Abstract
Using the single prolonged stress (SPS) animal model of post traumatic stress disorder (PTSD), previous studies suggest that enhanced glucocorticoid receptor (GR) expression leads to cued fear extinction retention deficits. However, it is unknown how the endogenous ligand of GRs, corticosterone (CORT), may contribute to extinction retention deficits in the SPS model. Given that CORT synthesis during fear learning is critical for fear memory consolidation and SPS enhances GR expression, CORT synthesis during fear memory formation could strengthen fear memory in SPS rats by enhancing GR activation during fear learning. In turn, this could lead to cued fear extinction retention deficits. We tested the hypothesis that CORT synthesis during fear learning leads to cued fear extinction retention deficits in SPS rats by administering the CORT synthesis inhibitor metyrapone to SPS and control rats prior to fear conditioning, and observed the effect this had on extinction memory. Inhibiting CORT synthesis during fear memory formation in control rats tended to decrease cued freezing, though this effect never reached statistical significance. Contrary to our hypothesis, inhibiting CORT synthesis during fear memory formation disrupted extinction retention in SPS rats. This finding suggests that even though SPS exposure leads to cued fear extinction memory deficits, CORT synthesis during fear memory formation enhances extinction retention in SPS rats. This suggests that stress-induced CORT synthesis in previously stressed rats can be beneficial.
Keywords: Single prolonged stress, PTSD, hippocampus, fear, anxiety, fear memory resistance
1The single prolonged stress (SPS) model consists of restraint, forced swim, and ether exposure, and is an established rat model of post traumatic stress disorder (PTSD) that reproduces a number of PTSD symptoms [1]. The similarity of certain PTSD symptoms to behavioral and physiological effects observed in the SPS model makes this model useful for examining neurobiological mechanisms that contribute to certain PTSD symptoms [1]. SPS exposure results in deficits in the ability to inhibit conditioned fear responding to a previously extinguished cued fear conditioned stimulus (i.e. cued fear extinction retention deficit) [2, 3] and leads to enhanced GR expression in the hippocampus and prefrontal cortex [3, 4]. Furthermore, these two effects may be linked [3]. Enhanced GR expression has been implicated in PTSD symptomatology [5] and deficits in cued fear extinction are also observed in PTSD patients [6].
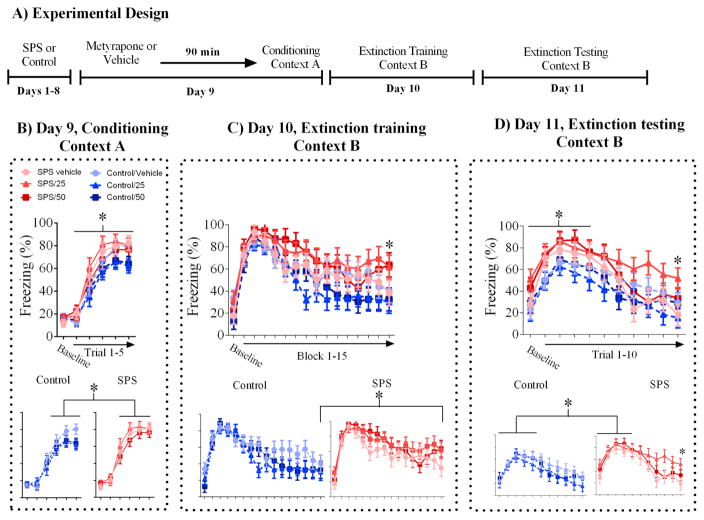
While previous reports suggest SPS-induced changes in GR expression could lead to extinction retention deficits in the SPS model, the role of the endogenous ligand for GRs, corticosterone (CORT), in mediating extinction retention deficits has received little attention. This is unfortunate since binding of CORT to GRs is the principal mechanism for activation of GRs. A number of previous studies have shown that enhanced CORT synthesis during fear learning strengthens fear memory consolidation [for review see 7, 8]. Given that SPS enhances GR expression, CORT synthesis during cued fear memory formation could lead to enhanced GR activation and strengthened cued fear memory. In turn, this could lead to cued fear extinction retention deficits in SPS-exposed rats. We tested the hypothesis that CORT synthesis during fear memory formation leads to extinction retention deficits in the SPS model by inhibiting CORT synthesis in SPS and control rats during cued fear memory formation. This was accomplished by systemically administering the CORT synthesis inhibitor metyrapone 90 minutes prior to fear conditioning. We predicted that inhibiting CORT synthesis during cued fear memory formation would weaken fear memory and attenuate SPS-induced extinction retention deficits. The general experimental design is illustrated in Figure 1A.
Figure 1.
Subcutaneous metyrapone administration prior to fear conditioning exacerbates extinction memory deficits in SPS rats. All the data for a behavioral session are represented in a single graph in the top of a panel, then broken down separately for SPS and control rats in the bottom of a respective panel. A) Experimental design implemented in this study. B) SPS enhanced acquisition of cued fear memory. C) SPS disrupted acquisition of cued extinction memory. D) Deficits in acquisition of cued extinction memory in SPS rats persisted into the extinction test. Enhanced baseline freezing during the extinction test was observed in SPS rats. Furthermore, SPS-induced cued extinction memory deficits were exacerbated in the SPS/25mg/kg metyrapone group. * - Denotes a statistically significant comparison.
Subjects were 63 male Sprague-Dawley rats obtained from Charles River (Portage, MI). Rats (postnatal day 42–45) were housed in pairs until separated after the SPS or control procedures. Animals were given two days to acclimate to the housing colony while allowed ad libitum access to food and water. After this, they were fed 23g/day of standard rat chow per the manufacturer’s recommendation and allowed ad libitum access to water. Experimental manipulations commenced after rats had been in the housing colony for greater than five days. Rats were on a 12 hour light/dark cycle. All experimental procedures were performed in the animals’ light cycle and all behavioral tests were conducted between 9:00 am and 12:00 pm. All experiments were approved by the University of Delaware Institutional Animal Care and Use Committee following guidelines established by the NIH.
SPS and control stress procedures were conducted as previously described [9]. Briefly, SPS consisted of 120 minutes of restraint, 20 minutes of forced swimming, and ether exposure until general anesthesia was induced. Control rats were left in a novel room in their home cages for the duration of the SPS procedure. Following the SPS or control procedure, rats were allowed a post-stress incubation period of seven days, during which they were left undisturbed. This post-stress incubation period is necessary to observe SPS-induced effects [2, 9].
All rats underwent fear conditioning in standard fear conditioning chambers as previously described [2, 3]. Fear conditioning was always conducted in a distinct context (Context A) and consisted of five presentations of a tone conditioned stimulus (CS, 2kHz, 80dB, 10s) that coterminated with a footshock unconditioned stimulus (UCS, 1s, 1mA). Extinction training consisted of 30 CS-only presentations and was always conducted 24 hours after fear conditioning and in another distinct context (Context B). Extinction retention testing consisted of 10 CS-only presentations and was always conducted 24 hours after extinction training in the extinction context (i.e. Context B). The context shifts were employed to examine cued fear and extinction memory processes, while reducing the impact of contextual fear conditioning [10]. Also, by measuring baseline freezing at the start of the extinction training and testing sessions, contextual fear memory discrimination can be examined. All behavioral sessions began with a 210s baseline period and had inter-stimulus intervals (ISIs) of 60s.
Rats were randomly assigned to drug treatment groups. Either vehicle comprised of 60% sterile physiological saline and 40% Polyethylene Glycol (Fisher Scientific) (SPS = 10, control = 12), 25 mg/kg of metyrapone dissolved in vehicle (SPS = 11, control = 12), or 50mg/kg of metyrapone dissolved in vehicle (SPS = 11, control = 12) were administered subcutaneously to rats 90 minutes prior to fear conditioning. Metyrapone inhibits CORT synthesis, and thus prevents stress-induced increases in CORT levels without affecting basal CORT levels [11, 12]. The doses of drug selected for this study were based on previous studies [11–13]. At the doses stated above, metyrapone administration has no effects on basal adrenocorticotropin releasing hormone levels or glucose metabolism [12]. In other words, these doses selectively attenuate evoked CORT synthesis without having unwanted non-specific effects. Metyrapone was purchased from Sigma-Aldrich Inc. After fear conditioning, extinction training, and extinction testing were conducted as previously described.
Freezing was scored with Any-maze software (Stoelting Inc.) as previously described [3]. Freezing during the CS presentation and the following ISI were blocked into one trial and converted into percentages for statistical analyses. For the extinction training sessions, freezing during trials were blocked into two-trial blocks. Cued freezing during fear conditioning, extinction training, and extinction testing was separately analyzed using a stress (SPS vs. control) x drug (vehicle, 25mg/kg, 50mg/kg) x trial or block (baseline, 1-n) mixed factor design. Main and simple effects were analyzed using analysis of variance (ANOVA) while main and simple comparisons were analyzed using t-test with Bonferroni corrections applied where necessary. P < .05 was set as the threshold to define statistical significance.
ANOVA of cued freezing during fear conditioning revealed a main effect of trial (F(5,260) = 215.517, p < .001) which suggested all rats acquired cued fear memory. SPS rats froze more during the CS presentations of the fear conditioning session, and this effect was most pronounced towards the end of the fear conditioning session (see Figure 1B). This finding was revealed by a significant main effect of stress (F(1,52) = 5.134, p = .028). There were no main or interaction effects of drug (analyses not shown). These findings suggest that acquisition of cued fear memory was enhanced in SPS rats.
ANOVA of cued freezing during extinction training yielded a significant main effect of blocked-trial (F(15,930) =46.431, p < .001) and a significant main effect of blocked-trial on the quadratic trend component (F(1,62) =24.645, p < .001). These findings suggest that all rats expressed cued fear memory and acquired cued extinction memory. There was a main effect of stress (F(1,62) = 4.992, p = .029), which suggested that cued freezing was higher in SPS rats when compared to control rats during the extinction training session. However, careful inspection of Figure 1C suggests that SPS enhancements in cued freezing during the extinction training session were not observed across the entire extinction training session. In order to better identify components of the extinction training session in which SPS enhanced cued freezing, we conducted further analyses. We subjected baseline freezing of the extinction training session to a t-test (SPS vs. control) to determine if SPS disrupted contextual fear memory discrimination. Baseline freezing between SPS and control rats was not statistically different (t(66) = .297, p = .767), which suggests SPS had no effects on contextual fear memory discrimination. Next, we subjected CS-induced freezing during the first two blocks of the extinction training session (i.e. four CS presentations) to a stress x trial factor design in order to determine if SPS enhanced cued fear memory retrieval. ANOVA of CS-induced freezing during the first two blocks of the extinction training session did not reveal any main or interaction effects of stress (analyses not shown), though there was a main effect of trial (F(1,62) = 64.243, p < .001), which reflected an increase in cued freezing from block 1 to block 2. Overall, this analysis suggests that cued fear memory retrieval was equivalent in SPS and control rats. Lastly, we subjected cued freezing during the last block of the extinction training session (i.e. last two CS presentations) to a t-test (SPS vs. control). This comparison was statistically significant (t(66) = 2.354, p = .022), which suggest SPS disrupted acquisition of cued fear extinction memory (see Figure 1C).
ANOVA of cued freezing during the extinction test revealed a main effect of stress (F(1,52) = 5.152, p = .027) and a stress x drug x trial interaction on the linear trend component (F(2,62) = 3.165, p= .049). These analyses suggest that SPS enhanced cued freezing during the extinction test and that metyrapone administration prior to fear conditioning had differential effects on cued freezing in SPS and control rats. To explore these differences further, we first subjected baseline freezing to a stress x drug factor design. ANOVA revealed a significant main effect of stress (F(1,62) = 4.422, p= .04), but no main or interaction effects of drug (analyses not shown). This analysis suggests that SPS rats developed contextual fear conditioning to the extinction context even though this context was never paired with footshocks (i.e. second order contextual fear conditioning). Next, we subjected CS-induced freezing during the first four CS presentations of the extinction test to a stress x drug x trial factor design. ANOVA revealed a main effect of stress (F(1,62) = 8.239, p = .006), but no main or interaction effects of drug (analyses not shown). This analysis suggests that deficits in acquisition of cued fear extinction memory persisted into the extinction test. Lastly, we subjected cued freezing during the last trial of the extinction test to a stress x drug factor design. There was a significant stress x drug interaction (F(2,62) = 3.215, p = .047). Furthermore, cued freezing in the SPS/25mg/kg group was enhanced when compared to the SPS/vehicle group (t(19) = 2.848, p = .02). Indeed, cued freezing in the SPS/25mg/kg group was higher than all other groups in the last trial of the extinction test. This drug effect was limited to SPS rats since cued freezing in the control/25mg/kg group was not significantly different from the control/vehicle group (t(12) = 1.12, p = .55), though cued freezing during the last trial was attenuated in the control/25mg/kg group relative to the control/vehicle group. The 50mg/kg dose of metyrapone had no effect in SPS or control rats (analyses not shown). These analyses suggest that cued fear extinction memory deficits induced by SPS were exacerbated in SPS rats that received 25mg/kg of metyrapone prior to fear conditioning (see Figure 1D).
Metyrapone administration prior to fear conditioning consistently decreased conditioned freezing during extinction training and testing in control rats. Even though this effect never reached statistical significance, the action of metyrapone in controls rats is consistent with previous studies that have demonstrated inhibiting CORT synthesis during fear learning tends to disrupt memory consolidation [7, 11, 13]. In this study, inhibiting CORT synthesis during fear memory formation in control rats may have led to a weaker fear memory and lower levels of cued freezing during extinction training and testing. In contrast to our hypothesis, systemic administration of 25mg/kg of metyrapone prior to cued fear conditioning exacerbated cued fear extinction memory deficits in SPS rats by disrupting cued fear extinction retention in these rats. This finding suggests that inhibiting CORT synthesis during fear memory formation leads to exacerbated cued fear extinction memory deficits in SPS-exposed rats.
Inhibiting CORT synthesis during fear memory formation did not enhance acquisition of cued fear memory or cued fear memory retrieval in SPS rats (see Figure 1). However, inhibiting CORT during cued fear memory formation could still exacerbate cued fear extinction memory deficits in SPS rats by enhancing fear memory resistance in these rats, because the neurobiological processes that facilitate fear memory resistance (i.e. resistance of the fear memory to the inhibitory extinction memory) can be different from neurobiological processes that mediate fear memory expression [14, 15]. Another possibility is that inhibiting CORT synthesis during cued fear memory formation in SPS rats disrupted neural activity in extinction circuits and rendered these circuits less effective at mediating extinction memory. To determine the physiological mechanisms via which inhibition of CORT synthesis during fear memory formation exacerbates cued fear extinction memory deficits in SPS rats, further research is needed.
The results suggest that CORT synthesis during fear memory formation leads to enhanced extinction retention in SPS, but not control, rats. Because enhanced extinction memory is considered adaptive [16], the results of this study are the first to raise the possibility that a prior acute stress episode (i.e. SPS) in adult animals can render CORT synthesis during fear memory formation adaptive. A previous study has shown that increasing CORT levels prior to acute stress can ameliorate the effects acute stress has on expression of anxiety-like behavior [17]. Thus, it would appear that CORT synthesis in previously stressed animals or prior to acute stress can be adaptive and/or beneficial. However, it must be noted that previous clinical and basic science studies have demonstrated that stress-induced CORT synthesis and stress-induced changes in GR function mediate pathology and/or confer susceptibility [5, 18–21]. Thus, further research is needed to understand when stress-induced CORT synthesis can lead to adaptive vs. maladaptive outcomes.
Unlike the 25mg/kg dose of metyrapone, the 50mg/kg dose of metyrapone had no effects on SPS-induced extinction memory deficits. This could be due to a number of reasons. Administration of 50mg/kg of metyrapone does not induce non-specific effects under basal conditions [12]. It could be that with the added stress of fear conditioning, administration of 50mg/kg of metyrapone produced non-specific effects (e.g. enhanced glucose metabolism). Alternatively, the results from a previous study suggests that systemic administration of 25mg/kg of metyrapone may specifically inhibit GR-CORT binding, while 50mg/kg of metyrapone may inhibit both GR-CORT binding and mineralcorticoid receptor (MR)-CORT binding [11]. For this reason as well, the 50mg/kg dose of metyrapone may not have selectively inhibited GR-CORT binding during fear memory formation.
We demonstrated that inhibiting CORT synthesis during cued fear memory formation disrupts extinction retention in SPS rats. Metyrapone administration does not alter basal CORT levels [11], and at basal conditions most MRs are occupied [11, 22, 23]. Thus, it is likely that administering 25mg/kg of metyrapone prior to cued fear conditioning inhibited GR-CORT binding during cued fear memory formation. This suggests that inhibiting GR-CORT binding during fear conditioning disrupts extinction retention in SPS rats, but not control rats. Because previous studies have shown that SPS alters GR expression in the prefrontal cortex and hippocampus [3], but has no effects on acute stress-induced CORT synthesis [9], the results of this study point to SPS-induced changes in GR function as being adaptive in the SPS model. Further research examining this hypothesis is needed. SPS exposure resulted in second order contextual fear conditioning. The relevance of this effect to extinction memory deficits in the SPS model also deserves further investigation.
HIGHLIGHTS.
SPS may induce extinction deficits by enhancing fear memory
CORT synthesis is critical for fear memory consolidation
CORT synthesis during fear conditioning may be critical for SPS extinction deficits
Inhibiting CORT synthesis during fear conditioning exacerbates SPS extinction deficits
CORT synthesis during fear conditioning may be beneficial in SPS rats
Acknowledgments
This work was supported by a University of Delaware Research Foundation award. We would like to thank Jordan Abrams and Kevin Savage for their contributions to this study.
Footnotes
ANOVA – Analysis of variance
CORT - corticosterone
CS – conditioned stimulus
GR – glucocorticoid receptor
ISI – interstimulus interval
PTSD – post traumatic stress disorder
SPS – single prolonged stress
UCS – Unconditioned stimulus
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Armario A, Escorihuela RM, Nadal R. Long-term neuroendocrine and behavioural effects of a single exposure to stress in adult animals. Neurosci Biobehav Rev. 2008;32:1121–35. doi: 10.1016/j.neubiorev.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Knox D, George SA, Fitzpatrick CJ, Rabinak CA, Maren S, Liberzon I. Single prolonged stress disrupts retention of extinguished fear in rats. Learn Mem. 2012;19:43–9. doi: 10.1101/lm.024356.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knox D, Nault T, Henderson C, Liberzon I. Glucocorticoid Receptors And Extinction Retention Deficits In The Single Prolonged Stress Model. Neuroscience. 2012;223:163–73. doi: 10.1016/j.neuroscience.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 4.George SA, Rodriguez-Santiago M, Riley J, Rodriguez E, Liberzon I. The effect of chronic phenytoin administration on single prolonged stress induced extinction retention deficits and glucocorticoid upregulation in the rat medial prefrontal cortex. Psychopharmacology (Berl) 2014 doi: 10.1007/s00213-014-3635-x. [DOI] [PubMed] [Google Scholar]
- 5.van Zuiden M, Geuze E, Willemen HL, Vermetten E, Maas M, Heijnen CJ, et al. Pre-existing high glucocorticoid receptor number predicting development of posttraumatic stress symptoms after military deployment. Am J Psychiatry. 2011;168:89–96. doi: 10.1176/appi.ajp.2010.10050706. [DOI] [PubMed] [Google Scholar]
- 6.Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res. 2008;42:515–20. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 8.Roozendaal B, Nguyen BT, Power AE, McGaugh JL. Basolateral amygdala noradrenergic influence enables enhancement of memory consolidation induced by hippocampal glucocorticoid receptor activation. Proc Natl Acad Sci U S A. 1999;96:11642–7. doi: 10.1073/pnas.96.20.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liberzon I, Krstov M, Young EA. Stress-restress: effects on ACTH and fast feedback. Psychoneuroendocrinology. 1997;22:443–53. doi: 10.1016/s0306-4530(97)00044-9. [DOI] [PubMed] [Google Scholar]
- 10.Chang CH, Knapska E, Orsini CA, Rabinak CA, Zimmerman JM, Maren S. Fear extinction in rodents. Curr Protoc Neurosci. 2009;Chapter 8(Unit8):23. doi: 10.1002/0471142301.ns0823s47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roozendaal B, Bohus B, McGaugh JL. Dose-dependent suppression of adrenocortical activity with metyrapone: effects on emotion and memory. Psychoneuroendocrinology. 1996;21:681–93. doi: 10.1016/s0306-4530(96)00028-5. [DOI] [PubMed] [Google Scholar]
- 12.Rotllant D, Ons S, Carrasco J, Armario A. Evidence that metyrapone can act as a stressor: effect on pituitary-adrenal hormones, plasma glucose and brain c-fos induction. Eur J Neurosci. 2002;16:693–700. doi: 10.1046/j.1460-9568.2002.02120.x. [DOI] [PubMed] [Google Scholar]
- 13.Barrett D, Gonzalez-Lima F. Behavioral effects of metyrapone on Pavlovian extinction. Neurosci Lett. 2004;371:91–6. doi: 10.1016/j.neulet.2004.08.046. [DOI] [PubMed] [Google Scholar]
- 14.Gogolla N, Caroni P, Luthi A, Herry C. Perineuronal nets protect fear memories from erasure. Science. 2009;325:1258–61. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- 15.Kim JH, Richardson R. A developmental dissociation of context and GABA effects on extinguished fear in rats. Behav Neurosci. 2007;121:131–9. doi: 10.1037/0735-7044.121.1.131. [DOI] [PubMed] [Google Scholar]
- 16.Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–43. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Rao RP, Anilkumar S, McEwen BS, Chattarji S. Glucocorticoids protect against the delayed behavioral and cellular effects of acute stress on the amygdala. Biol Psychiatry. 2012;72:466–75. doi: 10.1016/j.biopsych.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–77. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 19.Mizoguchi K, Ishige A, Aburada M, Tabira T. Chronic stress attenuates glucocorticoid negative feedback: involvement of the prefrontal cortex and hippocampus. Neuroscience. 2003;119:887–97. doi: 10.1016/s0306-4522(03)00105-2. [DOI] [PubMed] [Google Scholar]
- 20.Wilber AA, Southwood CJ, Wellman CL. Brief neonatal maternal separation alters extinction of conditioned fear and corticolimbic glucocorticoid and NMDA receptor expression in adult rats. Dev Neurobiol. 2009;69:73–87. doi: 10.1002/dneu.20691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ. Cortisol regulation in posttraumatic stress disorder and major depression: a chronobiological analysis. Biol Psychiatry. 1996;40:79–88. doi: 10.1016/0006-3223(95)00451-3. [DOI] [PubMed] [Google Scholar]
- 22.Lowy MT. Quantification of type I and II adrenal steroid receptors in neuronal, lymphoid and pituitary tissues. Brain Res. 1989;503:191–7. doi: 10.1016/0006-8993(89)91663-6. [DOI] [PubMed] [Google Scholar]
- 23.Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–11. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]