Abstract
That a minor injury can trigger a complex regional pain syndrome (CRPS) - multiple system dysfunction, severe and often chronic pain and disability - has fascinated scientists and perplexed clinicians for decades. However, substantial advances across several medical disciplines have recently increased our understanding of CRPS. Compelling evidence implicates biological pathways that underlie aberrant inflammation, vasomotor dysfunction, and maladaptive neuroplasticity in the clinical features of CRPS. Collectively, the evidence points to CRPS being a multifactorial disorder that is associated with an aberrant host response to tissue injury. Varying susceptibility to perturbed regulation of any of the underlying biological pathways probably accounts for the clinical heterogeneity of CRPS.
Introduction
Complex regional pain syndrome (CRPS) is characterized by pain, in combination with sensory, autonomic, trophic and motor abnormalities. A distinction is made between CRPS-1, in which a nerve lesion can not be identified, and CRPS-2, in which it can. This distinction is not without criticism however, because bone fracture or surgery will damage peripheral nerve fibres yet post-fracture and post-surgical CRPS are almost always called CRPS-1. Further, pathological studies on chronic CRPS-1 limbs that have been amputated, and skin biopsies of CRPS-1 limbs, clearly show degeneration of small (C and Aδ) nerve fibres,1–3 which serve nociceptive and autonomic functions. It remains to be determined if nerve degeneration is causally related to CRPS-1. Additionally, since other causes of neuropathic pain are frequently associated with a loss of C-fibre peripheral terminals,4 the specificity of these findings with respect to CRPS may be questioned.
The diagnosis of CRPS is based either on the Orlando criteria,5 endorsed by the International Association for the Study of Pain, or a modified version called the Budapest criteria (Fig. 16), which have higher specificity and also include the motor features of the syndrome. A recent international cohort study confirmed the validity of the Budapest criteria.6 The diagnosis according to the Budapest criteria is based on the grouping of signs and symptoms into four distinct categories, which were identified by factor analysis.7,8 A CRPS severity score to monitor longitudinal changes was subsequently developed.9
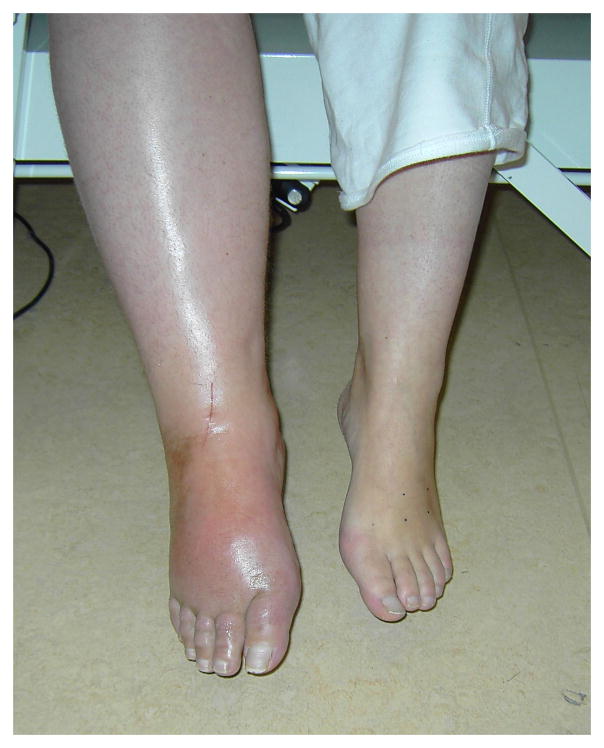
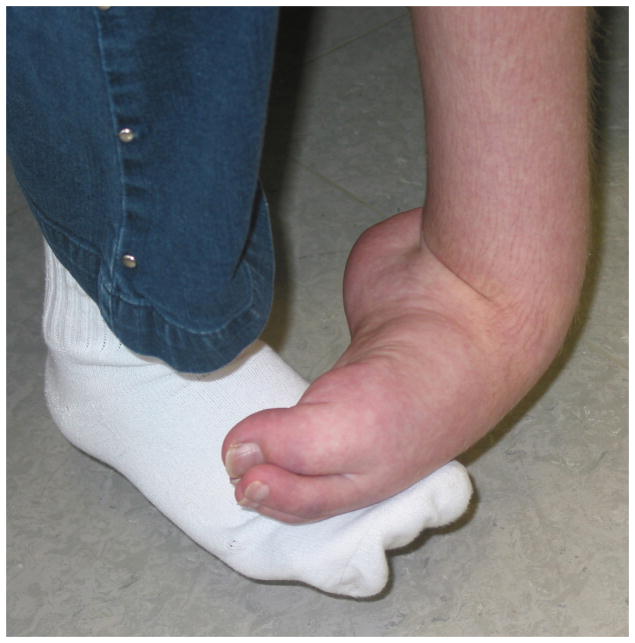
Figure 1. Photo’s: a) acute CRPS, b) chronic (cold) CRPS, c) CRPS dystonia.
Photographs of clinical CRPS cases. Panel (A): Acute CRPS with hyperemia, swelling and glossy skin. Panel (B): Chronic, cold type CRPS with blue discoloration of the fingers, glossy skin, and increased hair and nail growth. Panel (C): CRPS-related dystonia of the left ankle and foot with plantar flexion and inversion of the ankle, and flexion of the toes; edema and increased hair growth are also clearly visible.
Our understanding of CRPS has increased a great deal in the last decade. Three major pathophysiological pathways have been elucidated – aberrant inflammatory mechanisms, vasomotor dysfunction and maladaptive neuroplasticity. The clinical heterogeneity of this condition reflects between-individual variability in the activation of these pathways after tissue injury. Over the past 3–5 years significant and important developments have occurred in several fields and the inter-relationships between these developments are only now being identified. These developments are changing our understanding of CRPS rapidly and make it timely now to present a multidisciplinary overview that integrates these findings across all relevant fields and put them into perspective. Here we review the clinical and epidemiological features of CRPS and the role of inflammation, vasomotor dysfunction and maladaptive neuroplasticity in the development and persistence of this condition.
Search strategy and selection criteria
References for this review were identified from searches by the authors done over the past 30 years, as well as through exhaustive searches of PubMed by use of the search terms “complex regional pain syndrome”, “CRPS”, “reflex sympathetic dystrophy”, both alone and in combination with each of the following search terms: “epidemiology”, “incidence”, “risk factor”, “inflammation”, “sensory changes”, “cortical changes”, “sympathetic nervous system”, “sympathetically maintained pain”, “vasomotor control”, “vasomotor dysfunction”, “pain control”, “central sensitization”, “central sensitization”, “psychological factors”, “anxiety”, “depression”, “immobilization”, “immobilisation”, “genetic”, “endothelial dysfunction”, “neuroplasticity” and “dystonia”, from January 1980 until April 2011. Articles were also identified through searches of the references of articles and the authors’ own files. Only papers published in English, German and Dutch were reviewed. The final reference list was generated on the basis of relevance to the topics covered in this review.
Clinical presentation
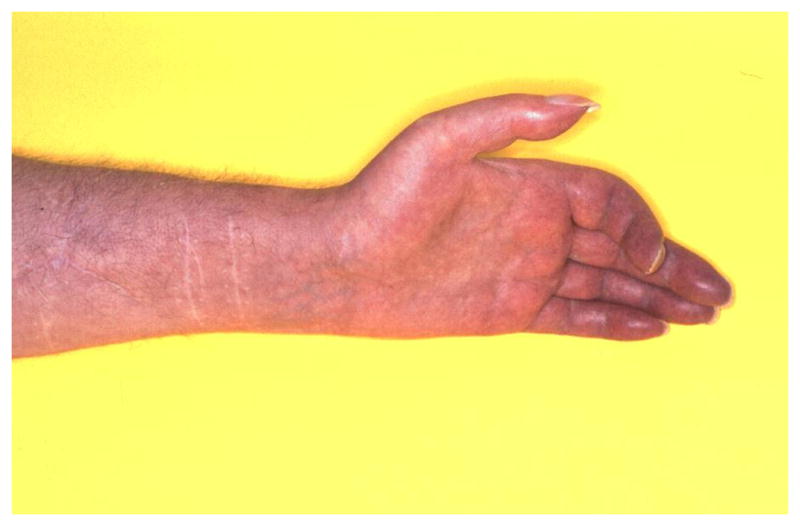
The typical CRPS patient presents after a minor or moderate tissue injury, for example a wrist fracture. In the acute phase, the injured limb is usually extremely painful, red, warm (although sometimes it quickly becomes relatively cold11) and swollen (Fig. 2A). Other features, also confined to the injured limb but not confined to the distribution of a specific nerve or nerve root, include allodynia (where usually non-painful stimuli evoke pain) and hyperalgesia (where painful stimuli evoke more intense pain than usual), changes in sweating, changes in hair and nail growth, and muscle weakness. Especially mechanical and thermal hyperalgesia are frequently present in CRPS.10,12,13 As the condition persists, pain does not subside but often spreads, voluntary motor control can reduce, hyperpathia may occur and negative sensory signs (hypoesthesia, hypoalgesia, hypothermesthesia)10,12,14 can develop. Thus, CRPS seems to be characterized by a mixture of noxious sensations (“positive symptoms”) and sensory loss (“negative symptoms”).12 Over months, the relatively warm limb often becomes relatively cold (Fig. 2B). Dystonia (Fig. 2C), tremor and myoclonus may also develop. Activity of the limb typically exacerbates signs and symptoms. Over time, clinical features spread proximally (but not distally15) and can even emerge on the opposite limb or the ipsilateral limb.16,17 In chronic cases with longer disease durations (>5 years) other features are sometimes noted, such as urological symptoms,18,19 syncope,20 and even mild cognitive deficits,21 although the latter probably is not specific for CRPS.
Figure 2. Fluorescence photomicrographs of NK1 receptor expression in keratinocytes in skin samples obtained from a CRPS patient.
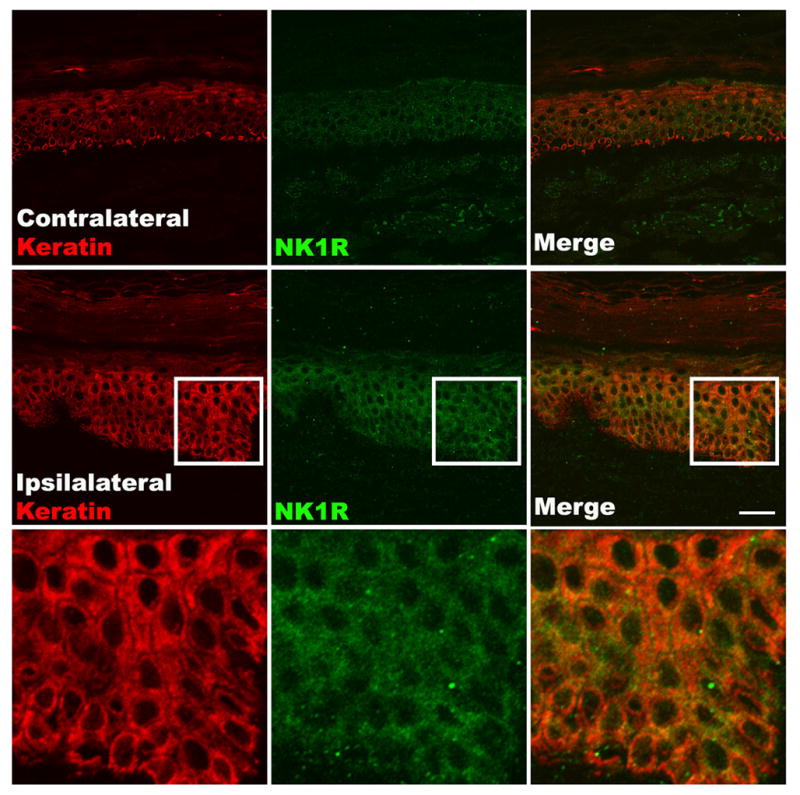
Representative fluorescence photomicrographs of NK1 receptor expression in keratinocytes in skin samples obtained from a CRPS patient at 3 months post-fracture. Co-immunostaining of NK1 (green) and keratin, (a keratinocyte marker, red) in the epidermis demonstrates greater NK1 receptor expression in keratinocytes in the affected limb than in the contralateral side. Also note the keratinocyte proliferation, epithelial thickening, and the increased thickness of the stratum corneum. Scale bar = 40 μm.
The feasibility to use the occurring sensory changes in the diagnosis of CRPS was recently evaluated. In a German study in which sensory function was quantitatively assessed in more than 1200 patients with various neuropathic pain syndromes - including 400 patients with CRPS - and where data were compared across diseases and with norm values, it was shown that CRPS patients display a mixture of gain and loss of sensory function.10 Gain in sensory function, particularly with respect to thermal and mechanical hyperalgesia, was observed more often in CRPS than in other neuropathic syndromes. However, a unique profile of change in sensory function could not be detected for any of the included neuropathic syndromes and the role of quantitative sensory testing in the diagnosis of CRPS therefore still needs further evaluation.
Epidemiology
The incidence of CRPS is unclear – the two best population-based studies yield very different data: 5.5 cases per 100,000 person years in the United States12 and 26.2 per 100,000 person years in The Netherlands.13 Based on these numbers one may expect that 20,000–80,000 new cases of CRPS are identified per year in the United States. The incidence increases with age until 70 and women are affected 3–4 times more often than men.22,23 The arm is affected in about 60% of cases and the leg in about 40% of cases.22 Resolution rate differs greatly between studies, ranging from 74% in the first year23 to 36% in 6 years.24 Resolution data are difficult to interpret though, because of heterogeneous study populations and inconsistency in diagnostic rigour and a lack of consensus on what defines recovery. Fractures (~45%), sprain (~18%) and elective surgery (~12%) are the most frequently reported triggering events.22 Spontaneous onsets, which present with a similar clinical picture,25 are uncommon (<10% of cases).11,22 CRPS is commonly associated with substantial disability, loss of quality of life and personal and societal economic burden.26,27
Risk factors and prognostic determinants
Nearly everyone experiences tissue trauma, yet few develop CRPS and the severity of trauma does not relate to the development of CRPS. Clearly then, some individuals are more susceptible than others. Identifying more proximal risk factors than injury alone may hint at underlying biological mechanisms and assist the development of preventive and treatment strategies. Epidemiological, genetic and experimental studies have attempted to identify factors that modulate the risk of developing and maintaining CRPS.
Psychological factors
The apparently unpredictable nature of CRPS underpins the popular presumption that a peculiar personality or psychological profile is a key risk factor. Most studies indeed provide compelling evidence that CRPS patients are more anxious and depressed than healthy controls. However, whether CRPS patients are more anxious or depressed than other chronic pain patients is unclear – available studies report contradictory results28 and cross-sectional studies tell us nothing about susceptibility to the condition. Only a few studies have attempted to obtain accurate information regarding the period before onset of CRPS.29–33 The two largest studies showed no evidence of psychological risk factors for CRPS onset. A large population-based case-control study33 found no difference in psychological variables between those who developed CRPS after trauma, and age, sex and trauma matched controls who did not.33 Another very recent study prospective multicenter cohort study including almost 600 consecutive patients with a single fracture showed that none of the psychological factors included in the Symptom Checklist-90 predicted the development of CRPS-1.32 Clearly, the popular presumption that anxiety and depression predispose to CRPS is incorrect. It remains to be determined whether other psychological factors contribute to the maintenance of CRPS - research into these topics is ongoing.
Immobilization
An injured limb is often immobilized, particularly if it is fractured, and early reports already indicated that immobilization may be a risk factor for CRPS.34 Several characteristic features of CRPS - changes in temperature, mechanosensitivity and thermosensitivity - can indeed be induced in a healthy limb by immobilisation alone.35 That is, healthy volunteers demonstrated mild signs of CRPS, although no pain, after four weeks limb immobilisation. Moreover, after topical application of capsaicin (which induces neurogenic inflammation – see below), mechanosensitivity, thermal sensitivity and perceptual disturbances are reported if the limb is subsequently immobilised for 24 hours, but they are not reported if the limb is not immobilised (Moseley et al UNDER REVIEW). The signs rapidly resolved once the limb was moved again. These experimental findings seem consistent with the idea that immobilisation is a risk factor for CRPS.
Epidemiological findings
A large population-based study identified the use of angiotensin-converting-enzyme (ACE) inhibitors at the time of trauma, and a history of migraine or asthma, to be associated with increased risk of developing CRPS.33,36 Migraine was also identified as risk factor for CRPS in another study.37 Both of these risk factors implicate inflammation - ACE inhibitors increase the availability of substance P (SP) and bradykinin, both important mediators of inflammation,36 and migraine and asthma share an underlying mechanism of neurogenic inflammation with CRPS (see below). Epidemiological studies have also identified several prognostic variables. Although it is the most common trigger of CRPS, fracture is associated with a more favourable course than soft tissue injury is.22,23 Gender does not seem to affect the prognosis in common types of CRPS.22,23 However, women have a higher risk of developing a more severe phenotype of CRPS - the female-to-male ratio is 5–6 to 1 in those with infections, ulcers, chronic oedema, or marked movement disorders, but 3–4 to 1 in the wider cohort.38,39 Finally, those who have ‘cold CRPS’, in which impaired thermoregulatory blood flow to the limb leaves it colder than its counterpart, have a worse prognosis than those with ‘warm CRPS’, in which excessive vasodilatation in the affected limb leaves it warmer than its counterpart.38
All these determinants have been identified on a group level but they are of limited use on an individual level; it is still unclear who will develop CRPS after trauma and who will not. In a recent longitudinal study, 1549 nearly consecutive patients presenting with wrist fracture and managed non-surgically were assessed within one week of their fracture and then followed up four months later. A simple assessment of pain intensity provided high discriminative ability (c index 0.98), such that average pain over the last two days of over 5/10 should be considered a “red flag” for CRPS (Moseley et al UNDER REVIEW).
Genetic findings
CRPS occurs in families and siblings of young onset cases have an increased risk of developing the syndrome,40–42 both of which implicate a potential genetic predisposition to CRPS. Consistent with this are associations between various CRPS phenotype characteristics and loci in the human leukocyte antigen region.43–48 Other genetic studies in CRPS involved polymorphisms in the ACE gene, although there are contrasting findings,42,49 and the tumour necrosis factor alpha (TNFα) gene.48 Although a picture of genetic predisposition to CRPS is emerging, it is important to note that associations have as yet not been replicated by independent studies and that small samples do not permit the exclusion of false positive findings. Nonetheless, the epidemiological and genetic findings to date consistently suggest that aspects of the individual’s response to tissue injury are important in the development of CRPS.
The role of inflammation
Human in-vivo experiments show that even minor tissue trauma is sufficient to amplify cytokine signalling in the traumatized tissue.50,51 Cytokines and nerve growth factor (NGF) can excite nociceptors and induce long-term peripheral sensitization.52,53 Moreover, in-vitro experiments suggest that cytokines54 and NGF55 enhance the release of inflammatory neuropeptides in primary afferent neurons. The activation of cutaneous nociceptors can induce retrograde depolarization of small-diameter primary afferents (“axon reflex”), causing the release of neuropeptides such as substance P (SP) and calcitonin-gene-related-peptide (CGRP) from sensory terminals in the skin.56 These neuropeptides evoke vasodilatation and protein extravasation in the tissue, and the resulting signs (reddening, warming, oedema) are labelled ‘neurogenic inflammation’.56
Most post-traumatic inflammatory changes observed in CRPS are mediated by two peptides – CGRP and SP. CGRP57 and SP58 serum levels are higher in CRPS patients than in healthy controls. Elevated CGRP release is probably responsible for the augmented flare response, as quantified by laser Doppler measurement of skin perfusion, in CRPS patients.59,60 Although C-fibres usually contain too little SP to generate electrically-evoked extravasation, significant protein extravasation is observed in the affected limb of patients with CRPS. Unlike the increased flare, which is observed experimentally on both unaffected and affected limbs in CRPS patients, increased protein extravasation is limited to the affected limb.61
The most likely mechanism underpinning facilitated neurogenic inflammation in CRPS is facilitated post-junction signalling, due to hampered inactivation of neuropeptides or increased receptor availability. When SP was perfused through dermal microdialysis fibres in CRPS patients and controls, it produced greater extravasation in both the affected and the unaffected limbs of CRPS patients than it did in controls.59 Increased SP signalling might also account for the increased cytokine expression seen in CRPS – SP stimulates keratinocytes to express cytokines in vitro62 and skin biopsies from CRPS affected limbs demonstrate increased SP receptor immunostaining in keratinocytes (Fig. 3).63 These findings support the hypothesis that facilitated cutaneous neuropeptide signalling contributes directly to the enhanced extravasation, limb oedema, and increased cytokine expression that are observed in CRPS. However, the details of how the immune and nervous system interact – particularly in the bones, the muscles and the connective tissue - are still not fully understood and warrant further research.
Figure 3. Cortical reorganization.128.
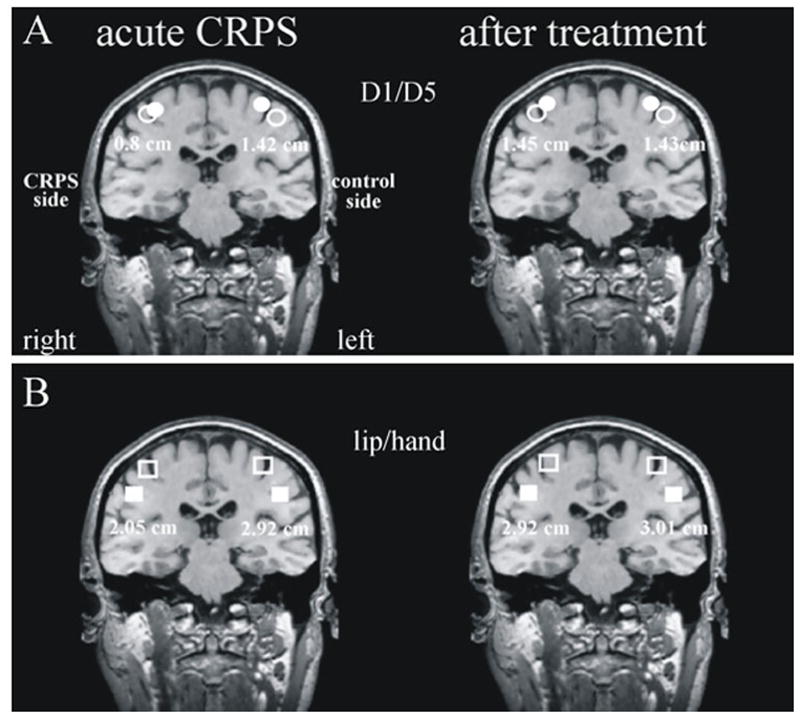
Cortical reorganization and maladaptive plasticity in patients with CRPS. Panel (A): The distance between the peak response over the contralateral somatosensory cortex to stimulation of the first digit (D1) and fifth digit (D5) on the affected and healthy hands in a patient with CRPS. Note that the distance between D1 and D5 is less for the affected limb. Panel (B): Normalisation of primary sensory cortex reorgnisation occurs in association with the resolution of CRPS symptoms. With friendly permission from Maihöfner et al.128
Levels of TNFα and interleukine 6 (IL-6) in blister fluid are greater on the CRPS-affected limb than the unaffected one64,65 and this difference only gradually resolves over six years post-injury.66 TNFα and IL-6 are also higher in skin biopsies from CRPS patients than they are in fracture patients who do not have CRPS.67 In serum, levels of soluble TNF receptors and the pro-inflammatory cytokines TNF, IL-1 and IL-8 are increased in early CRPS (average 3 months), whereas anti-inflammatory cytokines such as IL-4, IL-10, and TGFβ1 are decreased.58,68 These cytokine changes in blister fluid and serum are unrelated to clinical signs or to disease duration,68,69 but they do relate to the extent of mechanical hyperalgesia.70 Since mechanical hyperalgesia is a hallmark of central sensitization, it seems likely that inflammatory cytokines not only act locally in the limb but also in the spinal cord, probably by sensitization of secondary nociceptive neurons or by glial-neuronal interaction.71 Another very recent study supported the immune system involvement in CRPS. Findings in patients with longstanding CRPS showed that while the total monocyte count was not altered, the percentage of the pro-inflammatory CD14+CD16+ monocytes/macrophage subgroup was elevated as compared to age- and gender-matched healthy control individuals.72 Individuals with high percentage of CD14+CD16+ also demonstrated significantly lower plasma levels on the anti-inflammatory cytokine IL-10, supporting our previous finding.68 In view of the cross-sectional nature of those studies, it remains unclear if immune changes were already elevated prior to CRPS (in which case they could have facilitated onset of CRPS) or after developing CRPS (in which they may have played a role in the maintenance of the syndrome). The assumption that central sensitization by inflammatory cytokines might contribute to pain in CRPS, is supported by findings showing increased IL-1β and IL-6 cytokine levels in spinal fluid from chronic CRPS patients (average symptom duration 7–8 years),73,74 although these findings were not confirmed in another study that examined cytokines in spinal fluid of CRPS patients characterized by dystonia.75 indicating that further research in this field is required.
The aberrant inflammatory response to tissue injury observed in CRPS does not appear to be caused by a cellular mediated immune response. Sedimentation rates, antigen titres, autoimmune antibody levels, lymphocyte populations, activated T-cells, and blood cell counts are all normal. Moreover, histological studies demonstrate little or no inflammatory cell infiltrate in CRPS patients.11,58,76
Recent findings raise the possibility that autoimmune mechanisms might be involved in the pathophysiology of CRPS. About 35% of CRPS patients have surface-binding autoantibodies against sympathetic and mesenteric plexus neurons, and differentiated cholinergic type neuroblastoma cell lines.77,78 Recent results (Kohr et al. UNDER REVIEW) suggest that the antigens for these autoantibodies might be α-adrenoceptors and muscarinergic acetylcholine receptors. The significance of these findings is, however, unclear because the majority of patients lack clinical evidence of generalized autonomic failure that might be attributed to an autoimmune serum antibody involvement. Clearly further work is required.
The role of vasomotor dysfunction
Vasomotor dysfunction is common in CRPS.79 The affected limb is usually warmer than the healthy limb early-on, and colder than the healthy limb later on. This shift in relative temperature suggests that the activity in vasoconstrictor neurons changes over time in CRPS. This has been investigated experimentally using central sympathetic vasoconstrictor reflexes induced by whole-body warming and cooling, and by respiratory stimuli.80,81 Three distinct patterns were identified in people with CRPS: (i) In those with fewer than 6 months duration, the ‘warm’ type, the affected limb was warmer, and skin perfusion values were higher, than the contralateral limb. Even massive body cooling failed to activate sympathetic vasoconstrictor neurons.81 Consistently, norepinephrine levels from the venous effluent above the painful area were lower in the affected limb than in the contralateral one.81 (ii) An ‘intermediate type’ where temperature and perfusion were either warmer or colder, depending on the degree of sympathetic activity. (iii) In those with greater than 6 months duration, the ‘cold’ type, temperature and perfusion on the affected limb were consistently lower than those on the contralateral limb. Paradoxically though, norepinephrine levels were also lower on the affected side.80 These data suggest that, in addition to the inflammatory vasodilatation mentioned above, CRPS is associated with a unilateral inhibition of cutaneous sympathetic vasoconstrictor neurons, which leads to a warmer limb in the acute stage. It appears likely that this thermoregulatory impairment is caused by functional changes, triggered by the initial trauma, in the spinal cord, brainstem or brain. There are indications that the cutaneous sympathetic vasoconstrictor activity returns to normal as the condition persists,81 even though the limb becomes cold and bluish. Biopsies taken from people with chronic CRPS-1 suggest that this apparent paradox is most likely explained by increased density or responsiveness of α-adrenoceptors in the skin.82,83 Thus, although central disturbances in efferent sympathetic outflow seem to be predominant in the acute stage of CRPS, disturbed neurovascular transmission and development of hyper-reactivity of blood vessels to circulating catecholamines, seem to predominate in the chronic stage. However, not all CRPS cases pass through these different stages defined by skin temperature. About 20% of CRPS cases are cold right from the beginning. As it has been demonstrated recently, these patients not only differ in skin temperature but also in sensory symptoms and history.84
Apart from its effect on peripheral circulation in CRPS, the sympathetic nervous system may also contribute to pain. Clinical studies in CRPS patients support the idea that nociceptors develop catecholamine sensitivity,85,86 probably as a result of decreased activity of cutaneous sympathetic vasoconstrictor neurons. Norepinephrine released by the sympathetic nerve fibres may activate and/or sensitize the altered afferent neurons. This sympathetic-afferent coupling forms the theoretical basis for the clinical phenomenon of sympathetically maintained pain (SMP).87 In a study in CRPS-1 patients in which cutaneous sympathetic vasoconstrictor outflow to the painful extremity was activated to the highest possible physiological degree by whole body cooling,88 the intensity and area of spontaneous pain and mechanical hyperalgesia increased significantly in patients that had been classified as having SMP by positive sympathetic blocks, but not in patients with sympathetically independent pain. It is likely that, in addition to a coupling in the skin, a sympathetic-afferent interaction may also occur in tissues of the deep somatic domain such as bones, muscles or joints. Notably, however, one study suggests that, in some patients, arousal of sympathetic activity may still result in pain even after successful sympathetic blockade, which indicates that a central process - independent of the peripheral sympathetic nervous system - cannot be completely ruled out in all patients.89
Changes in the endothelium may also play a role in impaired peripheral circulation in chronic CRPS. Endothelial dysfunction is associated with a decreased ability to release endothelial nitric oxide, which leads to sustained vasoconstriction. Decreased levels of nitric oxide have been demonstrated in CRPS, both directly (impaired levels of nitric oxide in suction blister fluid of the affected as compared to the unaffected side in patients)65 and indirectly (by showing impaired acetylcholine-induced vasodilatation of the affected as compared to the unaffected side, and as compared to controls).90 However, it is unclear whether these changes are important in the development of CRPS or are a consequence of the trophic changes that affect the skin, muscles and bones in people with CRPS.
The role of the central nervous system
The central nervous system undergoes functional and structural changes in people with persistent pain and these changes are thought to be particularly important in CRPS.91 These persistent changes in the central nervous system lead to central sensitization, a process in which the excitability of neurons in the spinal cord is increased.92 The mechanism of central sensitization is not completely understood but may involve disinhibition of spinal and trigeminal nociceptive neurons or facilitation of nociceptive activity by excitatory neurons that project from the rostroventral medulla.93 Similar changes occur in structures involved in the emotional aspects of pain such as the amygdala, anterior cingulate gyrus, and prefrontal cortex,94,95 and these may represent a substrate for long-term cognitive and mood changes that are learned and retained, for example, conditioned fear and addictive behaviour.96 The sensitizing process appears to distort or suppress non-noxious sensations. Loss of an inhibitory influence of normal cutaneous sensations in the CRPS-affected limb may enhance the excitability of thalamocortical nociceptive networks, thereby establishing a vicious circle.97,98 One key in central sensitization is the activation and up-regulation of glutamate receptors which enhance signal transmission in the nociceptive circuitry from the spinal cord to the cortex.99 That is, sensitized spinal nociceptive neurons become more responsive to peripheral input and may even fire in the absence of such input. As such, central sensitization can cause chronic pain, hyperalgesia, and allodynia, as well as the spreading of pain to adjacent non-injured areas.92 It follows then, that antagonists of, for example, the N-methyl-D-aspartic-acid receptor (NMDAR), are expected to induce analgesia in CRPS. Indeed, intravenous infusion of the NMDAR antagonist ketamine over several days imparted a clinically significant pain reduction for 11 weeks in CRPS patients.100 This long-lasting analgesic effect in CRPS strongly suggests that ketamine imparts long-term desensitization of the NMDAR or, at least, an as yet unspecified NMDA-mediated downstream process.101 A recently identified and potentially important mechanism, ‘hyperalgesic priming’, may explain why a transient insult can trigger long-lasting changes in primary afferent nociceptors, which prime them to become hyperresponsive to future mild insults that would normally not evoke pain in the un-primed state.102 The epsilon isoform of protein kinase C in the primary afferent nociceptor plays multiple crucial roles in this phenomenon.103 Although the cellular mechanisms of hyperalgesic priming occur within the peripheral terminals of primary afferent nociceptors, the resultant abnormal afferent activity can trigger plastic changes in the central nervous system. Emerging evidence suggests that hyperalgesic priming may be not only a basic pathophysiological mechanism of chronic re-injury pain, but may also be key to understanding some of the most perplexing chronic pain conditions, including pain syndromes that are stress-related (e.g., fibromyalgia, irritable bowel syndrome, or post-traumatic stress disorder), and neuropathic (e.g., associated with diabetes or chemotherapy for cancer or AIDS).102
Another manifestation of central nervous system dysfunction in CRPS involves impaired motor function. Impaired motor function is common after most injuries, but this impairment generally resolves as the patient recovers. In CRPS however, susceptible patients develop marked movement disorders. Dystonia, the most prevalent movement disorder in CRPS, is characterized in the arm by persistent flexion postures of the fingers and wrist and in the leg by plantar flexion/inversion of the foot, with or without clawing of the toes (Fig. 2C).104 The onset of dystonia occurs after the acute stage, which suggests that it is not due to acute inflammatory mechanisms.11,39 The risk of dystonia spreading to additional extremities in CRPS patients increases with the number of extremities that are already dystonic.39 This accellerated disease course is a typical characteristic of maladaptive neuronal plasticity.39,105 Dystonia does not respond to intravenous ketamine, which implicates neuroplastic changes distinct to those associated with sensitisation. In fact, that central sensitisation is an important neurophysiological characteristic in CRPS patients whether they have dystonia or not, suggests that the mechanisms underpinning dystonia occur over and above central sensitisation.106–109 As to what those mechanisms are is not well understood, but enhancement of spinal inhibitory neurotransmission by intrathecal administration of the γ-aminobutyric-acid-receptor-B (GABAB) agonist baclofen but not glycine, improved dystonia.110,111 Thus, it seems likely that spinally mediated GABA-ergic mechanisms play a specific role in the dystonia associated with CRPS.110,111
There is accumulating evidence that supraspinal mechanisms are also involved in the pathophysiology of CRPS.112 For example, in a healthy participant, repetitive noxious electrical stimulation on the back of the hand will induce adaptation so that the pain evoked by the stimuli reduces, reflecting descending inhibition, and an area of hyperalgesic skin, reflecting descending facilitation. However, CRPS patients show less adaptation to such stimuli, regardless of whether they are stimulated on the affected hand or the unaffected hand, and a larger area of hyperalgesia.113 These data strongly suggest that descending inhibition is reduced and descending facilitation augmented in people with CRPS.
Maladaptive changes have also been observed in high-order cognitive representations. People with long-standing CRPS tend to perceive their affected limb to be larger than it really is.114 They also report distortions of the mental image of their limb, for example missing components, alterations in shape, posture and temperature of the whole limb, or of discrete parts of the limb.115 They can report feelings of hostility or disgust toward the affected limb, or feel as though it is a separate entity to themselves, a foreign body that they would like to have amputated.115 Two recent studies support the notion that these higher order disturbances are not simply a consequence of having CRPS, but may in fact exert a top-down effect on the limb itself. The first study showed that two cardinal signs of CRPS – limb specific disruption of temperature control and tactile dysfunction - can be evoked experimentally in healthy volunteers, by inducing an illusion of disownership over that limb.116 The second study demonstrated that the swelling and pain evoked by movement of the CRPS limb is more severe if patients view a magnified image of the limb – if it looks bigger, it hurts more and becomes more swollen.117 Critically, experimentally induced pain in healthy volunteers is decreased when the view of the limb is magnified,118 further pointing to cortical maladaptation in CRPS.
The perceptual disturbances that are relatively common in CRPS remind one of similar disturbances associated with unilateral neglect after stroke.119,120 There are several other phenomena common in both conditions: they can perceive touch on the affected limb if they watch the mirror image of the unaffected limb being touched;121 they perform poorly on left/right limb judgement tasks;122 and they show a bias in tactile processing away from the affected side, not the affected limb.123 That is, when they cross their limbs, tactile input from the affected limb, now on the unaffected side of the midline, is prioritised over that from the unaffected limb now held in the affected side of space. Moreover, the extent of this bias in tactile processing away from the affected side is positively related to the extent of cooling of the affected limb, relative to the unaffected one. In fact, recent work has clearly established that cool-type CRPS is associated with a cool side of space – crossing the arms so that the healthy hand is on the affected side of the midline, reduces the temperature of the healthy hand (Moseley et al UNDER REVIEW). The idea that brain-grounded maps of external space can influence thermal regulation is consistent with the recent proposal of a cortical body matrix, which integrates somatotopic and spatial representations, the sense of ownership and homeostatic regulation of the body.124 CRPS patients show ipsilateral hemisensory impairment15 and neglect is characterised by hemisensory impairment.125 Importantly however, patients with CRPS, unlike those with spatial neglect, are always aware of their altered feelings toward the limb and perform normally on clinical tasks such as the line bisection test.126 They invariably state that while they believe that the limb is theirs, they feel as though it is not. This clearly suggests that the neglect-like disturbances observed in CRPS reflect an implicit mechanism to avoid provocation of pain, or an altered representation of aspects of the limb, rather than a direct consequence of actual neural damage.
In line with these clinical findings, functional imaging techniques have shown a substantial reorganization of the somatotopic map within the primary somatosensory cortex (S1) contralateral to the affected limb in CRPS patients.112,120,127 The S1 representation of the affected hand is smaller than that of the opposite hand, and the S1 represent of the hand shifted towards the ipsilateral mouth. The extent of these changes is associated with spontaneous CRPS pain and mechanical hyperalgesia. Interestingly, when CRPS symptoms decrease subsequent to treatment, this S1 cortical reorganization also reversed (Fig. 4128).127 This cortical reorganization might explain some of the often puzzling clinical signs of CRPS, for example the spatial distribution of sensory disturbances in a glove-like or stocking-like distribution, the occurrence of tactile-induced referred sensations,129 the perception that the limb is bigger than it really is and the presence of hemisensory deficits.15
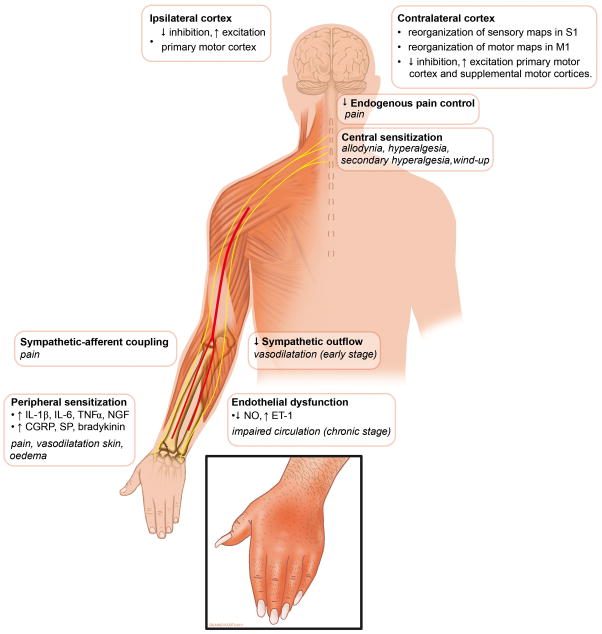
Figure 4. Illustration of clinical features and proposed pathophysiological model.
Illustration showing clinical features and their (assumed or demonstrated) underlying pathophysiological mechanisms. IL= interleukin; TNF = tumor necrosis factor; NGF = nerve growth factor; CGRP = Calcitonin-Gene-Related Peptide; SP = substance P; NO = nitric oxide; ET = endothelin.
Cortical changes also affect the primary motor cortex. Transcranial magnetic stimulation revealed decreased inhibitory mechanisms and increased excitability at the level of the contralateral primary motor cortex in CRPS patients.109 Interestingly, abnormalities of inhibitory mechanisms were also observed in the ipsilateral motor cortex of CRPS patients,109,130 which is consistent with reports of slight pre-clinical motor impairment of the unaffected limb.131 Further, bilateral cortical changes point to a widespread impairment of central motor processing in CRPS. An fMRI study on cortical activations during tapping movements of the CRPS-affected hand found that CRPS patients showed a significant reorganization of central motor circuits, with greater activation of primary motor and supplementary motor cortices than controls performing the same task.120 Furthermore, the ipsilateral motor cortex showed increased activation and the magnitude of motor dysfunction correlated with activations of the posterior parietal cortices, supplementary motor cortex and primary motor cortex. Clearly, there are widespread changes in cortical function in people with CRPS and although the exact role of these changes in the pathophysiology of CRPS has not been elucidated, it seems likely that they contribute to motor and sensory impairments commonly seen in CRPS.
Conclusions and future directions
Epidemiological, genetic and experimental studies strongly suggest that the pathophysiology of CRPS is multifactorial in nature and is characterized by an aberrant host response to tissue injury. We propose that (neurogenic) inflammation, nociceptive sensitization, vasomotor dysfunction and maladaptive neuroplasticity account for most or all of the clinical features of CRPS (Fig. 5). Inter-individual differences in the extent to which these mechanisms are affected account for the clinical heterogeneity of the condition. We argue that the responsible mechanisms can be conceptualized as a framework, each component of which should be considered in the management of these patients. That is, each of these components should be assessed, diagnosed and monitored. Finally, consideration of the multiple mechanisms implicated in the pathophysiology of CRPS should provide a basis for biomarker discovery and more targeted therapeutic interventions.
Table 1.
Budapest diagnostic criteria for CRPS.6
|
The validated Budapest criteria for the diagnoses of CRPS. There is a distinction between research diagnostic criteria (positive features in 4 symptom and 2 sign categories) and clinical diagnostic criteria (positive features in 3 symptom and 2 sign categories).6
A sign is counted only if observed at the time of diagnosis.
Acknowledgments
JM and JJvH participate in TREND, a Dutch knowledge consortium funded by the Netherlands’ Ministry of Economic Affairs (BSIK03016). GLM is supported by and receives project funding from the National Health & Medical Research Council of Australia (ID:571090;630431;63136;100817). FB is supported by the Rhineland Palatinate Foundation of Innovation (Project 936) and the Deutsche Forschungsgemeinschaft (GRK Neurowissenschaften Mainz). RB receives funding from the German Federal Ministry of Education and Research and the German Research Foundation (DFG). CM receives funding from the German Research Network “Neuropathic Pain” (BMBF) and the German Research Foundation (DFG; KFO 130). WSK receives funding from the Department of Veteran Affairs, Rehabilitation Research and Development Service (Grant F7137R).
Footnotes
Conflicts of interest
| JM: | none declared. |
| GLM: | none declared. |
| FB: | received fees as consultant and/or speaker from Pfizer, Grünenthal, Lilly, MedUpdate, Glaxo, and for expert testimony for Jerini. |
| RB: | received grants or research report from Pfizer, Genzyme, Grünenthal; is a member of the IMI “Europain” collaboration and industry members of this are: Astra Zenica, Pfizer, Esteve, UCB-Pharma, Sanofi Aventis, Grünenthal, Eli Lilly, Neuroscience technologies and Boehringer Ingelheim; received fees as consultant and/or speaker from Pfizer, Genzyme, Grünenthal, Mundipharma, Allergan, Sanofi Pasteur, Medtronic, Eisai, UCB BioSciences, Lilly, Boehringer Ingelheim, Astellas and Novartis. |
| CM: | received fees as consultant and/or speaker from Pfizer, Bionorica, Allergan and Grünenthal. |
| WK: | none declared. |
| JJvH: | received fees as consultant and speaker from Medtronic, GSK and Novartis. |
-
1Research project: A. Conception, B. Organization, C. Execution;
-
2Manuscript: A. Writing of the first draft, B. Review and critique
- JM: 1, 2
- GLM: 1A, 2
- FB: 1A, 2
- RB: 1A, 2
- CM: 1A, 2
- WK: 1A, 2
- JJvH: 1, 2
References
- 1.Albrecht PJ, Hines S, Eisenberg E, et al. Pathologic alterations of cutaneous innervation and vasculature in affected limbs from patients with complex regional pain syndrome. Pain. 2006;120:244–66. doi: 10.1016/j.pain.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 2.van der Laan L, ter Laak HJ, Gabreels-Festen A, Gabreels F, Goris RJ. Complex regional pain syndrome type I (RSD): pathology of skeletal muscle and peripheral nerve. Neurology. 1998;51:20–5. doi: 10.1212/wnl.51.1.20. [DOI] [PubMed] [Google Scholar]
- 3.Oaklander AL, Rissmiller JG, Gelman LB, et al. Evidence of focal small-fiber axonal degeneration in complex regional pain syndrome-I (reflex sympathetic dystrophy) Pain. 2006;120:235–43. doi: 10.1016/j.pain.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 4.Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain. 2008;131:1912–25. doi: 10.1093/brain/awn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merskey H, Bogduk N. Descriptions of chronic pain syndromes and definitions of pain terms. 2. Seattle: IASP Press; 1994. Classification of chronic pain. [Google Scholar]
- 6.Harden RN, Bruehl S, Perez RSGM, et al. Validation of proposed diagnostic criteria (the “Budapest Criteria”) for Complex Regional Pain Syndrome. Pain. 2010;150:268–74. doi: 10.1016/j.pain.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harden RN, Bruehl S, Galer BS, et al. Complex regional pain syndrome: are the IASP diagnostic criteria valid and sufficiently comprehensive? Pain. 1999;83:211–9. doi: 10.1016/s0304-3959(99)00104-9. [DOI] [PubMed] [Google Scholar]
- 8.Harden RN, Bruehl SP. Diagnostic Criteria: The Statistical Derivation of the Four Criterion Factors. In: Wilson PR, Stanton-Hicks M, Harden RN, editors. CRPS: Current Diagnosis and Therapy. 1. Seattle: IASP Press; 2005. pp. 45–58. [Google Scholar]
- 9.Harden RN, Bruehl S, Perez RS, et al. Development of a severity score for CRPS. Pain. 2010;151:870–6. doi: 10.1016/j.pain.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 10.Maier C, Baron R, Tolle TR, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain. 2010;150:439–50. doi: 10.1016/j.pain.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Veldman PH, Reynen HM, Arntz IE, Goris RJ. Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet. 1993;342:1012–6. doi: 10.1016/0140-6736(93)92877-v. [DOI] [PubMed] [Google Scholar]
- 12.Drummond PD. Sensory disturbances in complex regional pain syndrome: clinical observations, autonomic interactions, and possible mechanisms. Pain Med. 2010;11:1257–66. doi: 10.1111/j.1526-4637.2010.00912.x. [DOI] [PubMed] [Google Scholar]
- 13.Sethna NF, Meier PM, Zurakowski D, Berde CB. Cutaneous sensory abnormalities in children and adolescents with complex regional pain syndromes. Pain. 2007;131:153–61. doi: 10.1016/j.pain.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 14.Birklein F, Riedl B, Sieweke N, Weber M, Neundorfer B. Neurological findings in complex regional pain syndromes--analysis of 145 cases. Acta Neurol Scand. 2000;101:262–9. doi: 10.1034/j.1600-0404.2000.101004262x./. [DOI] [PubMed] [Google Scholar]
- 15.Rommel O, Gehling M, Dertwinkel R, et al. Hemisensory impairment in patients with complex regional pain syndrome. Pain. 1999;80:95–101. doi: 10.1016/s0304-3959(98)00202-4. [DOI] [PubMed] [Google Scholar]
- 16.Maleki J, LeBel AA, Bennett GJ, Schwartzman RJ. Patterns of spread in complex regional pain syndrome, type I (reflex sympathetic dystrophy) Pain. 2000;88:259–66. doi: 10.1016/S0304-3959(00)00332-8. [DOI] [PubMed] [Google Scholar]
- 17.van Rijn MA, Marinus J, Putter H, et al. Spreading of complex regional pain syndrome: not a random process. J Neural Transm. 2011 doi: 10.1007/s00702-011-0601-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chancellor MB, Shenot PJ, Rivas DA, Mandel S, Schwartzman RJ. Urological symptomatology in patients with reflex sympathetic dystrophy. J Urol. 1996;155:634–7. [PubMed] [Google Scholar]
- 19.Schwartzman RJ, Erwin KL, Alexander GM. The natural history of complex regional pain syndrome. Clin J Pain. 2009;25:273–80. doi: 10.1097/AJP.0b013e31818ecea5. [DOI] [PubMed] [Google Scholar]
- 20.Smith JA, Karalis DG, Rosso AL, et al. Syncope in complex regional pain syndrome. Clin Cardiol. 2011;34:222–5. doi: 10.1002/clc.20879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Libon DJ, Schwartzman RJ, Eppig J, et al. Neuropsychological deficits associated with Complex Regional Pain Syndrome. J Int Neuropsychol Soc. 2010;16:566–73. doi: 10.1017/S1355617710000214. [DOI] [PubMed] [Google Scholar]
- 22.de Mos M, de Bruijn AGJ, Huygen FJPM, et al. The incidence of complex regional pain syndrome: A population-based study. Pain. 2007;129:12–20. doi: 10.1016/j.pain.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Sandroni P, Benrud-Larson LM, McClelland RL, Low PA. Complex regional pain syndrome type I: incidence and prevalence in Olmsted county, a population-based study. Pain. 2003;103:199–207. doi: 10.1016/s0304-3959(03)00065-4. [DOI] [PubMed] [Google Scholar]
- 24.de Mos M, Huygen FJPM, van der Hoeven-Borgman M, et al. Outcome of the Complex Regional Pain Syndrome. Clin J Pain. 2009;25:590–7. doi: 10.1097/AJP.0b013e3181a11623. [DOI] [PubMed] [Google Scholar]
- 25.de Rooij AM, Perez RS, Huygen FJ, et al. Spontaneous onset of complex regional pain syndrome. Eur J Pain. 2010;14:510–3. doi: 10.1016/j.ejpain.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Galer BS, Henderson J, Perander J, Jensen MP. Course of symptoms and quality of life measurement in Complex Regional Pain Syndrome: a pilot survey. J Pain Symptom Manage. 2000;20:286–92. doi: 10.1016/s0885-3924(00)00183-4. [DOI] [PubMed] [Google Scholar]
- 27.Kemler MA, Raphael JH, Bentley A, Taylor RS. The cost-effectiveness of spinal cord stimulation for complex regional pain syndrome. Value Health. 2010;13:735–42. doi: 10.1111/j.1524-4733.2010.00744.x. [DOI] [PubMed] [Google Scholar]
- 28.Bruehl S. Complex Regional Pain Syndrome. Seattle: IASP Press; 2001. Do psychological factors play a role in the onset and maintenance of CRPS? [Google Scholar]
- 29.Field J, Gardner FV. Psychological distress associated with algodystrophy. J Hand Surg [Br ] 1997;22:100–1. doi: 10.1016/s0266-7681(97)80030-7. [DOI] [PubMed] [Google Scholar]
- 30.Harden RN, Bruehl S, Stanos S, et al. Prospective examination of pain-related and psychological predictors of CRPS-like phenomena following total knee arthroplasty: a preliminary study. Pain. 2003;106:393–400. doi: 10.1016/j.pain.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Puchalski P, Zyluk A. Complex regional pain syndrome type 1 after fractures of the distal radius: a prospective study of the role of psychological factors. J Hand Surg [Br ] 2005;30:574–80. doi: 10.1016/j.jhsb.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 32.Beerthuizen A, Stronks DL, Huygen FJ, et al. The association between psychological factors and the development of complex regional pain syndrome type 1 (CRPS1) - A prospective multicenter study. Eur J Pain. 2011 doi: 10.1016/j.ejpain.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 33.de Mos M, Huygen FJPM, Dieleman JP, et al. Medical history and the onset of complex regional pain syndrome (CRPS) Pain. 2008;139:458–66. doi: 10.1016/j.pain.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Schwartzman RJ, McLellan TL. Reflex sympathetic dystrophy. A review. Arch Neurol. 1987;44:555–61. doi: 10.1001/archneur.1987.00520170081028. [DOI] [PubMed] [Google Scholar]
- 35.Terkelsen AJ, Bach FW, Jensen TS. Experimental forearm immobilization in humans induces cold and mechanical hyperalgesia. Anesthesiology. 2008;109:297–307. doi: 10.1097/ALN.0b013e31817f4c9d. [DOI] [PubMed] [Google Scholar]
- 36.de Mos M, Huygen FJPM, Stricker BHC, Dieleman JP, Sturkenboom MCJM. The association between ACE inhibitors and the complex regional pain syndrome: Suggestions for a neuro-inflammatory pathogenesis of CRPS. Pain. 2009;142:218–24. doi: 10.1016/j.pain.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 37.Peterlin BL, Rosso AL, Nair S, Young WB, Schwartzman RJ. Migraine may be a risk factor for the development of complex regional pain syndrome. Cephalalgia. 2010;30:214–23. doi: 10.1111/j.1468-2982.2009.01916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Laan L, Veldman PHJM, Goris JA. Severe complications of reflex sympathetic dystrophy: Infection, ulcers, chronic edema, dystonia, and myoclonus. Arch Pys Med Rehabil. 1998;79:424–9. doi: 10.1016/s0003-9993(98)90144-7. [DOI] [PubMed] [Google Scholar]
- 39.van Rijn MA, Marinus J, Putter H, van Hilten JJ. Onset and progression of dystonia in Complex Regional Pain Syndrome. Pain. 2007;130:287–93. doi: 10.1016/j.pain.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 40.de Rooij AM, de MM, van Hilten JJ, et al. Increased risk of complex regional pain syndrome in siblings of patients? J Pain. 2009;10:1250–5. doi: 10.1016/j.jpain.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 41.de Rooij AM, de Mos M, Sturkenboom MCJM, et al. Familial occurrence of complex regional pain syndrome. Eur J Pain. 2009;13:171–7. doi: 10.1016/j.ejpain.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 42.Huhne K, Leis S, Schmelz M, Rautenstrauss B, Birklein F. A polymorphic locus in the intron 16 of the human angiotensin-converting enzyme (ACE) gene is not correlated with complex regional pain syndrome I (CRPS I) Eur J Pain. 2004;8:221–5. doi: 10.1016/j.ejpain.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 43.Kemler MA, van de Vusse AC, van den Berg-Loonen EM, et al. HLA-DQ1 associated with reflex sympathetic dystrophy. Neurology. 1999;53:1350–1. doi: 10.1212/wnl.53.6.1350. [DOI] [PubMed] [Google Scholar]
- 44.Mailis A, Wade J. Profile of Caucasian women with possible genetic predisposition to reflex sympathetic dystrophy: a pilot study. Clin J Pain. 1994;10:210–7. doi: 10.1097/00002508-199409000-00007. [DOI] [PubMed] [Google Scholar]
- 45.van de Beek WJ, Roep BO, van der Slik AR, Giphart MJ, van Hilten BJ. Susceptibility loci for complex regional pain syndrome. Pain. 2003;103:93–7. doi: 10.1016/s0304-3959(02)00444-x. [DOI] [PubMed] [Google Scholar]
- 46.van Hilten JJ, van de Beek WJ, Roep BO. Multifocal or generalized tonic dystonia of complex regional pain syndrome: a distinct clinical entity associated with HLA-DR13. Ann Neurol. 2000;48:113–6. doi: 10.1002/1531-8249(200007)48:1<113::aid-ana18>3.3.co;2-0. [DOI] [PubMed] [Google Scholar]
- 47.de Rooij AM, Florencia GM, Haasnoot GW, et al. HLA-B62 and HLA-DQ8 are associated with Complex Regional Pain Syndrome with fixed dystonia. Pain. 2009;145:82–5. doi: 10.1016/j.pain.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 48.Vaneker M, Laan Lvd, Allebes WA, Goris JA. Genetic factors associated with Complex Regional Pain Syndrome 1: HLA DRB and TNF alpha promotor gene polymorphism. Disability Medicine. 2002;2:69–74. [Google Scholar]
- 49.Kimura T, Komatsu T, Hosoda R, Nishiwaki K, Shimida Y. Angiotensin-Converting Enzyme Gene Polymorphism in Patients with Neuropathic Pain. In: Devor M, Rowbotham MC, Wiesenfeld-Hallin Z, editors. Proceedings of the 9th World Congress on Pain. Seattle: IASP Press; 2000. pp. 471–6. [Google Scholar]
- 50.Langberg H, Olesen JL, Gemmer C, Kjaer M. Substantial elevation of interleukin-6 concentration in peritendinous tissue, in contrast to muscle, following prolonged exercise in humans. J Physiol. 2002;542:985–90. doi: 10.1113/jphysiol.2002.019141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eberle T, Doganci B, Kramer H, et al. Mechanical but not painful electrical stimuli trigger TNF alpha release in human skin. Exp Neurol. 2010;221:246–50. doi: 10.1016/j.expneurol.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 52.Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361:184–7. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 53.Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–38. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- 54.Opree A, Kress M. Involvement of the proinflammatory cytokines tumor necrosis factor-alpha, IL-1 beta, and IL-6 but not IL-8 in the development of heat hyperalgesia: effects on heat-evoked calcitonin gene-related peptide release from rat skin. J Neurosci. 2000;20:6289–93. doi: 10.1523/JNEUROSCI.20-16-06289.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vedder H, Affolter HU, Otten U. Nerve growth factor (NGF) regulates tachykinin gene expression and biosynthesis in rat sensory neurons during early postnatal development. Neuropeptides. 1993;24:351–7. doi: 10.1016/0143-4179(93)90006-v. [DOI] [PubMed] [Google Scholar]
- 56.Holzer P. Neurogenic vasodilatation and plasma leakage in the skin. Gen Pharmacol. 1998;30:5–11. doi: 10.1016/s0306-3623(97)00078-5. [DOI] [PubMed] [Google Scholar]
- 57.Birklein F, Schmelz M, Schifter S, Weber M. The important role of neuropeptides in complex regional pain syndrome. Neurology. 2001;57:2179–84. doi: 10.1212/wnl.57.12.2179. [DOI] [PubMed] [Google Scholar]
- 58.Schinkel C, Gaertner A, Zaspel J, et al. Inflammatory mediators are altered in the acute phase of posttraumatic complex regional pain syndrome. Clin J Pain. 2006;22:235–9. doi: 10.1097/01.ajp.0000169669.70523.f0. [DOI] [PubMed] [Google Scholar]
- 59.Leis S, Weber M, Isselmann A, Schmelz M, Birklein F. Substance-P-induced protein extravasation is bilaterally increased in complex regional pain syndrome. Exp Neurol. 2003;183:197–204. doi: 10.1016/s0014-4886(03)00163-8. [DOI] [PubMed] [Google Scholar]
- 60.Leis S, Weber M, Schmelz M, Birklein F. Facilitated neurogenic inflammation in unaffected limbs of patients with complex regional pain syndrome. Neuroscience Letters. 2004;359:163–6. doi: 10.1016/j.neulet.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 61.Weber M, Birklein F, Neundorfer B, Schmelz M. Facilitated neurogenic inflammation in complex regional pain syndrome. Pain. 2001;91:251–7. doi: 10.1016/S0304-3959(00)00445-0. [DOI] [PubMed] [Google Scholar]
- 62.Dallos A, Kiss M, Polyanka H, et al. Effects of the neuropeptides substance P, calcitonin gene-related peptide, vasoactive intestinal polypeptide and galanin on the production of nerve growth factor and inflammatory cytokines in cultured human keratinocytes. Neuropeptides. 2006;40:251–63. doi: 10.1016/j.npep.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 63.Kingery WS. Role of neuropeptide, cytokine, and growth factor signaling in complex regional pain syndrome. Pain Med. 2010;11:1239–50. doi: 10.1111/j.1526-4637.2010.00913.x. [DOI] [PubMed] [Google Scholar]
- 64.Huygen FJPM, Ramdhani N, van Toorenenbergen A, Klein J, Zijlstra FJ. Mast cells are involved in inflammatory reactions during Complex Regional Pain Syndrome type 1. Immunology Letters. 2004;91:147–54. doi: 10.1016/j.imlet.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 65.Groeneweg JG, Huygen FJ, Heijmans-Antonissen C, Niehof S, Zijlstra FJ. Increased endothelin-1 and diminished nitric oxide levels in blister fluids of patients with intermediate cold type complex regional pain syndrome type 1. BMC Musculoskelet Disord. 2006;7:91. doi: 10.1186/1471-2474-7-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wesseldijk F, Huygen FJ, Heijmans-Antonissen C, Niehof SP, Zijlstra FJ. Six years follow-up of the levels of TNF-alpha and IL-6 in patients with complex regional pain syndrome type 1. Mediators Inflamm. 2008;2008:469439. doi: 10.1155/2008/469439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kramer HH, Eberle T, Uceyler N, et al. TNF-alpha in CRPS and ‘normal’ trauma - Significant differences between tissue and serum. Pain. 2011;152:285–90. doi: 10.1016/j.pain.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 68.Uceyler N, Eberle T, Rolke R, Birklein F, Sommer C. Differential expression patterns of cytokines in complex regional pain syndrome. Pain. 2007;132:195–205. doi: 10.1016/j.pain.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 69.Wesseldijk F, Huygen FJ, Heijmans-Antonissen C, Niehof SP, Zijlstra FJ. Tumor necrosis factor-[alpha] and interleukin-6 are not correlated with the characteristics of Complex Regional Pain Syndrome type 1 in 66 patients. Eur J Pain. 2008;12:716–21. doi: 10.1016/j.ejpain.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 70.Maihofner C, Handwerker HO, Neundorfer B, Birklein F. Mechanical hyperalgesia in complex regional pain syndrome: A role for TNF-alpha? Neurology. 2005;65:311–3. doi: 10.1212/01.wnl.0000168866.62086.8f. [DOI] [PubMed] [Google Scholar]
- 71.Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6:521–32. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- 72.Ritz BW, Alexander GM, Nogusa S, et al. Elevated blood levels of inflammatory monocytes (CD14(+) CD16(+) ) in patients with complex regional pain syndrome. Clin Exp Immunol. 2011;164:108–17. doi: 10.1111/j.1365-2249.2010.04308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alexander GM, van Rijn MA, van Hilten JJ, Perreault MJ, Schwartzman RJ. Changes in cerebrospinal fluid levels of pro-inflammatory cytokines in CRPS. Pain. 2005;116:213–9. doi: 10.1016/j.pain.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 74.Alexander GM, Perreault MJ, Reichenberger ER, Schwartzman RJ. Changes in immune and glial markers in the CSF of patients with Complex Regional Pain Syndrome. Brain Behav Immun. 2007;21:668–76. doi: 10.1016/j.bbi.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 75.Munts AG, Zijlstra FJ, Nibbering PH, et al. Analysis of cerebrospinal fluid inflammatory mediators in chronic complex regional pain syndrome related dystonia. Clin J Pain. 2008;24:30–4. doi: 10.1097/AJP.0b013e318156d961. [DOI] [PubMed] [Google Scholar]
- 76.Ribbers GM, Oosterhuis WP, van Limbeek J, de Metz M. Reflex sympathetic dystrophy: is the immune system involved? Arch Phys Med Rehabil. 1998;79:1549–52. doi: 10.1016/s0003-9993(98)90418-x. [DOI] [PubMed] [Google Scholar]
- 77.Blaes F, Schmitz K, Tschernatsch M, et al. Autoimmune etiology of complex regional pain syndrome (M. Sudeck) Neurology. 2004;63:1734–6. doi: 10.1212/01.wnl.0000143066.58498.ba. [DOI] [PubMed] [Google Scholar]
- 78.Kohr D, Tschernatsch M, Schmitz K, et al. Autoantibodies in complex regional pain syndrome bind to a differentiation-dependent neuronal surface autoantigen. Pain. 2009;143:246–51. doi: 10.1016/j.pain.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 79.Krumova EK, Frettlöh J, Klauenberg S, et al. Long-term skin temperature measurements - A practical diagnostic tool in complex regional pain syndrome. Pain. 2008;140:8–22. doi: 10.1016/j.pain.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 80.Wasner G, Schattschneider J, Heckmann K, Maier C, Baron R. Vascular abnormalities in reflex sympathetic dystrophy (CRPS I): mechanisms and diagnostic value. Brain. 2001;124:587–99. doi: 10.1093/brain/124.3.587. [DOI] [PubMed] [Google Scholar]
- 81.Wasner G, Heckmann K, Maier C, Baron R. Vascular abnormalities in acute reflex sympathetic dystrophy (CRPS I): complete inhibition of sympathetic nerve activity with recovery. Arch Neurol. 1999;56:613–20. doi: 10.1001/archneur.56.5.613. [DOI] [PubMed] [Google Scholar]
- 82.Arnold JM, Teasell RW, MacLeod AP, Brown JE, Carruthers SG. Increased venous alpha-adrenoceptor responsiveness in patients with reflex sympathetic dystrophy. Ann Intern Med. 1993;118:619–21. doi: 10.7326/0003-4819-118-8-199304150-00008. [DOI] [PubMed] [Google Scholar]
- 83.Drummond PD, Skipworth S, Finch PM. alpha 1-adrenoceptors in normal and hyperalgesic human skin. Clin Sci (Lond) 1996;91:73–7. doi: 10.1042/cs0910073. [DOI] [PubMed] [Google Scholar]
- 84.Eberle T, Doganci B, Kramer HH, et al. Warm and cold complex regional pain syndromes: Differences beyond skin temperature? Neurology. 2009;72:505–12. doi: 10.1212/01.wnl.0000341930.35494.66. [DOI] [PubMed] [Google Scholar]
- 85.Baron R, Levine JD, Fields HL. Causalgia and reflex sympathetic dystrophy: does the sympathetic nervous system contribute to the generation of pain? Muscle Nerve. 1999;22:678–95. doi: 10.1002/(sici)1097-4598(199906)22:6<678::aid-mus4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 86.Drummond PD. alpha(1)-Adrenoceptors augment thermal hyperalgesia in mildly burnt skin. Eur J Pain. 2009;13:273–9. doi: 10.1016/j.ejpain.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 87.Gibbs GF, Drummond PD, Finch PM, Phillips JK. Unravelling the pathophysiology of complex regional pain syndrome: focus on sympathetically maintained pain. Clin Exp Pharmacol Physiol. 2008;35:717–24. doi: 10.1111/j.1440-1681.2007.04862.x. [DOI] [PubMed] [Google Scholar]
- 88.Baron R, Schattschneider J, Binder A, Siebrecht D, Wasner G. Relation between sympathetic vasoconstrictor activity and pain and hyperalgesia in complex regional pain syndromes: a case-control study. Lancet. 2002;359:1655–60. doi: 10.1016/S0140-6736(02)08589-6. [DOI] [PubMed] [Google Scholar]
- 89.Drummond PD, Finch PM. Persistence of pain induced by startle and forehead cooling after sympathetic blockade in patients with complex regional pain syndrome. J Neurol Neurosurg Psychiatry. 2004;75:98–102. [PMC free article] [PubMed] [Google Scholar]
- 90.Schattschneider J, Hartung K, Stengel M, et al. Endothelial dysfunction in cold type complex regional pain syndrome. Neurology. 2006;67:673–5. doi: 10.1212/01.wnl.0000229931.40631.31. [DOI] [PubMed] [Google Scholar]
- 91.Del Valle L, Schwartzman RJ, Alexander G. Spinal cord histopathological alterations in a patient with longstanding complex regional pain syndrome. Brain Behav Immun. 2008;23:85–91. doi: 10.1016/j.bbi.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 92.Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–9. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- 93.Vera-Portocarrero LP, Zhang ET, Ossipov MH, et al. Descending facilitation from the rostral ventromedial medulla maintains nerve injury-induced central sensitization. Neuroscience. 2006;140:1311–20. doi: 10.1016/j.neuroscience.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 94.Fu Y, Han J, Ishola T, et al. PKA and ERK, but not PKC, in the amygdala contribute to pain-related synaptic plasticity and behavior. Mol Pain. 2008;4:26. doi: 10.1186/1744-8069-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pedersen LH, Scheel-Kruger J, Blackburn-Munro G. Amygdala GABA-A receptor involvement in mediating sensory-discriminative and affective-motivational pain responses in a rat model of peripheral nerve injury. Pain. 2007;127:17–26. doi: 10.1016/j.pain.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 96.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lebel A, Becerra L, Wallin D, et al. fMRI reveals distinct CNS processing during symptomatic and recovered complex regional pain syndrome in children. Brain. 2008;131:1854–79. doi: 10.1093/brain/awn123. [DOI] [PubMed] [Google Scholar]
- 98.Davis KD, Kiss ZH, Tasker RR, Dostrovsky JO. Thalamic stimulation-evoked sensations in chronic pain patients and in nonpain (movement disorder) patients. J Neurophysiol. 1996;75:1026–37. doi: 10.1152/jn.1996.75.3.1026. [DOI] [PubMed] [Google Scholar]
- 99.Kuner R. Central mechanisms of pathological pain. Nat Med. 2010;16:1258–66. doi: 10.1038/nm.2231. [DOI] [PubMed] [Google Scholar]
- 100.Sigtermans MJ, van Hilten JJ, Bauer MCR, et al. Ketamine produces effective and long-term pain relief in patients with Complex Regional Pain Syndrome Type 1. Pain. 2009;145:304–11. doi: 10.1016/j.pain.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 101.Sigtermans M, Noppers I, Sarton E, et al. An observational study on the effect of S+-ketamine on chronic pain versus experimental acute pain in Complex Regional Pain Syndrome type 1 patients. Eur J Pain. 2010;14:302–7. doi: 10.1016/j.ejpain.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 102.Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32:611–8. doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Parada CA, Yeh JJ, Reichling DB, Levine JD. Transient attenuation of protein kinase Cepsilon can terminate a chronic hyperalgesic state in the rat. Neuroscience. 2003;120:219–26. doi: 10.1016/s0306-4522(03)00267-7. [DOI] [PubMed] [Google Scholar]
- 104.van Hilten JJ, van de Beek WJ, Vein AA, van Dijk JG, Middelkoop HA. Clinical aspects of multifocal or generalized tonic dystonia in reflex sympathetic dystrophy. Neurology. 2001;56:1762–5. doi: 10.1212/wnl.56.12.1762. [DOI] [PubMed] [Google Scholar]
- 105.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–9. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 106.Avanzino L, Martino D, van de Warrenburg BP, et al. Cortical excitability is abnormal in patients with the “fixed dystonia” syndrome. Mov Disord. 2008;23:652. doi: 10.1002/mds.21801. [DOI] [PubMed] [Google Scholar]
- 107.Eisenberg E, Chistyakov AV, Yudashkin M, et al. Evidence for cortical hyperexcitability of the affected limb representation area in CRPS: a psychophysical and transcranial magnetic stimulation study. Pain. 2005;113:99–105. doi: 10.1016/j.pain.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 108.Schouten AC, van de Beek WJ, van Hilten JJ, Van der Helm FC. Proprioceptive reflexes in patients with reflex sympathetic dystrophy. Exp Brain Res. 2003;151:1–8. doi: 10.1007/s00221-003-1420-x. [DOI] [PubMed] [Google Scholar]
- 109.Schwenkreis P, Janssen F, Rommel O, et al. Bilateral motor cortex disinhibition in complex regional pain syndrome (CRPS) type I of the hand. Neurology. 2003;61:515–9. doi: 10.1212/wnl.61.4.515. [DOI] [PubMed] [Google Scholar]
- 110.Munts AG, van der Plas AA, Voormolen JH, et al. Intrathecal glycine for pain and dystonia in complex regional pain syndrome. Pain. 2009;146:199–204. doi: 10.1016/j.pain.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 111.van Rijn MA, Munts AG, Marinus J, et al. Intrathecal baclofen for dystonia of complex regional pain syndrome. Pain. 2009;143:41–7. doi: 10.1016/j.pain.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 112.Maihofner C, Handwerker HO, Neundorfer B, Birklein F. Patterns of cortical reorganization in complex regional pain syndrome. Neurology. 2003;61:1707–15. doi: 10.1212/01.wnl.0000098939.02752.8e. [DOI] [PubMed] [Google Scholar]
- 113.Seifert F, Kiefer G, DeCol R, Schmelz M, Maihofner C. Differential endogenous pain modulation in complex-regional pain syndrome. Brain. 2009;132:788–800. doi: 10.1093/brain/awn346. [DOI] [PubMed] [Google Scholar]
- 114.Moseley GL. Distorted body image in complex regional pain syndrome. Neurology. 2005;65:773. doi: 10.1212/01.wnl.0000174515.07205.11. [DOI] [PubMed] [Google Scholar]
- 115.Lewis JS, Kersten P, McCabe CS, McPherson KM, Blake DR. Body perception disturbance: a contribution to pain in complex regional pain syndrome (CRPS) Pain. 2007;133:111–9. doi: 10.1016/j.pain.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 116.Moseley GL, Zalucki NM, Wiech K. Tactile discrimination, but not tactile stimulation alone, reduces chronic limb pain. Pain. 2008;137:600–8. doi: 10.1016/j.pain.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 117.Moseley GL, Parsons TJ, Spence C. Visual distortion of a limb modulates the pain and swelling evoked by movement. Curr Biol. 2008;18:R1047–R1048. doi: 10.1016/j.cub.2008.09.031. [DOI] [PubMed] [Google Scholar]
- 118.Mancini F, Longo MR, Kammers MP, Haggard P. Visual Distortion of Body Size Modulates Pain Perception. Psychol Sci. 2011 doi: 10.1177/0956797611398496. [DOI] [PubMed] [Google Scholar]
- 119.Frettloh J, Huppe M, Maier C. Severity and specificity of neglect-like symptoms in patients with complex regional pain syndrome (CRPS) compared to chronic limb pain of other origins. Pain. 2006;124:184–9. doi: 10.1016/j.pain.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 120.Maihofner C, Baron R, DeCol R, et al. The motor system shows adaptive changes in complex regional pain syndrome. Brain. 2007;130:2671–87. doi: 10.1093/brain/awm131. [DOI] [PubMed] [Google Scholar]
- 121.Acerra NE, Moseley GL. Dysynchiria: Watching the mirror image of the unaffected limb elicits pain on the affected side. Neurology. 2005;65:751–3. doi: 10.1212/01.wnl.0000178745.11996.8c. [DOI] [PubMed] [Google Scholar]
- 122.Moseley GL. Why do people with complex regional pain syndrome take longer to recognize their affected hand? Neurology. 2004;62:2182–6. doi: 10.1212/01.wnl.0000130156.05828.43. [DOI] [PubMed] [Google Scholar]
- 123.Moseley GL, Gallace A, Spence C. Space-based, but not arm-based, shift in tactile processing in complex regional pain syndrome and its relationship to cooling of the affected limb. Brain. 2009;132:3142–51. doi: 10.1093/brain/awp224. [DOI] [PubMed] [Google Scholar]
- 124.Moseley GL, Gallace A, Spence C. Bodily illusions in health and disease: Physiological and clinical perspectives and the concept of a cortical ‘body matrix’. Neurosci Biobehav Rev. 2011 doi: 10.1016/j.neubiorev.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 125.von Giesen HJ, Schlaug G, Steinmetz H, et al. Cerebral network underlying unilateral motor neglect: evidence from positron emission tomography. J Neurol Sci. 1994;125:29–38. doi: 10.1016/0022-510x(94)90238-0. [DOI] [PubMed] [Google Scholar]
- 126.Forderreuther S, Sailer U, Straube A. Impaired self-perception of the hand in complex regional pain syndrome (CRPS) Pain. 2004;110:756–61. doi: 10.1016/j.pain.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 127.Pleger B, Tegenthoff M, Ragert P, et al. Sensorimotor returning in complex regional pain syndrome parallels pain reduction. Ann Neurol. 2005;57:425–9. doi: 10.1002/ana.20394. [DOI] [PubMed] [Google Scholar]
- 128.Maihofner C, Handwerker HO, Neundorfer B, Birklein F. Cortical reorganization during recovery from complex regional pain syndrome. Neurology. 2004;63:693–701. doi: 10.1212/01.wnl.0000134661.46658.b0. [DOI] [PubMed] [Google Scholar]
- 129.McCabe CS, Haigh RC, Halligan PW, Blake DR. Referred sensations in patients with complex regional pain syndrome type 1. Rheumatology (Oxford) 2003;42:1067–73. doi: 10.1093/rheumatology/keg298. [DOI] [PubMed] [Google Scholar]
- 130.Juottonen K, Gockel M, Silen T, et al. Altered central sensorimotor processing in patients with complex regional pain syndrome. Pain. 2002;98:315–23. doi: 10.1016/S0304-3959(02)00119-7. [DOI] [PubMed] [Google Scholar]
- 131.Ribbers GM, Mulder T, Geurts AC, den Otter RA. Reflex sympathetic dystrophy of the left hand and motor impairments of the unaffected right hand: impaired central motor processing? Arch Phys Med Rehabil. 2002;83:81–5. doi: 10.1053/apmr.2002.27331. [DOI] [PubMed] [Google Scholar]