychiatry 2001;50:377-82. [DOI] [PubMed]
- 8.Greenberg BD, Ziemann U, Cora-Locatelli G, Harmon A, Murphy DL, Keel JC, et al. Altered cortical excitability in obsessive-compulsive disorder. Neurology 2000;54:142-7. [DOI] [PubMed]
- 9.Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol 1996;40:367-78. [DOI] [PubMed]
- 10.Caramia MD, Palmieri MG, Desiato MT, Iani C, Scalise A, Telera S, et al. Pharmacological reversal of cortical hyperexcitability in patients with ALS. Neurology 2000;54:58-64. [DOI] [PubMed]
- 11.Ricker JH, Zafonte RD. Functional neuroimaging and quantitative electroencephalography in adult traumatic head injury: clinical applications and interpretive cautions. J Head Trauma Rehab 2000;15:859-68. [DOI] [PubMed]
- 12.Chistyakov AV, Soustiel JF, Hafner H, Trubnik M, Levy G, Feinsod M. Excitatory and inhibitory corticospinal responses to transcranial magnetic stimulation in patients with minor to moderate brain injury. J Neurol Neurosurg Psychiatry 2001;70:580-7. [DOI] [PMC free article] [PubMed]
- 13.Bryant RA. Early predictors of posttraumatic stress disorder. Biol Psychiatry 2003;53:789-95. [DOI] [PubMed]
- 14.Hull AM. Neuroimaging findings in post-traumatic stress disorder. Br J Psychiatry 2002;181:102-10. [PubMed]
- 15.Hathaway RS, McKinley JV. Manual for the Minnesota Multiphasic Personality Inventory. New York: Psychological Corporation; 1951.
- 16.Spielberg CD, Gorsuch RL, Lushene RE. Manual for the State–Trait Anxiety Inventory. Palo Alto (CA): Consulting Psychologist Press; 1983.
- 17.Evans RW. The postconcussion syndrome and the sequelae of mild head injury. In: Evans RW, editor. Neurology and trauma. Philadelphia: Saunders; 1996. p 91-116.
- 18.Caramia MD, Gigli G, Iani C, Desiato MT, Diomedi M, Palmieri MG, et al. Distinguishing forms of generalized epilepsy using magnetic brain stimulation. Electroenceph Clin Neurophysiol 1996;98:14-9. [DOI] [PubMed]
- 19.Rossini PM, Desiato MT, Lavaroni F, Caramia MD. Brain excitability and electroencephalographic activation: non-invasive evaluation in healthy humans via transcranial magnetic stimulation. Brain Res 1991;567:111-9. [DOI] [PubMed]
- 20.Rossini PM, Barker T, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Electroenceph Clin Neurophysiol 1994;91:79-92. [DOI] [PubMed]
- 21.Rothwell JC. Paired-pulse investigations of short-latency intracortical facilitation using TMS in humans. Electroenceph Clin Neurophysiol 1999;51:113-9. [PubMed]
- 22.Hallett M. Transcranial magnetic stimulation and the human brain. Nature 2000;406:147-50. [DOI] [PubMed]
- 23.Ridding MC, Inzelberg R, Rothwell JC. Changes in excitability of motor cortical circuitry in patients with Parkinson's disease. Ann Neurol 1995;37:181-8. [DOI] [PubMed]
- 24.Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry 1995;152:973-81. [DOI] [PMC free article] [PubMed]
- 25.Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, et al. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse - a preliminary report. Biol Psychiatry 1997;41:23-32. [DOI] [PMC free article] [PubMed]
- 26.Gurvits TV, Shenton ME, Hokama H, Ohta H, Lasko NB, Gilbertson MW, et al. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol Psychiatry 1996;11:1091-9. [DOI] [PMC free article] [PubMed]
- 27.Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med 1997;27:951-9. [DOI] [PubMed]
- 28.Villarreal G, Hamilton DA, Petropoulos H, Driscoll I, Rowland LM, Griego JA, et al. Reduced hippocampal volume and total white matter volume in posttraumatic stress disorder. Biol Psychiatry 2002;52:119-25. [DOI] [PubMed]
- 29.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko MB, Orr SP, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci 2002;5:1242-7. [DOI] [PMC free article] [PubMed]
- 30.De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, et al. A.E. Bennett Research Award. Developmental traumatology. Part II: brain development. Biol Psychiatry 1999;45:1271-84. [DOI] [PubMed]
- 31.De Bellis MD, Hall J, Boring AM, Frustaci K, Moritz G. A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry 2001;50:305-9. [DOI] [PubMed]
- 32.Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, et al. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol Psychiatry 2001;50:943-51. [DOI] [PubMed]
- 33.Bonne O, Brandes D, Gilboa A, Gomori JM, Shenton ME, Pitman RK, et al. Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. Am J Psychiatry 2001;158:1248-51. [DOI] [PMC free article] [PubMed]
- 34.Schuff N, Neylan TC, Lenoci MA, Du AT, Weiss DS, Marmar CR, et al. Decreased hippocampal N-acetylaspartate in the absence of atrophy in posttraumatic stress disorder. Biol Psychiatry 2001;50:952-9. [DOI] [PMC free article] [PubMed]
- 35.Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW. Hippocampal volume in patients with alcohol dependence. Arch Gen Psychiatry 1999;56:356-63. [DOI] [PubMed]
- 36.De Bellis MD, Keshavan MS, Frustaci K, Shifflett H, Iyengar S, Beers SR, et al. Superior temporal gyrus volumes in maltreated children and adolescents with PTSD. Biol Psychiatry 2002;51:544-52. [DOI] [PubMed]
- 37.Yamasue H, Kasai K, Iwanami A, Ohtani T, Yamada H, Abe O, et al. Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proc Natl Acad Sci U S A 2003;100:9039-43. [DOI] [PMC free article] [PubMed]
- 38.Pitman RK, Shin LM, Rauch SL. Investigating the pathogenesis of posttraumatic stress disorder with neuroimaging. J Clin Psychiatry 2001;62:47-54. [PubMed]
- 39.Villarreal G, King CY. Brain imaging in posttraumatic stress disorder. Semin Clin Neuropsychiatry 2001:6:131-45. [DOI] [PubMed]
- 40.Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, et al. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry 2001;50:932-42. [DOI] [PubMed]
- 41.Villarreal G, Petropoulos H, Hamilton DA, Rowland LM, Horan WP, Griego JA, et al. Proton magnetic resonance spectroscopy of the hippocampus and occipital white matter in PTSD: preliminary results. Can J Psychiatry 2002;47:666-70. [DOI] [PubMed]
- 42.Obrenovitch TP. Is high extracellular glutamate the key to excitotixicity in traumatic brain injury? J Neurotrauma 1997;14:677-98. [DOI] [PubMed]
- 43.Goforth PB, Ellis EF, Satin LS. Enhancement of AMPA-mediated current after traumatic injury in cortical neurons. J Neurosci 1999;19:7367-74. [DOI] [PMC free article] [PubMed]
- 44.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 1993;362:31-9. [DOI] [PubMed]
- 45.Charpier S, Deniau JM. In vivo activity-dependent plasticity at cortico-striatal connections: evidence for physiological long-term potentiation. Proc Natl Acad Sci U S A 1997;94:7036-40. [DOI] [PMC free article] [PubMed]
- 46.Kim JJ, Yoon KS. Stress: metaplastic effects in the hippocampus. Trends Neurosci 1998;21:505-9. [DOI] [PubMed]
- 47.Kim JJ, Lee HJ, Han JS, Packard MG. Amygdala is critical for stress-induced modulation of hippocampal long-term potentiation and learning. J Neurosci 2001;21:5222-8. [DOI] [PMC free article] [PubMed]
- 48.Blank T, Nijholt I, Eckart K, Spiess J. Priming of long-term potentiation in mouse hippocampus by corticotropin-releasing factor and acute stress: implications for hippocampus-dependent learning. J Neurosci 2002;22:3788-94. [DOI] [PMC free article] [PubMed]
- 49.Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron 2003;37:577-82. [DOI] [PubMed]
- 50.Hoffman RE, Cavus I. Slow transcranial magnetic stimulation, long-term depotentiation, and brain hyperexcitability disorders. Am J Psychiatry 2002;157:1093-102. [DOI] [PubMed]
- 51.Grisaru N, Amir M, Cohen H, Kaplan Z. Effect of transcranial magnetic stimulation in posttraumatic stress disorder: a preliminary study. Biol Psychiatry 1998;44:53-5. [DOI] [PubMed]
- 52.McCann UD, Kimbrell TA, Morgan CM, Geraci M, Benson BE, Wassermann EM, et al. Repetitive transcranial magnetic stimulation for posttraumatic stress disorder. Arch Gen Psychiatry 1998;55:277-9. [DOI] [PubMed]
- 53.Liepert J, Schwenkreis P, Tegenthoff M, Malin JP. The glutamate antagonist riluzole suppresses intracortical facilitation. J Neural Transm 1997;104:1207-14. [DOI] [PubMed]


