Abstract
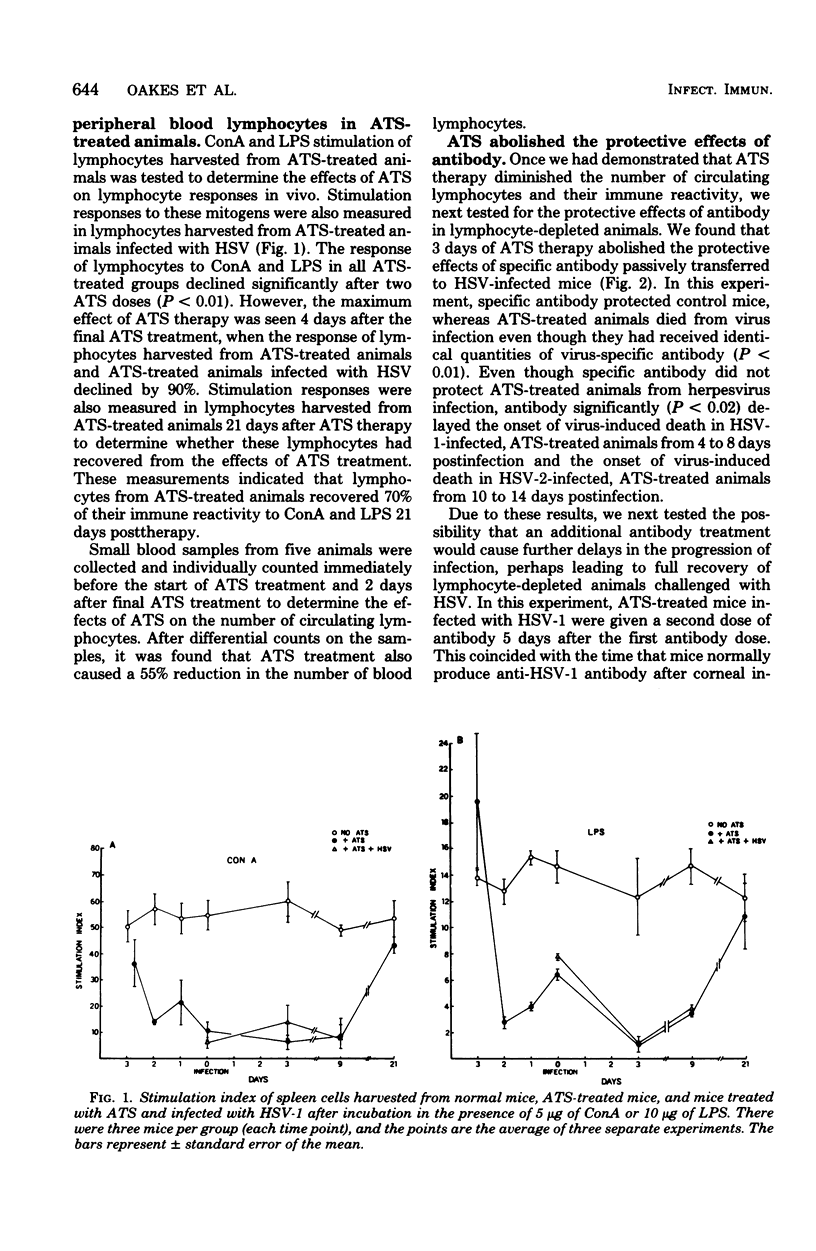
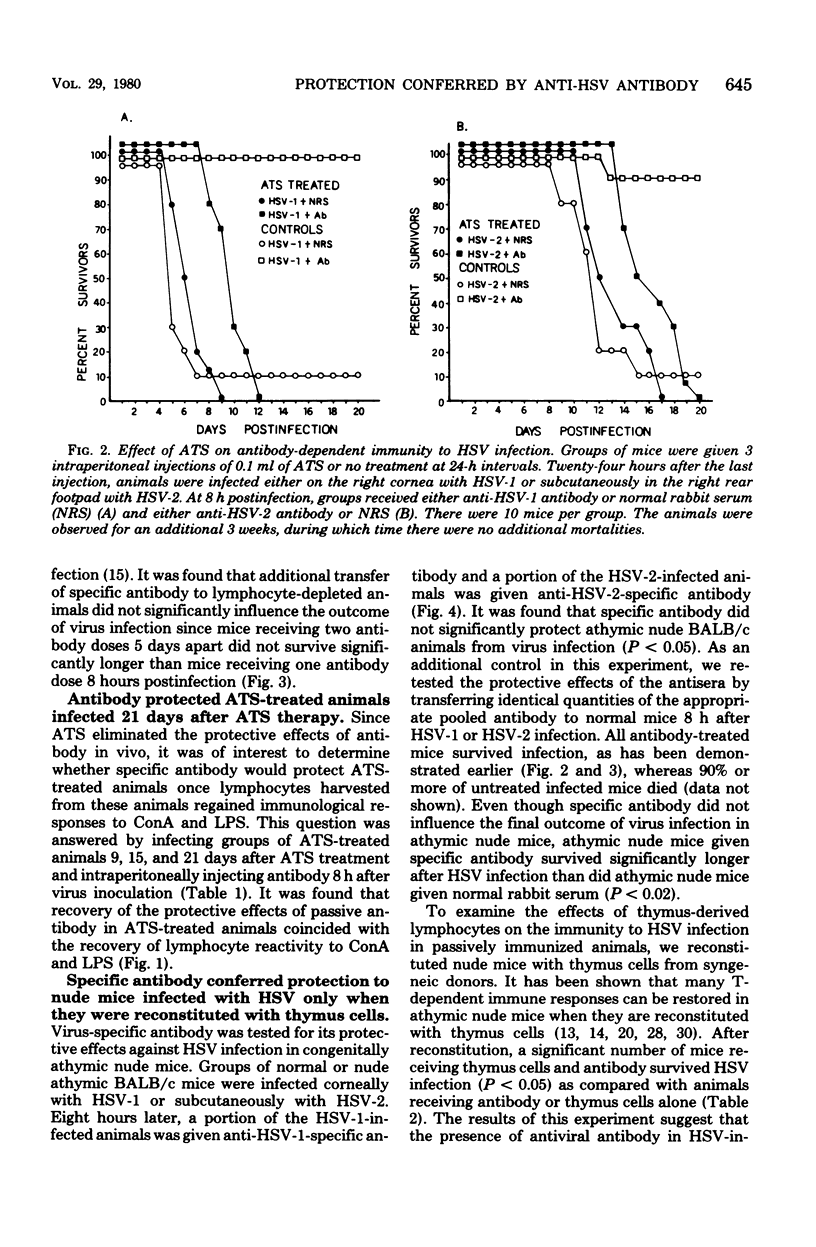
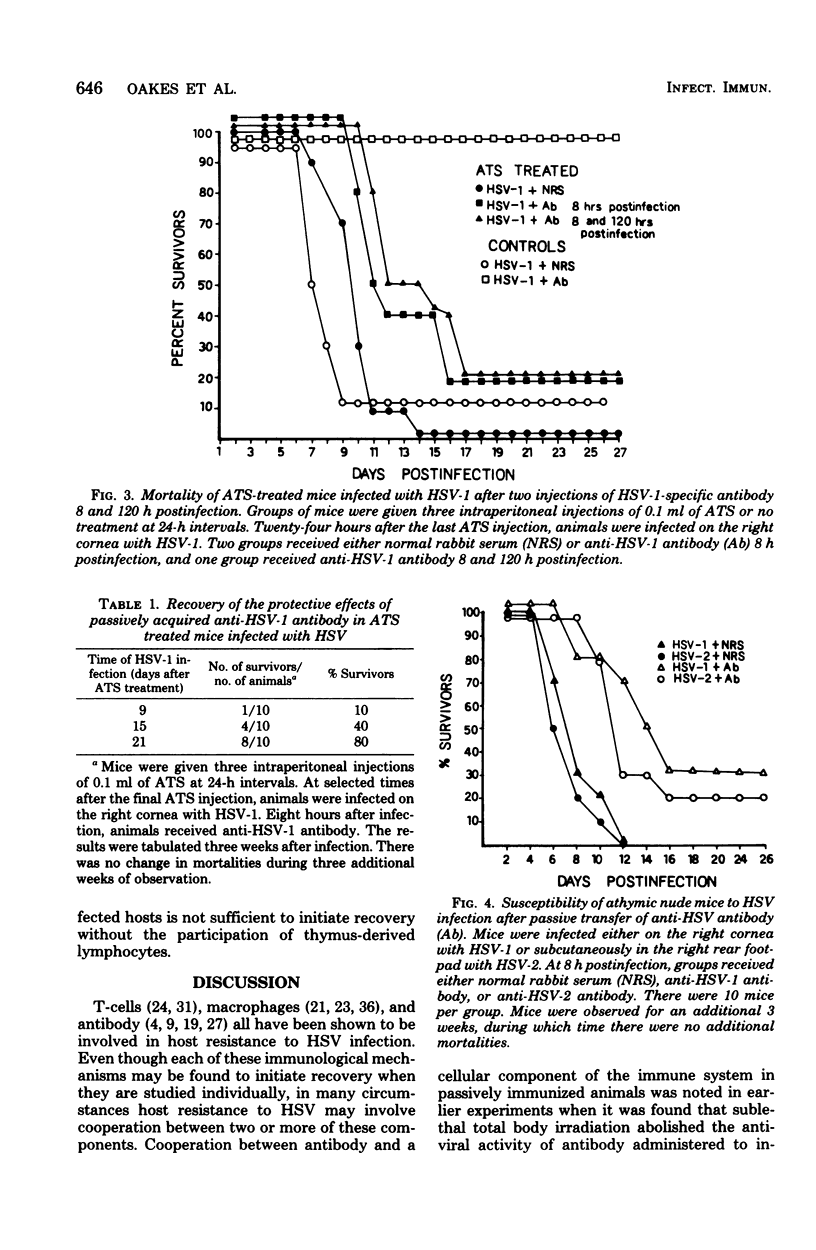
Passively acquired immunity to herpes simplex virus (HSV) was studied in antithymocyte serum (ATS)-treated mice and athymic nude mice to determine whether immunocompetent lymphocytes contribute to the protection observed after transfer of HSV-specific antibody to infected animals. Mice were given three intraperitoneal injections of 0.1 ml of ATS at 24-h intervals. This treatment reduced concanavalin A and lipopolysaccharide stimulation of lymphocytes harvested from these animals by 90% when compared with the stimulation of lymphocytes harvested from untreated animals. It was found that intraperitoneal injection of 0.5 ml of specific antibody 8 h after corneal HSV type 1 infection or subcutaneous HSV type 2 infection did not protect ATS-treated animals from virus infection. Specific antibody passively transferred to ATS-treated animals 8 and 120 h postinfection also failed to protect lymphocyte-depleted animals from HSV. However, ATS-treated animals were protected from HSV infection by passively acquired antibody when lymphocytes harvested from these animals regained 80% of their ability to be stimulated with concanavalin A and lipopolysaccharide. It was also found that specific antibody conferred protection to nude mice infected with HSV only if they were first reconstituted with syngeneic thymus cells 48 h before infection. The results suggest that both antiviral antibody and thymus-derived lymphocytes contribute to the recovery of HSV-infected hosts after passive immunization.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. E., Warner N. L. Ionizing radiation and the immune response. Adv Immunol. 1976;24:215–335. doi: 10.1016/s0065-2776(08)60331-4. [DOI] [PubMed] [Google Scholar]
- Andersson J., Möller G., Sjöberg O. Selective induction of DNA synthesis in T and B lymphocytes. Cell Immunol. 1972 Aug;4(4):381–393. doi: 10.1016/0008-8749(72)90040-8. [DOI] [PubMed] [Google Scholar]
- Baron S., Worthington M. G., Williams J., Gaines J. W. Postexposure serum prophylaxis of neonatal herpes simplex virus infection of mice. Nature. 1976 Jun 10;261(5560):505–506. doi: 10.1038/261505a0. [DOI] [PubMed] [Google Scholar]
- Blanden R. V. Mechanisms of recovery from a generalized viral infection: mousepox. I. The effects of anti-thymocyte serum. J Exp Med. 1970 Nov;132(5):1035–1054. doi: 10.1084/jem.132.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brier A. M., Wohlenberg C., Rosenthal J., Mage M., Notkins A. L. Inhibition or enhancement of immunological injury of virus-infected cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3073–3077. doi: 10.1073/pnas.68.12.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centifanto Y. M., Zam Z. S., Kaufman H. E. In vitro studies on the mechanism of herpesvirus plaque growth inhibition by sensitized lymphocytes. Infect Immun. 1977 Aug;17(2):350–355. doi: 10.1128/iai.17.2.350-355.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chare M. J., Boak J. L. Effect of antilymphocyte serum on macrophage activity. Clin Exp Immunol. 1970 May;6(5):655–659. [PMC free article] [PubMed] [Google Scholar]
- Davis W. B., Taylor J. A., Oakes J. E. Ocular infection with herpes simplex virus type 1: prevention of acute herpetic encephalitis by systemic administration of virus-specific antibody. J Infect Dis. 1979 Oct;140(4):534–540. doi: 10.1093/infdis/140.4.534. [DOI] [PubMed] [Google Scholar]
- Ennis F. A. Host defense mechanisms against Herpes simplex virus. I. Control of infection in vitro by senstized spleen cells and antibody. Infect Immun. 1973 Jun;7(6):898–904. doi: 10.1128/iai.7.6.898-904.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis F. A. Host defense mechanisms against herpes simplex virus. II. Protection conferred by sensitized spleen cells. J Infect Dis. 1973 Jun;127(6):632–638. doi: 10.1093/infdis/127.6.632. [DOI] [PubMed] [Google Scholar]
- Hampar B., Notkins A. L., Mage M., Keehn M. A. Heterogeneity in the properties of 7 S and 19S rabbit-neutralizing antibodies to herpes simplex virus. J Immunol. 1968 Mar;100(3):586–593. [PubMed] [Google Scholar]
- Kindred B. Antibody response in genetically thymus-less nude mice injected with normal thymus cells. J Immunol. 1971 Nov;107(5):1291–1295. [PubMed] [Google Scholar]
- Kindred B. The inception of the response to SRBC by nude mice injected with various doses of congenic or allogeneic thymus cells. Cell Immunol. 1975 May;17(1):277–284. doi: 10.1016/s0008-8749(75)80027-x. [DOI] [PubMed] [Google Scholar]
- Knotts F. B., Cook M. L., Stevens J. G. Pathogenesis of herpetic encephalitis in mice after ophthalmic inoculation. J Infect Dis. 1974 Jul;130(1):16–27. doi: 10.1093/infdis/130.1.16. [DOI] [PubMed] [Google Scholar]
- Kohl S., Cahall D. L., Walters D. L., Schaffner V. E. Murine antibody-dependent cellular cytotoxicity to herpes simplex virus-infected target cells. J Immunol. 1979 Jul;123(1):25–30. [PubMed] [Google Scholar]
- Liddell F. D. Evaluation of survival in challenge experiments. Microbiol Rev. 1978 Mar;42(1):237–249. doi: 10.1128/mr.42.1.237-249.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyet F., Samra D., Soneji A., Marks M. I. Passive immunization in experimental Herpesvirus hominis infection of newborn mice. Infect Immun. 1975 Dec;12(6):1258–1261. doi: 10.1128/iai.12.6.1258-1261.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKendall R. R., Klassen T., Baringer J. R. Host defenses in herpes simplex infections of the nervous system: effect of antibody on disease and viral spread. Infect Immun. 1979 Feb;23(2):305–311. doi: 10.1128/iai.23.2.305-311.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Vadas M. A., Whitelaw A., Gamble J. H-2 gene complex restricts transfer of delayed-type hypersensitivity in mice. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5095–5098. doi: 10.1073/pnas.72.12.5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen S. C., Andersen H. K. Role of activated macrophages in resistance of congenitally athymic nude mice to hepatitis induced by herpes simplex virus type 2. Infect Immun. 1978 Mar;19(3):792–798. doi: 10.1128/iai.19.3.792-798.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morahan P. S., Breinig M. C., McGeorge M. B. Immune responses to vaginal or systemic infection of BALB/c mice with herpes simplex virus type 2. J Immunol. 1977 Dec;119(6):2030–2036. [PubMed] [Google Scholar]
- Morahan P. S., Morse S. S., McGeorge M. G. Macrophage extrinsic antiviral activity during herpes simplex virus infection. J Gen Virol. 1980 Feb;46(2):291–300. doi: 10.1099/0022-1317-46-2-291. [DOI] [PubMed] [Google Scholar]
- Nagafuchi S., Oda H., Mori R., Taniguchi T. Mechanism of acquired resistance to herpes simplex virus infection as studied in nude mice. J Gen Virol. 1979 Sep;44(3):715–723. doi: 10.1099/0022-1317-44-3-715. [DOI] [PubMed] [Google Scholar]
- Oakes J. E. Invasion of the central nervous system by herpes simplex virus type 1 after subcutaneous inoculation of immunosuppressed mice. J Infect Dis. 1975 Jan;131(1):51–57. doi: 10.1093/infdis/131.1.51. [DOI] [PubMed] [Google Scholar]
- Oakes J. E. Role for cell-mediated immunity in the resistance of mice to subcutaneous herpes simplex virus infection. Infect Immun. 1975 Jul;12(1):166–172. doi: 10.1128/iai.12.1.166-172.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes J. E., Rosemond-Hornbeak H. Antibody-mediated recovery from subcutaneous herpes simplex virus type 2 infection. Infect Immun. 1978 Aug;21(2):489–495. doi: 10.1128/iai.21.2.489-495.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantelouris E. M. Observations on the immunobiology of 'nude' mice. Immunology. 1971 Feb;20(2):247–252. [PMC free article] [PubMed] [Google Scholar]
- Pavan P. R., Ennis F. A. The elimination of herpes simplex plaques by antibody and the emergence of resistant strains. J Immunol. 1977 Jun;118(6):2167–2175. [PubMed] [Google Scholar]
- Pritchard H., Micklem H. S. Immune responses in congenitally thymus-less mice. I. Absence of response to oxazolone. Clin Exp Immunol. 1972 Jan;10(1):151–161. [PMC free article] [PubMed] [Google Scholar]
- Rager-Zisman B., Allison A. C. Mechanism of immunologic resistance to herpes simplex virus 1 (HSV-1) infection. J Immunol. 1976 Jan;116(1):35–40. [PubMed] [Google Scholar]
- Rasmussen L., Merigan T. C. Role of T lymphocytes in cellular immune responses during herpes simplex virus infection in humans. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3957–3961. doi: 10.1073/pnas.75.8.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg G. L., Notkins A. L. Induction of cellular immunity to herpes simplex virus: relationship to the humoral immune response. J Immunol. 1974 Mar;112(3):1019–1025. [PubMed] [Google Scholar]
- Schlabach A. J., Martinez D., Field A. K., Tytell A. A. Resistance of C58 mice to primary systemic herpes simplex virus infection: macrophage dependence and T-cell independence. Infect Immun. 1979 Nov;26(2):615–620. doi: 10.1128/iai.26.2.615-620.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore S. L., Black C. M., Melewicz F. M., Wood P. A., Nahmias A. J. Antibody-dependent cell-mediated cytotoxicity to target cells infected with type 1 and type 2 herpes simplex virus. J Immunol. 1976 Jan;116(1):194–201. [PubMed] [Google Scholar]
- Shore S. L., Nahmias A. J., Starr S. E., Wood P. A., McFarlin D. E. Detection of cell-dependent cytotoxic antibody to cells infected with herpes simplex virus. Nature. 1974 Sep 27;251(5473):350–352. doi: 10.1038/251350a0. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Restriction of herpes simplex virus by macrophages. An analysis of the cell-virus interaction. J Exp Med. 1971 Jan 1;133(1):19–38. doi: 10.1084/jem.133.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M., Jr Cytotoxic cells induced during lymphocytic choriomeningitis virus infection of mice. I. Characterization of natural killer cell induction. J Exp Med. 1978 Jul 1;148(1):163–181. doi: 10.1084/jem.148.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawatzky R., Hilfenhaus J., Krichner H. Resistance of nude mice to herpes simplex virus and correlation with in vitro production of interferon. Cell Immunol. 1979 Oct;47(2):424–428. doi: 10.1016/0008-8749(79)90352-6. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Blanden R. V. Macrophage activation in mice lacking thymus-derived (T) cells. Experientia. 1975 May 15;31(5):591–593. doi: 10.1007/BF01932477. [DOI] [PubMed] [Google Scholar]



