Abstract
Background
The effect of maternal mood disorders on neonatal measurements is not well defined. The Fetal Growth Studies – Singletons provide a unique opportunity to evaluate the relationship between perceived maternal stress and neonatal growth measurements.
Objective
To determine whether perceived maternal stress during pregnancy is associated with anthropometric measurements in the neonate.
Study Design
This analysis was based on a prospective, multi-center longitudinal study of fetal growth. Women 18–40 years with BMI 19.0–29.9 kg/m2 were screened at 8+0 to 13+6 weeks’ gestation for low-risk status associated with optimal fetal growth (e.g. healthy, non-smoking), and underwent serial sonographic examination at six study visits throughout gestation. At each study visit, women completed the Cohen’s Perceived Stress Survey (PSS), which could have a score ranging from 0 – 40. We used a latent class trajectory model to identify distinct groupings (i.e., classes) of the PSS trajectories over pregnancy. Trend analysis was used to determine whether neonatal measurements including birth weight, length, head circumference, and abdominal circumference differed by PSS class, and whether this relationship was modified by maternal race/ethnicity, after adjusting for gestational age at delivery, maternal height, age and parity.
Results
Of the 2,334 women enrolled in the study, 1,948 had complete neonatal anthropometry and were included in the analysis. Latent class analysis identified three PSS trajectory classes, with mean PSS scores of 2.82 (low), 7.95 (medium) and 14.80 (high). Neonatal anthropometric measures of birth weight, length, head circumference and abdominal circumference were similar (P=0.78, P=0.10, P=0.18, and P=0.40 respectively) regardless of the participants’ PSS class. There was no effect modification by maternal race/ethnicity.
Conclusion
Neonatal measurements did not differ by levels of perceived stress among low-risk pregnant women.
Introduction
Major stressful life events during pregnancy, such as wars and natural disasters, have been demonstrated to be negatively associated with gestational age at birth, birth weight and length 1–5. A variety of psychosocial factors, including food insecurity, single-parent households, sedentary lifestyles and poor coping skills, also have been significantly associated with low birth weight at delivery6,7. The association between perceived maternal stress and pregnancy outcomes in populations who are not exposed to such catastrophic events is less clear. Rondo et al. (2003) discovered a nearly two-fold increased risk of low birth weight among 845 women with higher self-reported stress and anxiety during pregnancy, while other large cohort studies have failed to demonstrate an association8,9. It has been postulated that excessive maternal stress contributes to the development of fetal growth restriction through abnormal placental function10,11. For example, greater stress has been associated with higher levels of Epstein-Barr virus titers and C-Reactive Protein in the peripheral blood, and higher levels of these two biomarkers have been associated with increased placental inflammation11–14.
The uncertain relationship between maternal stress and fetal growth may be explained by the many confounding factors that exist and have not been adequately controlled for in prior studies. The NICHD Fetal Growth Studies-Singletons provide a unique opportunity to assess the relationship between maternal stress and neonatal anthropometric measurements in a group of healthy pregnant women in the U.S. Our objectives were to describe longitudinal changes in perceived stress throughout pregnancy, and to investigate whether perceived stress was associated with neonatal anthropometry including birth weight, length, head and abdominal circumferences. We hypothesized that neonatal anthropometric measurements would be smaller for women with greater perceived stress using the Cohen’s Perceived Stress Scale (PSS)5 as compared to those with lower levels of stress.
Methods
The NICHD Fetal Growth Studies – Singletons was a prospective cohort study in which pregnant women were recruited from 12 participating clinical sites from July 2009 through January 2013. Women were eligible for the study if they were at low risk of obstetric or medical complications. Psychiatric disorders, including an anxiety disorder currently requiring medication, depression, or bipolar disorder were exclusion criteria. Our protocol required concordance with the last menstrual period so that women requiring redating were not enrolled. Women underwent a screening ultrasound between 8 0/7 and 13 6/7 weeks, to ensure sonographic dating consistent with last menstrual period dating. The ultrasound estimate of gestation had to match the LMP-based gestational age within 5 days for women between 8w0d and 10w6d, within 6 days for those between 11w0d and 12w6d and within 7 days for participants between 13w0d and 13w6d. Gestational age was therefore based on the menstrual date. Consenting women were randomized to one of four serial sonography schedules for a total of six targeted visits throughout pregnancy. Full details of the protocol and study methods have been published previously15. Human subjects’ approval was obtained from all participating sites prior to initiation of the study and all women gave informed consent before enrollment and data collection.
During the study, research nurses conducted in-person interviews with participants that ascertained a variety of data, including demographic and psychosocial information. Women were administered the Cohen’s Perceived Stress Scale (PSS)5 at every visit. This is a 10-item validated survey in which each question is coded 0 to 4 and then summed to compute a total score ranging from 0 to 40. Higher scores indicate greater perceived stress.
Our trained research coordinators followed standardized protocols using uniform equipment, including a portable stadiometer (Seca Corporation, Hamburg, Germany; US office, Hanover, MD), at enrollment to measure height and weight. Recalled prepregnancy weight was also recorded and body mass index (BMI) calculated. Women’s race/ethnicity was categorized as non-Hispanic white, non-Hispanic black, Hispanic, and Asian or Pacific Islander. These categorizations were based on self-identified race/ethnicity provided by participants on their study questionnaire. Neonatal anthropometric measurements were conducted by trained research coordinators per protocol within 12 to 24 hours after delivery so as not to interfere with the hospital’s routine newborn care. Measurements were targeted closer to 24 hours if possible given that less head molding and flexion are present. The examination included measurements of the neonatal length, birth weight, head circumference (HC), and abdominal circumference (AC). Neonatal length was measured as the distance from the soles of the infant’s feet to the top of the head, with the infant supine and using an approved Infantometer (infant measuring board). The assistant positioned the infant’s head flush against the headboard, with the infant looking upward and with the head in the Frankfort Horizontal Plane. The Frankfort Plane runs through the inferior bones of the bony orbits and the upper margin of the auditory meatus. Supine, the plane should be perpendicular to the horizontal during measurement. The measurer held the infant’s legs flat as the footboard was moved flat against the infant’s heels. Birth weight was measured using an infant beam balance scale or an infant electronic (digital) scale, and recorded in pounds or grams. The head circumference was measured with a tape placed anteriorly on the forehead just above the eyebrows and posteriorly at the maximum protrusion of the occiput, so that the maximum head circumference was measured. The tape was pulled to be snug against the head but not tight and recorded to the nearest 0.1 cm. The AC was measured midway between the xiphoid process of the sternum and umbilicus. All measurements were taken in duplicate. If the two measurements differed by a prespecified tolerance limit, a third measurement was taken.
Statistical Analysis
To estimate the course of stress throughout the pregnancy, we used a latent-class trajectory model, a flexible semi-parametric method that can be used to discover patterns. This approach allows for multiple latent trajectories where each trajectory follows a linear mixed model. This method provides a data-driven approach to identify whether distinct individual patterns of stress exist and the corresponding probability of falling into each pattern (posterior probability). Subjects were then classified based on their highest posterior probability. We compared the fit of 2 to 4 trajectories by choosing the model with the lowest Bayesian Information Criterion (BIC) value. Latent – class trajectory analyses were conducted using R version 3.1.2, package LCMM16.
After the stress trajectories were estimated, we examined the proportion of women in each trajectory. Of the 2,334 women enrolled in the study, 1,962 had complete neonatal information. Among these women, we excluded pregnancies with missing covariate information (n=14). The final analysis, therefore, included 1948 women. Linear regression was used to assess whether an association was present between stress levels across pregnancy and neonatal anthropometry. In this analysis, the outcome variable was a neonatal anthropometric measurement, the independent variable was the stress class, and we adjusted for the following potential confounders: gestational age at delivery, neonatal measurement date, maternal height, maternal age, and parity. We tested whether the relationships were modified by maternal race/ethnicity using linear regression models with interaction terms between stress level and maternal race/ethnicity (likelihood ratio test conducted at the 0.05 significance level). In addition to accounting for the time between birth and neonatal measurements using the previously discussed regression analysis, we conducted a sensitivity analysis that excluded neonates (n=188) that had measurements more than 24 hours after birth.
Results
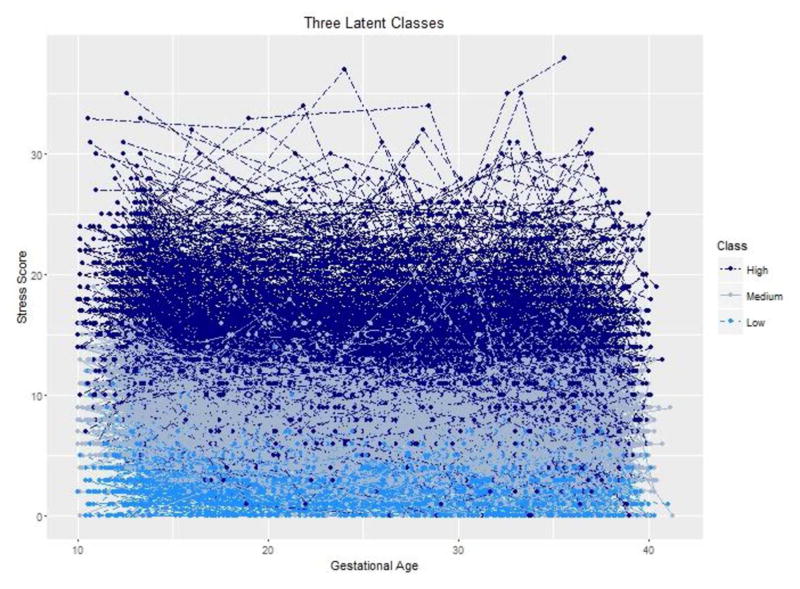
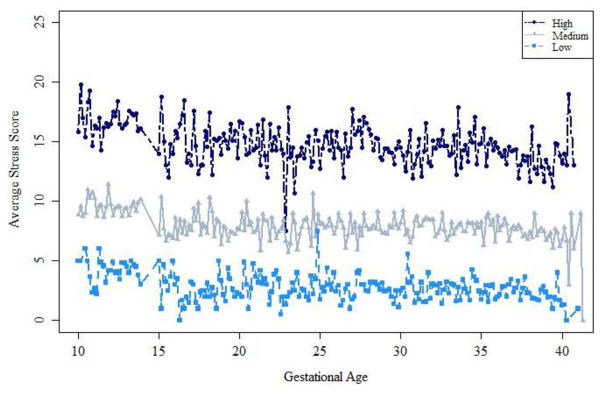
Characteristics of the study population are presented in Table 1. As illustrated, the women in the study population were racially and ethnically diverse, and represented a wide range of socioeconomic strata. We discriminated three separate groups of women who had low [n=336 (17.2%)], medium [n=871 (44.7%)] or high [n=741 (38.0%)] stress across gestation. (Figure 1) The mean perceived stress scores for each group were 2.82 (low), 7.95 (medium) and 14.80 (high) (Figure 1 and Table 2). Regardless of class, on average, stress was relatively constant, or slightly decreased, over the course of pregnancy (Figure 2). The group with the highest perceived stress was more likely to be younger, non-Hispanic black, heavier, multiparous, never married, receiving Medicaid, of lower educational status, and with lower income (Table 1).
Table 1.
Characteristics of the study population by Perceived Stress Latent Class
| Variable | Total (Overall) (N = 1948) | Class = Low (n = 336) | Class = Medium (n = 871) | Class = High (n = 741) | P-value |
|---|---|---|---|---|---|
| Maternal Age (years) | 28.23 (5.47) | 28.88 (5.07) | 28.69 (5.38) | 27.40 (5.64) | <0.0001 |
| Gestational Age at enrollment (weeks) | 12.69 (0.96) | 12.68 (0.92) | 12.68 (0.97) | 12.72 (0.97) | 0.38 |
| Gestational Age at delivery (weeks) | 39.32 (1.35) | 39.35 (1.20) | 39.35 (1.35) | 39.27 (1.41) | 0.42 |
| Height (cm) | 162.49 (6.96) | 162.37 (7.08) | 162.54 (6.94) | 162.48 (6.93) | 0.88 |
| Weight (kg) | 64.65 (10.55) | 64.27 (10.36) | 64.31 (10.28) | 65.21 (10.94) | 0.18 |
| Maternal pre-pregnancy BMI (kg/m2) | 23.63 (3.03) | 23.46 (2.91) | 23.46 (3.04) | 23.85 (3.05) | 0.02 |
| Race/Ethnicity | |||||
| Non-Hispanic White | 531 (27.3%) | 125 (37.2%) | 275 (31.6%) | 131 (17.7%) | <0.0001 |
| Non-Hispanic Black | 496 (25.5%) | 68 (20.2%) | 184 (21.1%) | 244 (32.9%) | |
| Hispanic | 539 (27.7%) | 84 (25.0%) | 229 (26.3%) | 226 (30.5%) | |
| Asian & Pacific Islander | 382 (19.6%) | 59 (17.6%) | 183 (21.0%) | 140 (18.9%) | |
| Parity | |||||
| Nulliparity N (%) | 952 (48.9%) | 167 (49.7%) | 446 (51.2%) | 339 (45.7%) | 0.09 |
| Parity ≥1 N (%) | 996 (51.1%) | 169 (50.3%) | 425 (48.8%) | 402 (54.3%) | |
| Marital status | |||||
| Never Married | 414 (21.3%) | 53 (15.8%) | 165 (19.0%) | 196 (26.5%) | <0.0001 |
| Married/Living as Married | 1481 (76.1%) | 279 (83.0%) | 690 (79.4%) | 512 (69.1%) | |
| Divorced/Separated/Widowed | 51 (2.6%) | 4 (1.2%) | 14 (1.6%) | 33 (4.5%) | |
| Education (Highest Level) | |||||
| Less than high school | 200 (10.3%) | 22 (6.5%) | 73 (8.4%) | 105 (14.2%) | <0.0001 |
| High school diploma or GED or equivalent | 346 (17.8%) | 50 (14.9%) | 139 (16.0%) | 157 (21.2%) | |
| Some college or Associate degree | 564 (29.0%) | 95 (28.3%) | 235 (27.0%) | 234 (31.6%) | |
| Bachelors degree | 479 (24.6%) | 101 (30.1%) | 234 (26.9%) | 144 (19.4%) | |
| Masters degree or Advanced degree | 359 (18.4%) | 68 (20.2%) | 190 (21.8%) | 101 (13.6%) | |
| Family income | |||||
| ≤$29,999 | 462 (27.5%) | 48 (16.6%) | 182 (23.7%) | 232 (37.4%) | <0.0001 |
| $30,000–49,999 | 284 (16.9%) | 42 (14.5%) | 117 (15.2%) | 125 (20.1%) | |
| $50,000–$74,999 | 202 (12.0%) | 34 (11.8%) | 101 (13.1%) | 67 (10.8%) | |
| $75,000–$99,999 | 232 (13.8%) | 41 (14.2%) | 109 (14.2%) | 82 (13.2%) | |
| ≥$100,000 | 499 (29.7%) | 124 (42.9%) | 260 (33.8%) | 115 (18.5%) | |
| Health insurance | |||||
| Private/managed care | 1132 (58.1%) | 213 (63.4%) | 545 (62.6%) | 374 (50.5%) | <0.0001 |
| Medicaid; other | 771 (39.6%) | 120 (35.7%) | 304 (34.9%) | 347 (46.8%) | |
| Self pay | 45 (2.3%) | 3 (0.9%) | 22 (2.5%) | 20 (2.7%) |
GED: General Education Diploma
Data presented as n (%) or mean (SD) [n].
Figure 1. Longitudinal Derived Cohen’s Perceived Stress Trajectories for the NICHD Fetal Growth Studies.
each dot-dashed line reflects an individual stress trajectories. The lines are color coded to indicate the subject’s class.
Table 2.
Posterior Classifications Representing Probability of Falling Into Each Perceived Stress Class
| Low | Medium | High | |
|---|---|---|---|
| n | 336 | 871 | 741 |
| % | 17.2 | 44.7 | 38.0 |
| Average Probability | 0.77 | 0.70 | 0.84 |
| Perceived Stress Score | 2.82 | 7.95 | 14.79 |
Figure 2.
Average Derived Cohen’s Perceived Stress trajectories from the NICHD Fetal Growth Studies
Average measurements for those classified in the High, Medium or Low class. Subjects were classified into these groups via highest posterior probability.
Table 3 illustrates the individual biometric parameters of birth weight, length, HC, and AC stratified by PSS score classes. There was no significant difference in the mean (SD) birthweight among low [3274 (432.17) g], medium [3300 (457.43) g], and high [3268 (474.34) g] stress groups. Neonatal length, head circumference, and abdominal circumference followed a similar pattern. Maternal race/ethnicity did not modify any of the above relations, suggesting that the relationship between perceived stress and neonatal anthropometry was similar across race/ethnicity groups (Table 3). In a sensitivity analysis excluding the neonates for whom the anthropometric measurements were obtained more than 24 hours after birth, the findings were consistent with the main analysis (data not shown).
Table 3.
Mean Neonatal Measurements of Perceived Stress Classes
| Low | Medium | High | Adjusted Pa | |
|---|---|---|---|---|
| Birth weight (g) | 3274 (432.17) | 3300 (457.43) | 3268 (474.34) | 0.80 |
| Length (cm) | 50.29 (2.46) | 50.18 (2.52) | 49.95 (2.54) | 0.08 |
| Head circumference (cm) | 34.10 (1.40) | 34.11 (1.48) | 33.91 (1.55) | 0.12 |
| Abdominal circumference (cm) | 33.14 (2.16) | 33.16 (2.26) | 32.95 (2.30) | 0.38 |
P adjusted for measurement date, maternal age, race/ethnicity height and parity.
Measurements represented as mean with standard deviation in ( ).
The current analysis excluded voluntary termination of pregnancies (n=7, <1%), miscarriages (n=23,1%), women who moved away from the study catchment area (n=26, 1%), refused to continue prior to delivery (n=85, 4%), did not meet the inclusion criteria after enrollment (n=14, <1%), had unknown birth outcomes if the participant delivered at home or another hospital and medical records could not be obtained (n=11, <1%), had at least one neonatal anthropometry variable not measured (n=29, 1%), measurements were not collected because the newborn was in the NICU or the patient was discharged before measurements could be collected (n=10, <1%) or were missing for other reasons (n=167, 7%). The final sample size for this analysis consisted of 1,962 (84%) women with low risk singleton pregnancies.
Comment
We discriminated three separate longitudinal trajectories of perceived stress in pregnancy. Higher scores on the PSS throughout pregnancy were not associated with alterations in neonatal anthropometry including birth weight, length, head and abdominal circumferences, even after accounting for important confounders. Further, this lack of association was similar regardless maternal race/ethnicity. Our results are consistent with previous findings from the same study population regarding the lack of association of sonographic trajectories of fetal biometry with either maternal perceived stress or depression (Grobman et al, In Progress). This study however, did not assess actual neonatal measurement.
An inverse association between psychosocial burden and neonatal measurements has been reported inconsistently in the literature. Zhu et al. (2010)17 studied 1800 women who delivered after 32 weeks and found that each unit increase of perceived life events stress during the first trimester was associated with a 99-gram decrease in infant birth weight. Similarly, Khashan et al. (2014)18 found a significant association between antenatal perceived stress scores and the risk of small-for-gestational age birth (adjusted odds ratio 1.01, 95% confidence interval 1.01–1.02) in a longitudinal prospective cohort investigation performed in the Australia, New Zealand and parts of the United Kingdom Other European studies have echoed these results19,20. Yet, other studies such as ours have not been able to reproduce these associations. Broekman et al. (2014)21 found no relation of anxiety and depression in pregnancy at 26 weeks of gestation with birth weight, although they did note an association with birth length in a large cohort of Asian women. This last report has been cited by some investigators as evidence that birth length is a more sensitive marker of fetal growth than is birth weight4. Explanations for the conflicting results include differing sample sizes, study designs and measures of maternal mental health.
There are several strengths of our investigation. Exposure data related to stress were obtained prospectively and serially from participants. The neonatal exams were performed using standardized equipment and protocols by trained research personnel. Because the inclusion criteria for NICHD Fetal Growth Studies – Singletons were designed to capture a healthy obstetrical population, our subjects were without histories of previous adverse pregnancy outcomes or other preexisting comorbidities, other extrinsic factors such as smoking, alcohol or illicit substance ingestion, or extreme poverty. Women with all these factors have been present in other studies, which could contribute to perturbations in perinatal growth but which are at best difficult to quantify in statistical modeling.
Even if greater perceived stress during pregnancy has no association with alterations in neonatal anthropometric measurements, the possibility exists that the psychosocial environment affects pregnancy outcomes, especially when the levels of perceived stress are more extreme than observed here or when the women are less physically healthy. We assessed perceived stress because its relationship with neonatal birth parameters is biologically plausible and suggested by other observational studies6,17,22. However, other unmeasured psychosocial constructs may have stronger associations with pregnancy outcomes6,7,17. The stress survey used assessed events and feelings that were relatively acute and proximate to the pregnancy. Yet, it may be that other elements such as chronic stress and affective symptoms, which were not measured in the present study, are the etiologic factors in the psychosocial domain more likely responsible for adverse pregnancy outcomes23.
There was no evidence in this longitudinal cohort study that perceived stress translated into reductions in overall neonatal weight, length or individual biometric parameters. The similarity in neonatal measures existed whether women experienced the exposure of interest relatively early in pregnancy or persistently throughout pregnancy, due to the fact that our observed stress trajectories were relatively flat. Moreover, race/ethnicity did not explain the lack of association. From this investigation, we conclude that perceived stress alone is not sufficient to result in altered neonatal anthropometric parameters. This should be reassuring to pregnant women. Future studies are necessary to delineate whether a greater psychosocial burden, either alone or in combination with maternal health or other environmental factors, and experienced at critical times in pregnancy, contributes to impairments in neonatal biometry.
Acknowledgments
Funding: This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (Contract Numbers: HHSN275200800013C; HHSN275200800002I; HHSN27500006; HHSN275200800003IC; HHSN275200800014C; HHSN275200800012C; HHSN275200800028C; HHSN275201000009C)
Footnotes
Disclosure: The authors report no conflict of interest.
These findings were presented at the 37th Annual Meeting of the Society of Maternal Fetal Medicine: Pregnancy Meeting in Las Vegas, Nevada –January 23rd–28th, 2017.
Reprints will not be available.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Khashan AS, McNamee R, Abel KM, et al. Reduced infant birthweight consequent upon maternal exposure to severe life events. Psychosomatic medicine. 2008;70(6):688–694. doi: 10.1097/PSY.0b013e318177940d. [DOI] [PubMed] [Google Scholar]
- 2.Khashan AS, McNamee R, Abel KM, et al. Rates of preterm birth following antenatal maternal exposure to severe life events: a population-based cohort study. Human reproduction (Oxford, England) 2009;24(2):429–437. doi: 10.1093/humrep/den418. [DOI] [PubMed] [Google Scholar]
- 3.Class QA, Lichtenstein P, Långström N, D’Onofrio BM. Timing of prenatal maternal exposure to severe life events and adverse pregnancy outcomes: a population study of 2.6 million pregnancies. Psychosomatic medicine. 2011;73(3):234–241. doi: 10.1097/PSY.0b013e31820a62ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dancause KN, Laplante DP, Oremus C, Fraser S, Brunet A, King S. Disaster-related prenatal maternal stress influences birth outcomes: project Ice Storm. Early Hum Dev. 2011;87(12):813–820. doi: 10.1016/j.earlhumdev.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 6.Borders AE, Grobman WA, Amsden LB, Holl JL. Chronic stress and low birth weight neonates in a low-income population of women. Obstetrics and gynecology. 2007;109(2 Pt 1):331–338. doi: 10.1097/01.AOG.0000250535.97920.b5. [DOI] [PubMed] [Google Scholar]
- 7.Savard N, Levallois P, Rivest LP, Gingras S. Impact of individual and ecological characteristics on small for gestational age births: an observational study in Quebec. Chronic Dis Inj Can. 2014;34(1):46–54. [PubMed] [Google Scholar]
- 8.Andersson L, Sundström-Poromaa I, Wulff M, Aström M, Bixo M. Neonatal outcome following maternal antenatal depression and anxiety: a population-based study. Am J Epidemiol. 2004;159(9):872–881. doi: 10.1093/aje/kwh122. [DOI] [PubMed] [Google Scholar]
- 9.Henrichs J, Schenk JJ, Roza SJ, et al. Maternal psychological distress and fetal growth trajectories: the Generation R Study. Psychological medicine. 2010;40(4):633–643. doi: 10.1017/S0033291709990894. [DOI] [PubMed] [Google Scholar]
- 10.Lewis AJ, Austin E, Galbally M. Prenatal maternal mental health and fetal growth restriction: a systematic review. J Dev Orig Health Dis. 2016;7(4):416–428. doi: 10.1017/S2040174416000076. [DOI] [PubMed] [Google Scholar]
- 11.Entringer S, Buss C, Wadhwa PD. Prenatal stress, development, health and disease risk: A psychobiological perspective-2015 Curt Richter Award Paper. Psychoneuroendocrinology. 2015;62:366–375. doi: 10.1016/j.psyneuen.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christian LM, Iams JD, Porter K, Glaser R. Epstein-Barr virus reactivation during pregnancy and postpartum: effects of race and racial discrimination. Brain Behav Immun. 2012;26(8):1280–1287. doi: 10.1016/j.bbi.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borders AE, Wolfe K, Qadir S, Kim KY, Holl J, Grobman W. Racial/ethnic differences in self-reported and biologic measures of chronic stress in pregnancy. J Perinatol. 2015;35(8):580–584. doi: 10.1038/jp.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ernst LM, Grobman WA, Wolfe K, et al. Biological markers of stress in pregnancy: associations with chronic placental inflammation at delivery. Am J Perinatol. 2013;30(7):557–564. doi: 10.1055/s-0032-1329187. [DOI] [PubMed] [Google Scholar]
- 15.Buck Louis GM, Grewal J, Albert PS, et al. Racial/ethnic standards for fetal growth: the NICHD Fetal Growth Studies. American journal of obstetrics and gynecology. 2015;213(4):449.e441–449.e441. doi: 10.1016/j.ajog.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.lcmm. Extended Mixed Models Using Latent Classes and Latent Processes, R package version: 1.7.5. 2016 [Google Scholar]
- 17.Zhu P, Tao F, Hao J, Sun Y, Jiang X. Prenatal life events stress: implications for preterm birth and infant birthweight. American journal of obstetrics and gynecology. 2010;203(1):34.e31–38. doi: 10.1016/j.ajog.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 18.Khashan AS, Everard C, McCowan LM, et al. Second-trimester maternal distress increases the risk of small for gestational age. Psychol Med. 2014;44(13):2799–2810. doi: 10.1017/S0033291714000300. [DOI] [PubMed] [Google Scholar]
- 19.Bödecs T, Horváth B, Szilágyi E, Gonda X, Rihmer Z, Sándor J. Effects of depression, anxiety, self-esteem, and health behaviour on neonatal outcomes in a population-based Hungarian sample. Eur J Obstet Gynecol Reprod Biol. 2011;154(1):45–50. doi: 10.1016/j.ejogrb.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 20.Maina G, Saracco P, Giolito MR, Danelon D, Bogetto F, Todros T. Impact of maternal psychological distress on fetal weight, prematurity and intrauterine growth retardation. J Affect Disord. 2008;111(2–3):214–220. doi: 10.1016/j.jad.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 21.Broekman BF, Chan YH, Chong YS, et al. The influence of anxiety and depressive symptoms during pregnancy on birth size. Paediatr Perinat Epidemiol. 2014;28(2):116–126. doi: 10.1111/ppe.12096. [DOI] [PubMed] [Google Scholar]
- 22.Khashan AS, McNamee R, Henriksen TB, et al. Risk of affective disorders following prenatal exposure to severe life events: a Danish population-based cohort study. J Psychiatr Res. 2011;45(7):879–885. doi: 10.1016/j.jpsychires.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Messer LC, Kaufman JS. Invited commentary: the socioeconomic causes of adverse birth outcomes. Am J Epidemiol. 2010;172(2):135–137. doi: 10.1093/aje/kwq107. discussion 138–139. [DOI] [PMC free article] [PubMed] [Google Scholar]