Abstract
Objectives
Telomeres are repetitive DNA at chromosomes ends that shorten with age due to cellular replication and oxidative stress. As telomeres shorten, this can eventually place limits on cell replication and contribute to senescence. Infections are common during early development and activate cellular immune responses that involve clonal expansion and oxidative stress. As such, a high infectious disease burden might shorten blood telomere length (BTL) and accelerate the pace of immune senescence.
Methods
To test this, BTL measured in young adults (21.7 ± 0.3 years old) from the Philippines (N=1,759) were linked to prospectively collected early life data on infectious burden.
Results
As predicted, increased early life diarrheal prevalence was associated with shorter adult BTL. The association was most marked for infections experienced from 6–12 months, which corresponds with weaning and maximal diarrheal burden. A standard deviation increase in infections at 6–12 m predicts a 45 bp decrease in BTL, equivalent to 3.3 years of adult telomeric aging in this population. Contrary to expectations, breastfeeding duration was not associated with BTL, nor did effects vary by sex.
Conclusions
These findings show that infancy diarrheal disease predicts a marker of cellular aging in adult immune cells. These findings suggest that early life infectious burden may influence late life health, or alternatively, that short TL in early life increases infectious disease susceptibility.
Keywords: senescence, aging, DOHaD, immune function, infection
Introduction
In many high income countries, human life expectancies have been continually increasing for over a hundred years (Oeppen and Vaupel, 2002). While many factors likely contribute to these increases, one prominently considered explanation is improved hygiene, including the control and treatment of common infections in infancy (reviewed in Kuzawa and Eisenberg, 2014). Indeed, above and beyond socioeconomic measures, individuals who grow up with a high infectious disease burden have been shown to have shorter lifespans, suggesting possible lingering biological influences of early life exposures (Bengtsson and Lindström, 2003; Finch and Crimmins, 2004). However, the mechanisms linking early life infection to increased late life morbidity and mortality are presently unknown. Shortening of telomeres is one potential pathway.
Telomeres are repeating DNA sequences at the ends of chromosomes that protect and buffer chromosomes from nucleotide loss as cells divide (Blackburn and Gall, 1978). In many tissues, telomeres are shortened by cellular proliferation, and, as a result, TL tends to decline with age (Olovnikov, 1971; Watson, 1972; Kimura et al., 2008; Ishii et al., 2006). Reduced TL is thought to contribute to senescence because cell replication ceases when telomeres reach a critically short length and production of new cells is required for tissue repair and immune function (Harley, 1991). Consistent with this, individuals with immune cell telomeres that are relatively short for their age have been shown to have reduced life expectancies (Cawthon et al., 2003; Ehrlenbach et al., 2009; Ilmonen et al., 2008).
Early life infections may result in repeated activation of cellular immune responses, each involving clonal expansion of lymphocytes and thus TL shortening. As well, infection also increases oxidative stress (reviewed in Dowling and Simmons, 2009), another cause of TL shortening (e.g. Richter and Zglinicki, 2007; Oikawa and Kawanishi, 1999). Telomerase, an enzyme that re-builds telomeres, is upregulated in response to at least some immune challenges and infections, but this is apparently insufficient to maintain TL, particularly with exposure to persistent immune challenges (Maini et al., 1999; Reed et al., 2004; Plunkett et al., 2001; Hiyama and Hiyama, 2007).
In mice, and birds experimental infections have been shown to cause shortened TLs (Ilmonen et al., 2008; Asghar et al., 2015; Asghar et al., 2016). In humans, studies examining associations between infectious diseases and blood or lymphocyte TL have primarily been limited to adults in clinical contexts from high income countries. These studies have shown that shorter blood TL (BTL) is associated with HIV (Gianesin et al., 2016; Pommier et al., 1997; Effros et al., 1996; Zanet et al., 2014; Malan-Müller et al., 2013; Pathai et al., 2013; Srinivasa et al., 2014; but see Giesbrecht et al., 2014; Imam et al., 2012), hepatitis C (Zanet et al., 2014), and Helicobacter pylori infection (Hou et al., 2009). Infection also causes upregulation of inflammatory markers. Markers of inflammation such as C-reactive protein (CRP), tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) often, but not always, predict shorter BTL (Sampson et al., 2006; Carrero et al., 2008; O'Donovan et al., 2009; Aviv et al., 2006; Farzaneh-Far et al., 2010; O'Donovan et al., 2011; Solorio et al., 2011; Sanders et al., 2012; Salpea et al., 2010; Bendix et al., 2010; Olivieri et al., 2009; Adaikalakoteswari et al., 2007).
While BTL declines with age, the rate of decrease is far greater in infancy and childhood than in later life—likely due to the heightened pace of cellular proliferation related to immune system development and rapid somatic growth (Eisenberg, 2011). This points to a potential sensitive period in early life in which inflammation and oxidative stress secondary to infection might have a greater impact on TL attrition because of these increased cell replication rates. This, coupled with high mortality rates from infectious diseases in infancy and early childhood in low income countries suggests that early life infections could be particularly influential on TL—with lingering effects into adulthood that may ultimately influence disease risk. Similarly, breastfeeding is known to provide protection to infants from infections due to immunoglobulins and other factors in breast milk, as well as the fact that breastfed babies are less likely to consume contaminated food and liquids (Popkin et al., 1990; McDade and Worthman, 1998; Meremikwu et al., 1997; VanDerslice et al., 1994). This along with the likely lingering positive effects of breastfeeding into adult life (Horta and Victora, 2013) led us to predict that longer breastfeeding duration should predict longer adult BTL. To our knowledge, no study has evaluated the association of early life infection with adult TL and only one study has evaluated the association of breastfeeding duration and TL (Wojcicki et al., 2016). We examine these questions using longitudinal data collected from the Philippines. In particular we address three inter-related hypotheses:
Hypothesis 1 (H1). Increased early life diarrheal morbidity will predict shorter BTL measured in adulthood. Diarrheal diseases are the second leading cause of death in children under five, and particularly prevalent in low income contexts with underdeveloped public health infrastructures (Fischer Walker et al., 2012). Thus, diarrheal morbidity is an important marker of infectious disease exposure and immunological activation which might influence TL.
H2. Longer exclusive breastfeeding duration is expected to be protective against BTL shortening due to immunoglobulins and other protective factors in breast milk, as well as the fact that breastfed babies are less likely to consume contaminated food and liquids (Popkin et al., 1990; McDade and Worthman, 1998; Meremikwu et al., 1997; VanDerslice et al., 1994) and that one previous study found an association between breastfeeding and longer TL (Wojcicki et al., 2016).
H3. Increased early life diarrheal morbidity and decreased breastfeeding duration will be associated with shorter BTL in adulthood to a greater degree in males than in females. Experimental infection has a greater effect on TL in male than female mice (Ilmonen et al., 2008), human males have higher infant mortality rates from infections than females (Drevenstedt et al., 2008), and females display higher levels of estrogens (Kuiri-Hänninen et al., 2013; but see Soldin et al., 2005; Ji et al., 2008) which may be protective against BTL attrition (Aviv, 2002; Misiti et al., 2000). These suggest that males might experience greater lingering biological costs from infection and subsequent BTL shortening than females.
Methods
Data collection
Data came from the Cebu Longitudinal Health and Nutrition Survey (CLHNS), a birth cohort study in Metropolitan Cebu, Philippines that began with enrollment of 3,327 pregnant mothers in 1983–1984 (Adair et al., 2011). Longitudinal data are available for download at https://dataverse.unc.edu/dataverse/cebu. Mothers came from randomly-selected rural and urban neighborhoods. The mothers, and their 1983–1984 born offspring have been surveyed since. These surveys consisted of bimonthly surveys for the first two years of the offsprings’ life followed by surveys in 1991, 1994, 1998, 2002 and 2005. In the bimonthly surveys mothers were asked about their breastfeeding practices and about whether their infant had diarrhea in the last day and last week. In 2005 venous blood was drawn from all the mothers and their 1983–84 born offspring that had remained in the survey (21.7 ± 0.3 years old). Descriptive statistics for key variables used in analyses are given in Table 1.
Table 1.
Descriptive statistics of key variables
| Variable | N | Mean | SD |
|---|---|---|---|
| Diarrhea Birth–6 m | 1742 | 0.13 | 0.21 |
| Diarrhea 6–12 m | 1711 | 0.24 | 0.27 |
| Diarrhea 12–24 m | 1713 | 0.20 | 0.20 |
| Breastfeeding (days) | 1662 | 58.51 | 39.17 |
| Age (years) | 1774 | 21.68 | 0.35 |
| Assets 83 | 1776 | 2.49 | 1.88 |
| Assets 86 | 1680 | 2.60 | 1.90 |
| Assets 05 | 1772 | 5.30 | 1.95 |
| Log income 83 | 1765 | 5.46 | 0.71 |
| Log income 86 | 1672 | 5.35 | 0.65 |
| Log income 05 | 1772 | 6.15 | 0.72 |
| Maternal years of education | 1776 | 7.83 | 3.76 |
| Paternal years of education | 1750 | 8.28 | 3.95 |
| Urbanicity | 1776 | 35.61 | 12.51 |
| Maternal height (cm) | 1776 | 151.46 | 4.96 |
| Father's age at conception | 1733 | 28.54 | 6.73 |
| Sex (% Male) | 1776 | 52.70 |
Informed consent was obtained from all participants and data collection was conducted with approval and oversight from the Institutional Review Boards of the University of North Carolina at Chapel Hill and Northwestern University. Telomere measurement and analysis of de-identified samples and data was not considered human subjects research by Northwestern University’s Institutional Review Board.
Telomere length measurement
Automated and manual DNA extraction (Puregene, Gentra) was conducted on the venous blood collected in 2005 from 1,779 offspring. DNA isolated from blood by these methods is widely considered to be of leukocyte origin. While this is no doubt mostly true, there are other possible cellular and non-cellular sources of DNA found in blood (Eisenberg, 2011; Hermansen, 2001; Stachon et al., 2004; Lo et al., 1998; Lo et al., 2010; Chan et al., 2006) necessitating a distinction be drawn between BTL and TL exclusively leukocyte in origin. Telomere lengths were measured using the monochrome multiplex quantitative polymerase chain reaction assay (2009) with the following modifications. Reactions were run with telomere primers (telg/telc) at 500 nM each and albumin (single-copy control) primers (albd/albu) at 300 nM each on a Bio-Rad iCycler iQ thermocycler with a modified thermo-profile: internal well factor collection for 1.5 min at 95°C, denaturation and Taq activation for 13.5 m at 95°, 2 repeats of: 2 s at 98° followed by 30 s at 49°, 34 repeats of: 2 s at 98°, 30 s at 59°, 15 s at 74° with signal acquisition, 30 s at 84°, 15 s at 85° with signal acquisition, followed by a melt curve for PCR product verification. Data were analyzed with a per-well efficiency calculation method (Ehrlenbach et al., 2009; Ehrlenbach et al., 2010; Willeit et al., 2010) using LinRegPCR version 12.7 (Ramakers et al., 2003; Ruijter et al., 2009). All T/S ratios were normalized to (divided by) the same control sample run with six replicates per 96 well plate. Further details on methodology and validation of these TL measures have been described previously, including a correction for systematic variation due to well position on the thermocycler (Eisenberg et al., 2015).
A subsample of 190 of these samples show a correlation between MMQPCR measures and southern blot of terminal restriction fragments (r = 0.663) that is on par with recent qPCR TL validation efforts (Eisenberg et al., 2015; Elbers et al., 2013). Since the coefficient of variation (CV) has recently been recognized to be an invalid statistic to assess TL measurement reliability, we instead use the intraclass correlation coefficient (ICC) (Eisenberg, 2016; Verhulst et al., 2015) which estimates the percent of variation attributable to individuals versus to measurement error. Individual and average ICCs were calculated using a one-way random effects model to calculate absolute agreement between the averages of the same samples run in triplicate on different runs with the ICC command in Stata 14.1. Individual and average ICC values correspond to ICC(1) and ICC(k) in McGraw and Wong (1996). Individual ICC gives an estimate of the reliability of measures of samples analyzed on one run (in triplicate), while average ICC gives an estimate of the reliability of the average TL estimate of a sample measured across multiple runs. While considerable numbers of samples in these analyses were included on multiple runs, these samples were re-run because of initially high intra-assay CVs. 873 of the samples were run separately in triplicate on two separate runs and had an individual ICC of 0.81 (95% CI: 0.79–0.84) and average ICC of 0.89 (95% CI 0.88–0.91). 118 of the samples were run separately in triplicate on three separate runs and had an individual ICC of 0.87 (95% CI: 0.83–0.91) and average ICC of 0.95 (95% CI: 0.94–0.97). Conventional rules of thumb suggest that these ICC values are good (Cicchetti, 1994). Since this ICC represents a subset of more noisy measures than the population of measures, this ICC is an under-estimate of the true inter-run reliability and it is not surprising that it is slightly lower than the only other reports of ICC values in the telomere biology coming from results from the lab which originated the qPCR assay (Eisenberg et al., 2016).
Statistical analysis
Maternally reported diarrhea of the infant at each bimonthly survey was used as an estimate of diarrheal prevalence in a similar manner as in Kuzawa et al (2010). First, a dichotomous variable was created for each survey, scoring whether the child had reported any cases of diarrhea within the past 24 hours and/or the past week or no reported episodes. Then these values were averaged to create indices for the first half year of life (surveys at 2, 4 and 6 months of age), second half year (surveys at 8, 10 and 12 months) and for the second year of age (14, 16, 18, 20, 22, 24 months old). These average values can be interpreted as estimated individual weekly diarrheal prevalence during each time period (see Figure 1 and Table 1). All three diarrheal morbidity variables are used simultaneously as predictors of TL in a regression model. To address multiple-testing issues, Wald joint significance tests are utilized (Cohen et al., 2003). That is, the null hypothesis that diarrheal morbidity has no association with telomere length was tested by calculating the joint significance of all of the three diarrheal measures simultaneously (Table 2: Line 9).
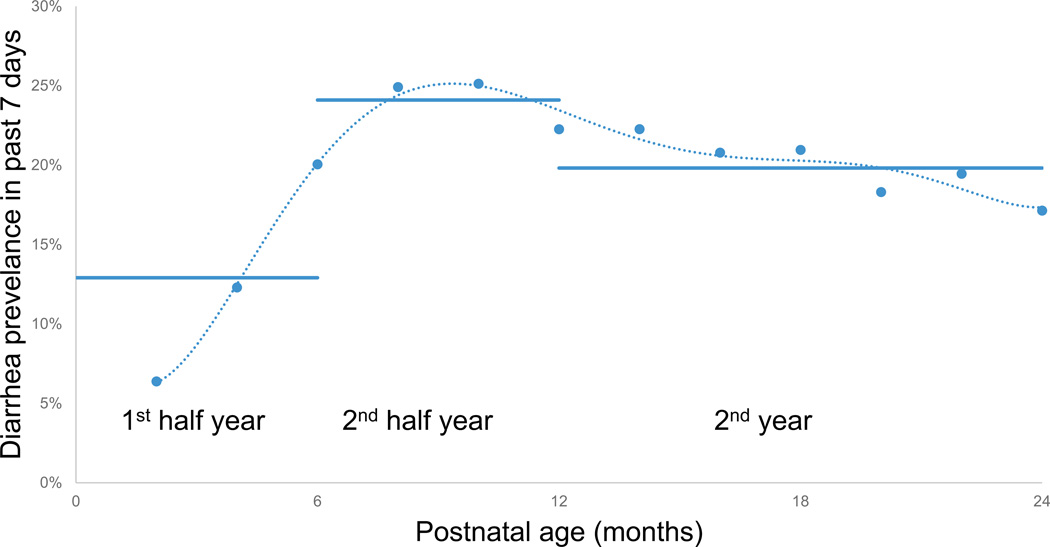
Figure 1.
Average diarrheal prevalence reported in the past seven days at each bimonthly survey (n = 1,660 to 1,724). Horizontal lines show means of weekly diarrheal prevalence for each period. From figure for diarrheal episodes in Cebu.xlsx
Table 2.
Imputation OLS regression models of diarrheal prevalence and breastfeeding duration predicting blood telomere lengths measured in 1,759 young adults.
| Line | Variable Model: | Minimum1 | Maximum2 |
|---|---|---|---|
| 1 | Diarrhea Birth–6 m | 0.0077 | 0.00082 |
| 2 | Diarrhea 6–12 m | −0.064** | −0.053* |
| 3 | Diarrhea 12–24 m | −0.019 | −0.030 |
| 4 | Breastfeeding | 0.00012 | 0.00015 |
| 5 | Diarrhea Birth–6 m*Sex | −0.035 | −0.022 |
| 6 | Diarrhea 6–12 m*Sex | 0.061+ | 0.045 |
| 7 | Diarrhea 12–24 m*Sex | 0.023 | 0.017 |
| 8 | Breastfeeding*Sex | −0.00029 | −0.00021 |
| 9 | Wald joint sig 1–3 p value: | 0.026 | 0.048 |
Values above the line are β coefficients;
p < 0.10,
p < 0.05,
p < 0.01,
p < 0.001
controls for age in 2005, sex, and age × sex.
additionally controls for logged household income and assets, at birth, two years old and 22 years old, maternal years of education, paternal years of education, maternal height, urbanicity, paternal age at birth, and the first ten principal components of genetic variation. Complete regression statistics including for control variables are included in Supplementary Table 1.
To measure the immune-protective benefits of breastfeeding, the duration of exclusive breastfeeding (with allowances for supplementation with non-nutritive liquids such as teas, brews and plain water) which has been previously shown to predict diarrheal morbidity (VanDerslice et al., 1994), is used as a predictor of adult TL. Like reported diarrhea, breastfeeding data come from the bimonthly surveys for the first two years of life where mothers gave a 24 hour recall of what the child consumed.
Control variables included sex, deflated household income and assets in 1983, 1986 (coincident with diarrheal morbidity measures) and 2005, average urbanicity score between 1983 and 2005 (Dahly and Adair, 2007) and age in 2005 (when blood collection for TL analysis occurred), years of maternal and paternal education, maternal height and paternal age at birth (Eisenberg et al., 2012). The income variables were logged reflecting a probable multiplicative rather than additive effect. Additionally, to control for potential population structure effects, principal components (PCs) of genome-wide genetic variation were considered. The derivation of these principal components have been described previously (Wu et al., 2011; Croteau-Chonka et al., 2012; Croteau-Chonka et al., 2011). As in previous analyses (Bethancourt et al., 2015), the bivariate association between the first ten principal components and TL were tested. The top principal components up to and including the last one showing a significant bivariate association with TL were retained as control variables. Results of regression models with the key variables of interest are shown in Table 2 while complete regression results including statistics for control variables are provided in Supplementary Table 1. All tests were two-tailed with α = 0.05.
To improve statistical power and minimize potential bias in analyses we used imputation of missing data (multiple imputation by chained equations in Stata 13.1). To accommodate tests of statistical interactions with sex, imputations were conducted separately in males and females. Indices of average diarrheal prevalence in the first half year of life, second half year and for the second year of age and of average urbanicity were generated uniquely in each imputation (‘mi passive’ command). Following standard imputation methods, variables used for the imputation phase included all of those used in the regression models plus 30 auxiliary variables. All variables used in imputation models and their percent missing are given in Supplementary Table 2. 30 imputations were conducted each with a burn-in period of 20. Monte Carlo errors for all significant associations reported in Table 2 were smaller than recommended thresholds for betas, T-statistics and P-values (White et al., 2011). While results from imputation models are reported here because they are the most reliable estimates of effects, we note that regression results using the more common “complete case only” method yields virtually identical results.
Results
Diarrheal prevalence was measured through 12 prospective bimonthly surveys in the first two years of children’s lives. Maternally reported diarrheal prevalence over the previous week was 6.4% at 2 months postpartum, increased to 25.1% by 10 months and then gradually declined to 17.1% by 24 months (Figure 1). Hypothesis 1, that increased early life diarrheal morbidity predicts adult BTL, was tested in regression models with BTL measured in 1,759 individuals. With both minimum and maximum statistical controls, diarrheal prevalence in the first two years of life significantly predicted adult telomere length (Table 2: Line 9). Examined more closely in different time periods, prevalence from 6–12 months, when diarrheal morbidity was at its peak (Figure 1) appeared to be driving the association of diarrheal prevalence with shorter adult BTL (Table 2: Line 2 and Figure 2). The effect was also in the expected direction for the second year of life (Table 2: Line 3 and Figure 2), but was not significant (p = 0.31 in maximum controlled model).
Figure 2.
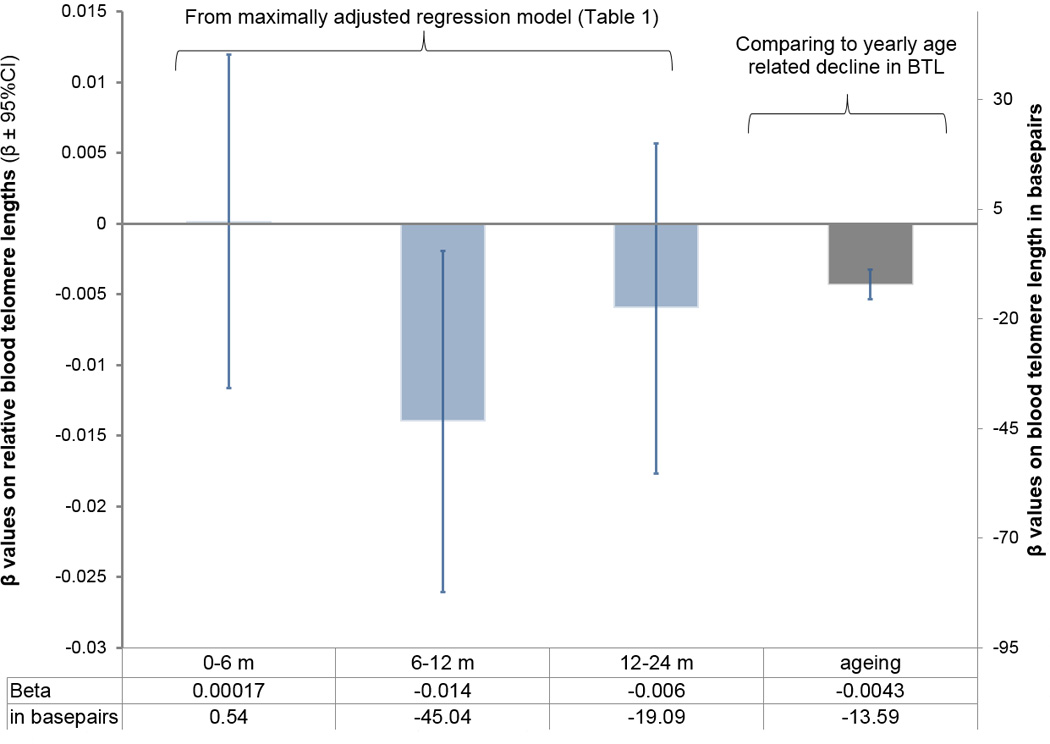
Comparing effect sizes of 1 SD changes in diarrheal morbidity on adult BTL with age related decline in adult BTL (from maximum controlled model in Table 1).
Contrary to our prediction (H2) breastfeeding duration did not predict adult TL (Table 2: Line 4). Also contrary to our predictions (H3), there were no significant interactions between sex and diarrheal morbidity nor sex and breastfeeding duration (Table 2: Lines 5–8). There was a trend towards significance of diarrheal morbidity in 6–12 months varying by sex in the minimally controlled model (p = 0.053; Line 6), but this effect was opposite the expected direction and the p value increased to 0.14 in the maximally controlled model.
To quantify the effect size of early life diarrheal morbidity on adult BTL, we converted our values into base pairs (bp) using southern blot measures from a subset of these data (Eisenberg et al., 2015) and compared these effects to the age related decline in TL. As illustrated in Figure 2, in the maximum controlled model, there is an estimated 45.0 bp decrease in adult TL for a single SD increase in diarrhea prevalence at 6–12 m (SD = 0.27). The age related decline in BTL in 36–69 year old women in this population was previously found to be 0.0043 relative telomere length units (n = 1,845, p = 7.19 × 10−16)(Eisenberg et al., 2012) or 13.6 bp per year. This implies that a 1 SD increase in diarrheal morbidity is equivalent to 3.3 years of telomeric aging in adulthood.
Discussion
We predicted that increased diarrheal morbidity early in life causes decreased BTL that lingers into adulthood (H1), that longer breastfeeding duration—which generally is protective against diarrhea—would be associated with increased BTL (H2) and that both effects (in H1 and H2) would be greater in males than females (H3). Using prospectively collected longitudinal data from the Philippines, we find support for H1, that increased diarrheal morbidity predicts shorter adult BTL, but no support for H2 or H3. Our lack of support for an association between breastfeeding and adult BTL (H2) is inconsistent with a recent study focusing on a small sample (Wojcicki et al., 2016). The association of early life infectious disease with adult BTL (H1) fits within a broader literature on the developmental origin of health and disease (DOHaD) and suggest that telomeres are a pathway by which early life environment and health might influence adult morbidity and mortality.
Early life diarrheal morbidity shows the predicted association with adult TL. When parsed out by age, the association is driven by morbidity during months 6–12 of age. This is noteworthy because this period roughly corresponds to the typical timing of weaning and increased child mobility and exploration. These developmental shifts mark the first time when pathogens tend to be introduced en masse without the immunological protective effects of breast milk and as a result infectious diseases reach a lifetime peak in Cebu and most other populations (Kuzawa, 1998; Victora et al., 1989; Mølbak et al., 1994; Yoon et al., 1996; Meremikwu et al., 1997). The much greater BTL attrition rate in the first few years of life than in adulthood (Frenck et al., 1998; Zeichner et al., 1999), viewed alongside our findings, raises the possibility that the first few years of life represent a sensitive period for influencing TL throughout life.
The magnitudes of changes in TL predicted by diarrheal morbidity may be large enough to have important biological effects. Our results suggest that a one standard deviation increase in diarrheal prevalence during 6–12 months causes a shortening of adult BTL by 45 bp—or the equivalent to 3.3 years of telomeric aging in adulthood. In comparison, smoking is thought to cause an estimated 3 bp/year steeper decline in BTL with age (Benetos et al., 2013). Recent evidence from experimental infection of a bird with avian malaria showed TL shortening in not just blood, but also liver, lung, spleen, heart, kidney and brain (Asghar et al., 2016). BTL is a marker of the TL of hematopoietic stem cells and shorter hematopoietic stem cell TL could have important effects on leukocyte and erythrocyte production. However, if infection also causes TL shortening in other organs in humans, as in this bird model, the physiological effects could be far broader.
There are clear reasons to believe that the observed association between early life diarrheal morbidity and adult BTL in this study reflects a causal role of infection on BTL. Telomeres are known to shorten with each round of cell division, especially in the presence of oxidative stress. Infection causes increased immune cell proliferation and oxidative stress which should thus shorten BTL. However, we cannot rule out the alternative that this association is due entirely or in part to individuals with shorter TL in infancy being more susceptible to infectious diseases. Indeed, in late life, shorter TL predicts increased infectious disease related mortality (Cawthon et al., 2003), and even in young adulthood, those with shorter TL are more susceptible to developing infection after experimental exposure to the cold virus (Cohen et al., 2013). Since TL is highly heritable (Hunt et al., 2008; Vasa-Nicotera et al., 2005; Andrew et al., 2006; Slagboom et al., 1994; Bakaysa et al., 2007; Bischoff et al., 2005), much of the population variation in TL is evident at birth (Benetos et al., 2013), and adults with short TL at baseline tend to have relatively short TL later in life (Benetos et al., 2013), it is possible that those with shorter TL early in life retain this trait into adulthood. In this regard, it is notable that the lack of association between breastfeeding duration and adult TL is potentially consistent with TL being causally related to early life infection susceptibility. Future longitudinal studies including BTL measured before and after early life infections, or Mendelian randomization studies examining whether genetic polymorphisms associated with TL predict infant morbidity and mortality rates, could help clarify the direction of causation.
In sum, we find that diarrheal morbidity at the age of weaning and peak infectious disease burden in this Filipino sample predict shorter BTL measured two decades later in young adulthood. This finding is consistent with our hypothesis that increased early life immune activation leads to an accelerated pace of telomere shortening in immune cells, which speculatively could manifest as increased susceptibility to infection later in life. Research with longitudinal blood sampling or Mendelian randomization approaches will be needed to rule out the competing hypothesis that short TL at birth increases susceptibility to infection.
Supplementary Material
Acknowledgments
We thank Karen Mohlke for sharing aliquots of extracted DNA and genetic information. Amy Klegarth, Hilary Bethancourt, Dan Grunspan, Erin Masterson, Tiffany Pan, Tim Moore and Robert Tennyson provided valuable feedback on earlier iterations of this manuscript. We especially thank the many researchers at the USC-Office of Population Studies Foundation, Inc., University of San Carlos, Cebu, Philippines, for their central role in study design and data collection, and the Filipino participants, who provided their time and samples for this study.
Grants sponsorship: qPCR laboratory analysis were funded by NSF (Doctoral Dissertation Improvement Grant BCS-0962282), the Wenner-Gren Foundation (Gr. 8111) and institutional support from Northwestern University; data and sample collection funded by the NIH (grants TW05596, DK078150, RR20649, ES10126, and DK056350).
Footnotes
Author contributions
DTAE carried out the telomere length analyses, conducted the data analysis, participated in the design of the study and drafted the manuscript; JBB had a key role in collection of field data and contributed to the manuscript; MGH participated in the design of the study, oversaw lab work, and contributed to the manuscript. CWK participated in the design of the study, oversaw collection of field data and contributed to the manuscript.
References
- Adaikalakoteswari A, Balasubramanyam M, Ravikumar R, Deepa R, Mohan V. Association of telomere shortening with impaired glucose tolerance and diabetic macroangiopathy. Atherosclerosis. 2007;195(1):83–89. doi: 10.1016/j.atherosclerosis.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Adair LS, Popkin BM, Akin JS, Guilkey DK, Gultiano S, Borja J, Perez L, Kuzawa CW, McDade T, Hindin MJ. Cohort profile: the Cebu longitudinal health and nutrition survey. Int J Epidemiol. 2011;40(3):619–625. doi: 10.1093/ije/dyq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew T, Aviv A, Falchi M, Surdulescu GL, Gardner JP, Lu X, Kimura M, Kato BS, Valdes AM, Spector TD. Mapping Genetic Loci That Determine Leukocyte Telomere Length in a Large Sample of Unselected Female Sibling Pairs. The American Journal of Human Genetics. 2006;78(3):480–486. doi: 10.1086/500052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar M, Hasselquist D, Hansson B, Zehtindjiev P, Westerdahl H, Bensch S. Hidden costs of infection: Chronic malaria accelerates telomere degradation and senescence in wild birds. Science. 2015;347(6220):436–438. doi: 10.1126/science.1261121. [DOI] [PubMed] [Google Scholar]
- Asghar M, Palinauskas V, Zaghdoudi-Allan N, Valkiūnas G, Mukhin A, Platonova E, Färnert A, Bensch S, Hasselquist D. Parallel telomere shortening in multiple body tissues owing to malaria infection. Proceedings of the Royal Society of London B: Biological Sciences. 2016;283(1836) doi: 10.1098/rspb.2016.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv A. Telomeres, sex, reactive oxygen species, and human cardiovascular aging. J Mol Med. 2002;80(11):689–695. doi: 10.1007/s00109-002-0377-8. [DOI] [PubMed] [Google Scholar]
- Aviv A, Valdes A, Gardner JP, Swaminathan R, Kimura M, Spector TD. Menopause Modifies the Association of Leukocyte Telomere Length with Insulin Resistance and Inflammation. J Clin Endocrinol Metab. 2006;91(2):635–640. doi: 10.1210/jc.2005-1814. [DOI] [PubMed] [Google Scholar]
- Bakaysa SL, Mucci LA, Slagboom PE, Boomsma DI, McClearn GE, Johansson B, Pedersen NL. Telomere length predicts survival independent of genetic influences. Aging Cell. 2007;6(6):769–774. doi: 10.1111/j.1474-9726.2007.00340.x. [DOI] [PubMed] [Google Scholar]
- Bendix L, Horn PB, Jensen UB, Rubelj I, Kolvraa S. The load of short telomeres, estimated by a new method, Universal STELA, correlates with number of senescent cells. Aging Cell. 2010;9(3):383–397. doi: 10.1111/j.1474-9726.2010.00568.x. [DOI] [PubMed] [Google Scholar]
- Benetos A, Kark JD, Susser E, Kimura M, Sinnreich R, Chen W, Steenstrup T, Christensen K, Herbig U, von Bornemann Hjelmborg J, et al. Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell. 2013;12(4):615–621. doi: 10.1111/acel.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson T, Lindström M. Airborne infectious diseases during infancy and mortality in later life in southern Sweden, 1766–1894. Int J Epidemiol. 2003;32(2):286–294. doi: 10.1093/ije/dyg061. [DOI] [PubMed] [Google Scholar]
- Bethancourt HJ, Kratz M, Hayes MG, Kuzawa CW, Borja JB, Duazo PL, Beresford SAA, Eisenberg DTA. No Association between Blood Telomere Length and Longitudinally-Assessed Diet or Adiposity or Diet in a Young Adult Filipino Population. Eur J Nutr. 2015 doi: 10.1007/s00394-015-1080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff C, Graakjaer J, Petersen HC, Hjelmborg JV, Vaupel JW, Bohr V, Koelvraa S, Christensen K. The heritability of telomere length among the elderly and oldest-old. Twin Res Hum Genet. 2005;8(5):433–439. doi: 10.1375/183242705774310141. [DOI] [PubMed] [Google Scholar]
- Blackburn EH, Gall JG. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J Mol Biol. 1978;120(1):33–53. doi: 10.1016/0022-2836(78)90294-2. [DOI] [PubMed] [Google Scholar]
- Carrero JJ, Stenvinkel P, Fellstrom B, Qureshi AR, Lamb K, Heimburger O, Barany P, Radhakrishnan K, Lindholm B, Soveri I, et al. Telomere attrition is associated with inflammation, low fetuin-A levels and high mortality in prevalent haemodialysis patients. J Intern Med. 2008;263(3):302–312. doi: 10.1111/j.1365-2796.2007.01890.x. [DOI] [PubMed] [Google Scholar]
- Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009;37(3):e21. doi: 10.1093/nar/gkn1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. The Lancet. 2003;361(9355):393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- Chan CW, Garruto RM, Lum JK. Paleoparasite Populations from Archived Sera: Insights into Chloroquine Drug Resistance in Papua New Guinea. J Infect Dis. 2006;194(7):1023–1024. doi: 10.1086/507433. [DOI] [PubMed] [Google Scholar]
- Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess. 1994;6(4):284. [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. xxviii. Mahwah, N.J.: L. Erlbaum Associates; 2003. p. 703. [Google Scholar]
- Cohen S, Janicki-Deverts D, Turner RB, Casselbrant ML, Li-Korotky HS, Epel ES, Doyle WJ. Association between telomere length and experimentally induced upper respiratory viral infection in healthy adults. JAMA. 2013;309(7):699–705. doi: 10.1001/jama.2013.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau-Chonka DC, Marvelle AF, Lange EM, Lee NR, Adair LS, Lange LA, Mohlke KL. Genome-wide association study of anthropometric traits and evidence of interactions with age and study year in Filipino women. Obesity (Silver Spring) 2011;19(5):1019–1027. doi: 10.1038/oby.2010.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau-Chonka DC, Wu Y, Li Y, Fogarty MP, Lange LA, Kuzawa CW, McDade TW, Borja JB, Luo J, AbdelBaky O, et al. Population-specific coding variant underlies genome-wide association with adiponectin level. Hum Mol Genet. 2012;21(2):463–471. doi: 10.1093/hmg/ddr480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahly DL, Adair LS. Quantifying the urban environment: A scale measure of urbanicity outperforms the urban–rural dichotomy. Soc Sci Med. 2007;64(7):1407–1419. doi: 10.1016/j.socscimed.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling DK, Simmons LW. Reactive oxygen species as universal constraints in life-history evolution. Proceedings of the Royal Society B: Biological Sciences. 2009;276(1663):1737–1745. doi: 10.1098/rspb.2008.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevenstedt GL, Crimmins EM, Vasunilashorn S, Finch CE. The rise and fall of excess male infant mortality. Proc Natl Acad Sci. 2008;105(13):5016–5021. doi: 10.1073/pnas.0800221105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros RB, Allsopp R, Chiu C-p, Hausner MA, Hirji K, Wang L, Harley CB, Villeponteau B, West MD, Giorgi JV. Shortened telomeres in the expanded CD28-CD8+ cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS. 1996;10(8):F17–F22. doi: 10.1097/00002030-199607000-00001. [DOI] [PubMed] [Google Scholar]
- Ehrlenbach S, Willeit P, Kiechl S, Willeit J, Reindl M, Schanda K, Kronenberg F, Brandstatter A. Influences on the reduction of relative telomere length over 10 years in the population-based Bruneck Study: introduction of a well-controlled high-throughput assay. Int J Epidemiol. 2009;38(6):1725–1734. doi: 10.1093/ije/dyp273. [DOI] [PubMed] [Google Scholar]
- Ehrlenbach S, Willeit P, Kiechl S, Willeit J, Reindl M, Schanda K, Kronenberg F, Brandstatter A. Raising the bar on telomere epidemiology. Int J Epidemiol. 2010;39(1):308–309. doi: 10.1093/ije/dyp383. [DOI] [PubMed] [Google Scholar]
- Eisenberg DT, Hayes MG, Kuzawa CW. Delayed paternal age of reproduction in humans is associated with longer telomeres across two generations of descendants. Proc Natl Acad Sci. 2012;109(26):10251–10256. doi: 10.1073/pnas.1202092109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg DT, Kuzawa CW, Hayes MG. Improving qPCR telomere length assays: Controlling for well position effects increases statistical power. Am J Hum Biol. 2015;27(4):570–575. doi: 10.1002/ajhb.22690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg DTA. An evolutionary review of human telomere biology: The thrifty telomere hypothesis and notes on potential adaptive paternal effects. Am J Hum Biol. 2011;23(2):149–167. doi: 10.1002/ajhb.21127. [DOI] [PubMed] [Google Scholar]
- Eisenberg DTA. Telomere length measurement validity: the coefficient of variation is invalid and cannot be used to compare quantitative polymerase chain reaction and Southern blot telomere length measurement techniques. Int J Epidemiol. 2016 doi: 10.1093/ije/dyw191. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Eisenberg DTA, Tackney J, Cawthon RM, Cloutier CT, Hawkes K. Paternal and grandpaternal ages at conception and descendant telomere lengths in chimpanzees and humans. Am J Phys Anthropol. 2016 doi: 10.1002/ajpa.23109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbers CC, Garcia ME, Kimura M, Cummings SR, Nalls MA, Newman AB, Park V, Sanders JL, Tranah GJ, Tishkoff SA, et al. Comparison Between Southern Blots and qPCR Analysis of Leukocyte Telomere Length in the Health ABC Study. J of Gerontol Ser A: Bio Sci and Med Sci. 2013;69(5):527–531. doi: 10.1093/gerona/glt121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzaneh-Far R, Lin J, Epel E, Lapham K, Blackburn E, Whooley MA. Telomere length trajectory and its determinants in persons with coronary artery disease: longitudinal findings from the heart and soul study. PLoS ONE. 2010;5(1):e8612. doi: 10.1371/journal.pone.0008612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE, Crimmins EM. Inflammatory exposure and historical changes in human life-spans. Science. 2004;305(5691):1736–1739. doi: 10.1126/science.1092556. [DOI] [PubMed] [Google Scholar]
- Fischer Walker CL, Aryee MJ, Boschi-Pinto C, Black RE. Estimating Diarrhea Mortality among Young Children in Low and Middle Income Countries. PLoS ONE. 2012;7(1):e29151. doi: 10.1371/journal.pone.0029151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenck RW, Jr, Blackburn EH, Shannon KM. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci U S A. 1998;95(10):5607–5610. doi: 10.1073/pnas.95.10.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianesin K, Noguera-Julian A, Zanchetta M, Del Bianco P, Petrara MR, Freguja R, Rampon O, Fortuny C, Camós M, Mozzo E. Premature aging and immune senescence in HIV-infected children. AIDS. 2016;30(9):1363–1373. doi: 10.1097/QAD.0000000000001093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesbrecht CJ, Thornton AE, Hall-Patch C, Maan EJ, Côté HCF, Money DM, Murray M, Pick N. Select Neurocognitive Impairment in HIV-Infected Women: Associations with HIV Viral Load, Hepatitis C Virus, and Depression, but Not Leukocyte Telomere Length. PLoS ONE. 2014;9(3):e89556. doi: 10.1371/journal.pone.0089556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley C. Telomere loss: mitotic clock or genetic time bomb? Mutat Res. 1991;256(2–6):271–282. doi: 10.1016/0921-8734(91)90018-7. [DOI] [PubMed] [Google Scholar]
- Hermansen MC. Nucleated red blood cells in the fetus and newborn. Arch Dis Child Fetal Neonatal Ed. 2001;84(3):F211–F215. doi: 10.1136/fn.84.3.F211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama E, Hiyama K. Telomere and telomerase in stem cells. Br J Cancer. 2007;96(7):1020–1024. doi: 10.1038/sj.bjc.6603671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horta BL, Victora CG. Long-term effects of breastfeeding-a systematic review. World Health Organization; 2013. [Google Scholar]
- Hou L, Savage SA, Blaser MJ, Perez-Perez G, Hoxha M, Dioni L, Pegoraro V, Dong LM, Zatonski W, Lissowska J, et al. Telomere length in peripheral leukocyte DNA and gastric cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18(11):3103–3109. doi: 10.1158/1055-9965.EPI-09-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt SC, Chen W, Gardner JP, Kimura M, Srinivasan SR, Eckfeldt JH, Berenson GS, Aviv A. Leukocyte telomeres are longer in African Americans than in whites: the National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging Cell. 2008;7(4):451–458. doi: 10.1111/j.1474-9726.2008.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilmonen P, Kotrschal A, Penn DJ. Telomere Attrition Due to Infection. PLoS ONE. 2008;3(5):e2143. doi: 10.1371/journal.pone.0002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam T, Jitratkosol MHJ, Soudeyns H, Sattha B, Gadawski I, Maan E, Forbes JC, Alimenti A, Lapointe N, Lamarre V, et al. Leukocyte Telomere Length in HIV-Infected Pregnant Women Treated With Antiretroviral Drugs During Pregnancy and Their Uninfected Infants. JAIDS J Acquir Immune Defic Syndr. 2012;60(5):495–502. doi: 10.1097/QAI.0b013e31825aa89c. 410.1097/QAI.1090b1013e31825aa31889c. [DOI] [PubMed] [Google Scholar]
- Ishii A, Nakamura K, Kishimoto H, Honma N, Aida J, Sawabe M, Arai T, Fujiwara M, Takeuchi F, Kato M, et al. Telomere shortening with aging in the human pancreas. Exp Gerontol. 2006;41(9):882–886. doi: 10.1016/j.exger.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Ji C, Huang X-w, Yang R-w, Wang X, Zhao Z-y. Gonadotropins and sex hormones in healthy Chinese infants. Indian Pediatr. 2008;45(6):489. [PubMed] [Google Scholar]
- Kimura M, Cherkas LF, Kato BS, Demissie S, Hjelmborg JB, Brimacombe M, Cupples A, Hunkin JL, Gardner JP, Lu X, et al. Offspring's leukocyte telomere length, paternal age, and telomere elongation in sperm. PLoS Genet. 2008;4(2):e37. doi: 10.1371/journal.pgen.0040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiri-Hänninen T, Haanpää M, Turpeinen U, Hämäläinen E, Seuri R, Tyrväinen E, Sankilampi U, Dunkel L. Postnatal Ovarian Activation Has Effects in Estrogen Target Tissues in Infant Girls. The Journal of Clinical Endocrinology & Metabolism. 2013;98(12):4709–4716. doi: 10.1210/jc.2013-1677. [DOI] [PubMed] [Google Scholar]
- Kuzawa CW. Adipose tissue in human infancy and childhood: An evolutionary perspective. Yearbook of Physical Anthropology. 1998;41 – 1998:177–209. doi: 10.1002/(sici)1096-8644(1998)107:27+<177::aid-ajpa7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Kuzawa CW, Eisenberg DTA. The Long Reach of History: Intergenerational Pathways to Plasticity in Human Lifespan. In: Weinstein M, Lane MA, editors. Sociality, hierarchy, health comparative biodemography : a collection of papers. Washington, DC: The National Academies Press; 2014. pp. 65–94. [PubMed] [Google Scholar]
- Kuzawa CW, McDade TW, Adair LS, Lee N. Rapid weight gain after birth predicts life history and reproductive strategy in Filipino males. Proc Natl Acad Sci. 2010;107(23):16800–16805. doi: 10.1073/pnas.1006008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo YM, Chan KC, Sun H, Chen EZ, Jiang P, Lun FM, Zheng YW, Leung TY, Lau TK, Cantor CR, et al. Maternal Plasma DNA Sequencing Reveals the Genome-Wide Genetic and Mutational Profile of the Fetus. Sci Transl Med. 2010;2(61):61ra91. doi: 10.1126/scitranslmed.3001720. [DOI] [PubMed] [Google Scholar]
- Lo YMD, Tein MSC, Lau TK, Haines CJ, Leung TN, Poon PMK, Wainscoat JS, Johnson PJ, Chang AMZ, Hjelm NM. Quantitative Analysis of Fetal DNA in Maternal Plasma and Serum: Implications for Noninvasive Prenatal Diagnosis. The American Journal of Human Genetics. 1998;62(4):768–775. doi: 10.1086/301800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maini MK, Soares MV, Zilch CF, Akbar AN, Beverley PC. Virus-induced CD8+ T cell clonal expansion is associated with telomerase up-regulation and telomere length preservation: a mechanism for rescue from replicative senescence. J Immunol. 1999;162(8):4521–4526. [PubMed] [Google Scholar]
- Malan-Müller S, Hemmings SMJ, Spies G, Kidd M, Fennema-Notestine C, Seedat S. Shorter Telomere Length - A Potential Susceptibility Factor for HIV-Associated Neurocognitive Impairments in South African Woman. PLoS ONE. 2013;8(3):e58351. doi: 10.1371/journal.pone.0058351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, Worthman CM. The weanling's dilemma reconsidered: A biocultural analysis of breastfeeding ecology. J Dev Behav Pediatr. 1998;19(4):286–299. doi: 10.1097/00004703-199808000-00008. [DOI] [PubMed] [Google Scholar]
- McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol Methods. 1996;1(1):30. [Google Scholar]
- Meremikwu M, Asindi A, Antia-Obong O. The influence of breast feeding on the occurrence of dysentery, persistent diarrhoea and malnutrition among Nigerian children with diarrhoea. West Afr J Med. 1997;16(1):20. [PubMed] [Google Scholar]
- Misiti S, Nanni S, Fontemaggi G, Cong Y-S, Wen J, Hirte HW, Piaggio G, Sacchi A, Pontecorvi A, Bacchetti S, et al. Induction of hTERT Expression and Telomerase Activity by Estrogens in Human Ovary Epithelium Cells. Mol Cell Biol. 2000;20(11):3764–3771. doi: 10.1128/mcb.20.11.3764-3771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mølbak Kr, Aaby P, Højlyng N, da Silva APJ. Risk Factors for Cryptosporidlum Diarrhea in Early Childhood: A Case Study from Guinea-Blssau, West Africa. Am J Epidemiol. 1994;139(7):734–740. doi: 10.1093/oxfordjournals.aje.a117064. [DOI] [PubMed] [Google Scholar]
- O'Donovan A, Lin J, Tillie J, Dhabhar FS, Wolkowitz OM, Blackburn EH, Epel ES. Pessimism correlates with leukocyte telomere shortness and elevated interleukin-6 in post-menopausal women. Brain Behav Immun. 2009;23(4):446–449. doi: 10.1016/j.bbi.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan A, Pantell MS, Puterman E, Dhabhar FS, Blackburn EH, Yaffe K, Cawthon RM, Opresko PL, Hsueh W-C, Satterfield S, et al. Cumulative Inflammatory Load Is Associated with Short Leukocyte Telomere Length in the Health, Aging and Body Composition Study. PLoS ONE. 2011;6(5):e19687. doi: 10.1371/journal.pone.0019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeppen J, Vaupel JW. Demography. Broken limits to life expectancy. Science. 2002;296(5570):1029–1031. doi: 10.1126/science.1069675. [DOI] [PubMed] [Google Scholar]
- Oikawa S, Kawanishi S. Site-specific DNA damage at GGG sequence by oxidative stress may accelerate telomere shortening. FEBS Lett. 1999;453(3):365–368. doi: 10.1016/s0014-5793(99)00748-6. [DOI] [PubMed] [Google Scholar]
- Olivieri F, Lorenzi M, Antonicelli R, Testa R, Sirolla C, Cardelli M, Mariotti S, Marchegiani F, Marra M, Spazzafumo L, et al. Leukocyte telomere shortening in elderly Type2DM patients with previous myocardial infarction. Atherosclerosis. 2009;206(2):588–593. doi: 10.1016/j.atherosclerosis.2009.03.034. [DOI] [PubMed] [Google Scholar]
- Olovnikov AM. Principle of marginotomy in template synthesis of polynucleotides. Dokl Akad Nauk SSSR. 1971;201(6):1496–1499. [PubMed] [Google Scholar]
- Pathai S, Lawn SD, Gilbert CE, McGuinness D, McGlynn L, Weiss HA, Port J, Christ T, Barclay K, Wood R, et al. Accelerated biological ageing in HIV-infected individuals in South Africa: a case-control study. AIDS. 2013;27(15):2375–2384. doi: 10.1097/QAD.0b013e328363bf7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plunkett FJ, Soares MV, Annels N, Hislop A, Ivory K, Lowdell M, Salmon M, Rickinson A, Akbar AN. The flow cytometric analysis of telomere length in antigen-specific CD8+ T cells during acute Epstein-Barr virus infection. Blood. 2001;97(3):700–707. doi: 10.1182/blood.v97.3.700. [DOI] [PubMed] [Google Scholar]
- Pommier J-P, Gauthier L, Livartowski J, Galanaud P, Boué F, Dulioust A, Marcé D, Ducray C, Sabatier L, Lebeau J, et al. Immunosenescence in HIV Pathogenesis. Virology. 1997;231(1):148–154. doi: 10.1006/viro.1997.8512. [DOI] [PubMed] [Google Scholar]
- Popkin BM, Adair L, Akin JS, Black R, Briscoe J, Flieger W. Breast-feeding and diarrheal morbidity. Pediatrics. 1990;86(6):874–882. [PubMed] [Google Scholar]
- Ramakers C, Ruijter JM, Deprez RH, Moorman AF. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett. 2003;339(1):62–66. doi: 10.1016/s0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- Reed JR, Vukmanovic-Stejic M, Fletcher JM, Soares MV, Cook JE, Orteu CH, Jackson SE, Birch KE, Foster GR, Salmon M, et al. Telomere erosion in memory T cells induced by telomerase inhibition at the site of antigenic challenge in vivo. J Exp Med. 2004;199(10):1433–1443. doi: 10.1084/jem.20040178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter T, Zglinicki T. A continuous correlation between oxidative stress and telomere shortening in fibroblasts. Exp Gerontol. 2007;42(11):1039–1042. doi: 10.1016/j.exger.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Ruijter JM, Ramakers C, Hoogaars WM, Karlen Y, Bakker O, van den Hoff MJ, Moorman AF. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009;37(6):e45. doi: 10.1093/nar/gkp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpea KD, Talmud PJ, Cooper JA, Maubaret CG, Stephens JW, Abelak K, Humphries SE. Association of telomere length with type 2 diabetes, oxidative stress and UCP2 gene variation. Atherosclerosis. 2010;209(1):42–50. doi: 10.1016/j.atherosclerosis.2009.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson MJ, Winterbone MS, Hughes JC, Dozio N, Hughes DA. Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care. 2006;29(2):283–289. doi: 10.2337/diacare.29.02.06.dc05-1715. [DOI] [PubMed] [Google Scholar]
- Sanders JL, Fitzpatrick AL, Boudreau RM, Arnold AM, Aviv A, Kimura M, Fried LF, Harris TB, Newman AB. Leukocyte telomere length is associated with noninvasively measured age-related disease: The Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2012;67(4):409–416. doi: 10.1093/gerona/glr173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slagboom PE, Droog S, Boomsma DI. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1994;55(5):876–882. [PMC free article] [PubMed] [Google Scholar]
- Soldin OP, Hoffman EG, Waring MA, Soldin SJ. Pediatric reference intervals for FSH, LH, estradiol, T3, free T3, cortisol, and growth hormone on the DPC IMMULITE 1000. Clin Chim Acta. 2005;355(1):205–210. doi: 10.1016/j.cccn.2005.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solorio S, Murillo-Ortíz B, Hernández-González M, Guillén-Contreras J, Arenas-Aranda D, Solorzano-Zepeda FJ, Ruiz-Avila R, Mora-Villalpando C, de la Roca-Chiapas JM, Malacara-Hernández JM. Association Between Telomere Length and C-Reactive Protein and the Development of Coronary Collateral Circulation in Patients with Coronary Artery Disease. Angiology. 2011;62(6):467–472. doi: 10.1177/0003319710398007. [DOI] [PubMed] [Google Scholar]
- Srinivasa S, Fitch KV, Petrow E, Burdo TH, Williams KC, Lo J, Cote HC, Grinspoon SK. Soluble CD163 is associated with shortened telomere length in HIV-infected patients. J Acquir Immune Defic Syndr. 2014;67(4):414–418. doi: 10.1097/QAI.0000000000000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachon A, Holland-Letz T, Krieg M. High in-hospital mortality of intensive care patients with nucleated red blood cells in blood. Clin Chem Lab Med. 2004;42(8):933–938. doi: 10.1515/CCLM.2004.151. [DOI] [PubMed] [Google Scholar]
- VanDerslice J, Popkin B, Briscoe J. Drinking-water quality, sanitation, and breast-feeding: their interactive effects on infant health. Bull World Health Organ. 1994;72(4):589. [PMC free article] [PubMed] [Google Scholar]
- Vasa-Nicotera M, Brouilette S, Mangino M, Thompson JR, Braund P, Clemitson J-R, Mason A, Bodycote CL, Raleigh SM, Louis E, et al. Mapping of a Major Locus that Determines Telomere Length in Humans. The American Journal of Human Genetics. 2005;76(1):147–151. doi: 10.1086/426734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst S, Susser E, Factor-Litvak PR, Simons MJ, Benetos A, Steenstrup T, Kark JD, Aviv A. Commentary: The reliability of telomere length measurements. Int J Epidemiol. 2015 doi: 10.1093/ije/dyv166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora CG, Smith PG, Vaughan JP, Nobre LC, Lombardi C, Teixeira AMB, Fuchs SC, Moreira LB, Gigante LP, Barros FC. Infant feeding and deaths due to diarrhea. Am J Epidemiol. 1989;129(5):1032–1041. doi: 10.1093/oxfordjournals.aje.a115207. [DOI] [PubMed] [Google Scholar]
- Watson JD. Origin of concatemeric T7 DNA. Nat New Biol. 1972;239(94):197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- Willeit P, Willeit J, Mayr A, Weger S, Oberhollenzer F, Brandstatter A, Kronenberg F, Kiechl S. Telomere length and risk of incident cancer and cancer mortality. JAMA. 2010;304(1):69–75. doi: 10.1001/jama.2010.897. [DOI] [PubMed] [Google Scholar]
- Wojcicki JM, Heyman MB, Elwan D, Lin J, Blackburn E, Epel E. Early exclusive breastfeeding is associated with longer telomeres in Latino preschool children. The American Journal of Clinical Nutrition. 2016 doi: 10.3945/ajcn.115.115428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, McDade T, Kuzawa C, Borja J, Li Y, Adair L, Mohlke K, Lange L. Genome-wide Association with C-Reactive Protein Levels in CLHNS: Evidence for the CRP and HNF1A Loci and their Interaction with Exposure to a Pathogenic Environment. Inflammation. 2011:1–10. doi: 10.1007/s10753-011-9348-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon PW, Black RE, Moulton LH, Becker S. Effect of Not Breastfeeding on the Risk of Diarrheal and Respiratory Mortality in Children under 2 Years of Age in Metro Cebu, The Philippines. Am J Epidemiol. 1996;143(11):1142–1148. doi: 10.1093/oxfordjournals.aje.a008692. [DOI] [PubMed] [Google Scholar]
- Zanet DL, Thorne A, Singer J, Maan EJ, Sattha B, Le Campion A, Soudeyns H, Pick N, Murray M, Money DM, et al. Association Between Short Leukocyte Telomere Length and HIV Infection in a Cohort Study: No Evidence of a Relationship With Antiretroviral Therapy. Clin Infect Dis. 2014;58(9):1322–1332. doi: 10.1093/cid/ciu051. [DOI] [PubMed] [Google Scholar]
- Zeichner SL, Palumbo P, Feng Y, Xiao X, Gee D, Sleasman J, Goodenow M, Biggar R, Dimitrov D. Rapid telomere shortening in children. Blood. 1999;93(9):2824–2830. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.