Abstract
Objectives
Vitamin A (VA) is an essential micronutrient required for a range of biological functions throughout life. VA deficiency (VAD) claims an estimated 1 million preschool children’s lives annually. Human milk is enriched with VA (retinol) from the maternal blood, which originates from the hepatic reserve and dietary intake. Secreting retinol into milk will benefit the nursing infant through breast milk, but retaining retinol is also important for the maternal health. Previous studies found that the public health intervention of high-dose VA supplementation to lactating mothers did not significantly lower child mortality. WHO recently acknowledged that our understanding about the principle of VA allocation within the maternal system and the secretion into milk is too incomplete to devise an effective intervention.
Methods
We present a secondary analysis of data collected among lactating mothers in VAD endemic northern Kenya (n=171), examining nutritional, inflammatory, and ecological factors that might associate with maternal retinol allocation. Regression models were applied using the outcome milk-retinol allocation index: .
Results
Ten percent of the sample was identified as VAD. The average milk retinol concentration was 0.1 μmo/L, grossly below what is considered minimally necessary for an infant (1 μmol/L). VAD mothers and mothers with inflammation did not seem to compromise their milk retinol even though their serum retinol was lower than non-VAD and non-inflammation mothers. Breastmilk fat concentration positively correlated with milk retinol but not with serum retinol.
Conclusions
This exploratory study contributes toward an understanding of maternal retinol allocation.
Keywords: Human milk, liver retinol, maternal buffering, reproductive investment, vitamin A deficiency
Introduction
Vitamin A (VA) is an essential vitamin humans must obtain from dietary sources because we are incapable of synthesizing it in our body. VA is necessary for a broad range of biological functions, including vision, immunity, cell differentiation, growth, and reproduction (McLaren and Frigg, 2001). VA includes both retinol, the preformed VA exclusively available from foods of animal origin and highly bioavailable (absorbed in the human intestine), and pro-VA carotenoids which are VA precursors, widely present in both plant- and animal-based foods but capable of providing lower levels of VA. Mother’s milk is the best source of VA, more specifically retinol, for human infants because other foods suitable for infants tend to have lower retinol contents and lower bioavailability (Stoltzfus and Underwood, 1995). Consuming ample amounts of retinol is important for infants because they have elevated requirements for VA not only for sustaining their growth and development but also for building hepatic reserves which will be crucial during and beyond the weaning transition when their intake of maternal milk will be diminished and eventually terminated.
Globally, vitamin A deficiency (VAD) claims an estimated 1 million children under 5 years of age every year (Micronutrient Initiative and UNICEF, 2004), with the burden falling heavily on children in the weaning transition. Young children in the weaning transition are most at risk of VAD-related mortality (Williams, 1983) especially in endemically deficient regions of the world. This is because mothers who are VA deficient tend to have breast milk with low retinol levels. These levels may still be sufficient to supply an infant’s immediate physiological needs, but tend to fall short of facilitating an infant’s hepatic retinol reserves to last through the weaning transition and beyond (Ettyang et al., 2004; Newman, 1993).
Faced with this public health problem for the past two decades, the World Health Organization recommended that all mothers in endemically deficient areas be administered a high-dose VA supplementation capsule. This recommendation was based on the belief that improving maternal VA status postpartum should improve their breast milk VA content and therefore lower the morbidity and mortality of their children. Resulting intervention programs yielded a transient improvement in breast milk retinol yet with no sustainable benefits for child/infant mortality (review of this literature see WHO, 2011, 2014). Because of this, coupled with undesirable side-effects of high-dose VA supplementation such as headaches, WHO has recently abandoned this recommendation (WHO, 2011) and revised their nutrition guidelines to emphasize dietary solutions rather than pharmaceutical ones (WHO, 2014). At the same time, they acknowledged that our scientific understanding about the principles of VA distribution within and between the human maternal somatic system and the secretion into milk was, and still is, too incomplete (WHO, 2014) to devise an effective intervention.
One of the reasons for this knowledge gap is that it is invasive and/or logistically challenging to collect all the data necessary to operationalize retinol distribution or allocation. Because retinol can be stored in the human body, it is necessary to simultaneously assess the levels of body (often liver) reserve, circulating blood retinol, and breast milk retinol. The assessment of hepatic stores is particularly challenging, requiring either liver biopsies or other less invasive, but logistically similarly demanding, surrogate measures such as the relative-dose response (RDR) test requiring two repeated blood samples with a 5-hour interval from each person. These challenges have generally forced many researchers to confine their studies to more logistically feasible and less invasive data, such as dietary intake recalls or a single observation of retinol concentration in blood and/or breast milk. These studies have facilitated our understanding of correlations or associations between maternal blood retinol, milk retinol and other factors, but these studies fell short of clarifying the concept of retinol distribution or allocation within the lactating mother. The few studies that managed to incorporate hepatic retinol reserve tended to place a primary focus on the infant outcomes rather than investigate what may be involved in the distribution of retinol within the maternal system and the allocation of retinol between the maternal soma and her breastmilk.
Here, we present a secondary analysis of data collected among lactating mothers residing in an endemically VAD region of Kenya. By utilizing a new index variable representing the degree of milk-retinol allocation, this study examines maternal nutritional, inflammatory, and ecological factors that might associate with maternal retinol allocation between maternal blood and milk. Our motivation was to explore the concept of maternal retinol allocation in a small, anthropological population in a specific cultural and ecological context, with a hope that this rare dataset may contribute to closing the knowledge gap that hinders advancement in public health solutions to VAD.
Breast Milk Vitamin A Allocation
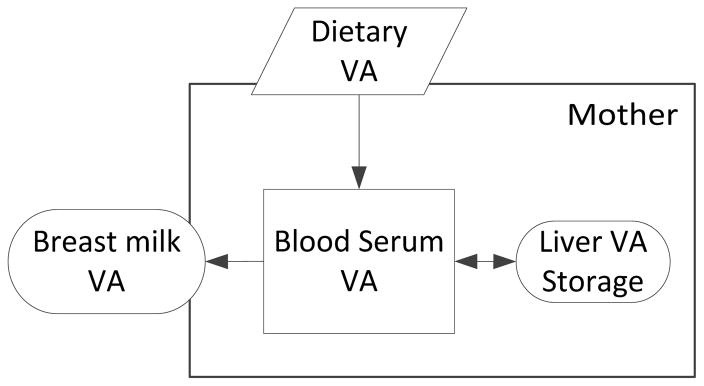
The model of the basic flow of VA in the maternal system is well established in the nutrition literature (Figure 1; Fujita et al., 2011). Dietary intake of VA positively contributes to milk retinol in two routes. First, a small fraction of VA from dietary intake promptly contributes to milk retinol via blood circulation into the mammary for milk synthesis (Stoltzfus and Underwood, 1995). The majority of ingested VA not immediately required for maternal physiological purposes will be diverted to, and reserved in, the liver store. As the need arises, this reserve is mobilized into the blood circulation bound to retinol-binding protein (a transporter protein of VA), to reach the target tissues, including the mammary for milk synthesis (Newman, 1993; WHO, 1996). Because the latter route is quantitatively greater, the mother’s VA status (assessed at the liver or serum level) is known to influence the retinol concentration in milk (Stoltzfus and Humphrey, 2002).
Figure 1.
Flow of vitamin A in maternal system (modified from Fujita et al., 2011)
Beyond this basic specification, however, the current model of the maternal VA flow is incomplete as to what circumstances (other than maternal VA nutriture) may enhance or diminish each of these flows, ultimately affecting the degree of retinol replenishment in milk. Epidemiological research shows that factors such as the duration of lactation, gestation age at birth, parity, and oral contraceptive use are all associated with retinol concentrations in human milk (Newman, 1993). Maternal nutritional factors that may influence the retinol concentration in milk include dietary intake of VA (Gebre-Medhin et al., 1976; Stoltzfus and Underwood, 1995), liver stores of retinol (Stoltzfus and Humphrey, 2002), and general nutrition (protein-energy malnutrition can diminish the availability of retinol-binding protein; Newman, 1993). How these factors may be physiologically linked to milk synthesis is unclear.
In a VA deficiency endemic population such as the one studied here, lactating mothers must allocate limited retinol between the competing biological needs of the mother and infant (Fujita, 2008; Fujita et al., 2011). Maternal blood and hepatic retinol are important for maternal physiological needs and future reproduction while milk VA is crucial for the infant. Prior to this study, no researcher has attempted to quantify the concept of maternal allocation of retinol to breast milk. Often attempts to measure reproductive efforts rely upon the analysis of “those isolated characteristics most obviously associated with reproduction” that are only crude approximations of the true costs of reproduction (Lombardi, 1998). Milk retinol is only a fraction of the total maternal reproductive effort and therefore may not approximate the true costs. Still, by focusing on retinol, an essential micronutrient that is indispensable for human survival and reproduction, we are able to utilize the single currency of retinol to measure the current reproductive effort (milk retinol) in relation to somatic effort or future reproductive effort (blood serum retinol; Fujita, 2008).
We previously evaluated the impacts of maternal life-history factors such as postpartum time and maternal parity (the number of previous live births) on the relationship between retinol in maternal hepatic reserve and breast milk (Fujita, 2008; Fujita et al., 2011). We found that the relationship between liver retinol and milk retinol varied in a manner consistent with life-history theoretical predictions (Fujita et al., 2011). In that study, however, we did not quantify the theoretical concept of maternal allocation. Rather, we essentially examined the presence or absence of the tradeoff relationship between milk retinol and maternal hepatic retinol store. These previous analyses (Fujita, 2008, Fujita et al., 2011) suffered an important limitation of excluding maternal serum retinol because the primary focus was on testing the trade-off relationships between milk retinol and hepatic reserve. As shown in Figure 1, serum retinol is an important immediate pool of maternal resource that can serve the maternal somatic effort and reproductive effort. The exclusion of serum retinol in our previous study was because of serum retinol having the potential to serve a wide range of biological functions, we thought it would blur the boundary between different biological domains within the mother. Here, we use a new index in an attempt to overcome this limitation and more directly address the concept of maternal allocation of retinol from blood serum to milk.
Materials & Methods
Study Population
The study population is made up of Ariaal mothers in agro-pastoral communities in northern Kenya. This population was originally selected because women of reproductive ages in this population were found to feature a wide range of VA statuses in our pilot survey study (Fujita, 2006), spanning from clinical deficiency (night blindness), to sub-clinical deficiency (low serum retinol), to normal status. Mothers in this population have a wide variance in VA nutriture, considered ideal for examining the relationship between maternal VA and breast milk VA. The Ariaal people have long adapted to this drought-ridden, arid region through semi-nomadic mixed-species pastoralism. During the last 50 years, many Ariaal families have become sedentary to take advantage of, or are forced into, additional economic opportunities such as cultivation of dry-land maize and/or utilization of drought relief and other food distribution programs (Fratkin, 1998). Previous research among sedentary agro-pastoral Ariaal people and closely affiliated Rendille people indicates marginal nutritional status among women and children (Fratkin, Roth, and Nathan, 2004; Fujita et al., 2012; Fujita et al., 2014; Miller, 2011; Shell-Duncan and Yung, 2004).
Sample and Data Collection
Cross-sectional data collected in 2006 from 171 breastfeeding mothers within 1.5 years postpartum were used. These mothers were a convenience sub-sample of the 241 mothers enrolled in the original study via stratified random sampling where strata were postpartum-time groups of 0–3, 3–6, 6–9, 9–12, 12–15 months (Fujita, 2008; Fujita et al., 2011). These mothers had residence in 3 locations (communities) - Karare, Kituruni, and Korr. The former two communities are highland agro-pastoral communities, respectively located on the southern and western/northern facing slopes of the Mount Marsabit where the land is arable. Korr is a lowland desert community where agriculture is not possible.
The exclusion criteria for the original study were: 1) postpartum time beyond 20 months, 2) non-lactating, 3) using oral contraceptives, and 4) clinical signs (or reports) of liver or kidney diseases or acute infections. During specimen transportation within Kenya, approximately 20% of milk vials either accidentally broke or lost identification labels, reducing the sample size. Of the remaining sample, 171 mothers had complete data for variables relevant for this study, including: milk retinol (μmol/L), serum retinol (μmol/L), RDR to determine VAD status, anthropometric measurements, dietary intake of VA (retinol and beta-carotene respectively in μg), breastfeeding frequency, socio-demographic information, and the subclinical inflammatory status (acute phase response or APR). The sample was not significantly different from the original 241 mothers in terms of age, postpartum time, or anthropometry.
Of the 171 mothers, 17 mothers had VAD (defined as low liver stores; RDR>14%, see below) and another 17 mothers had APR (serum C-reactive protein >10 mg/L, determined using Brindle et al., 2010). The acute subclinical infection or inflammation is known to transiently reduce the hepatic synthesis of retinol-binding protein and therefore the secretion of the retinol-retinol-binding protein complex into the blood (Rosales et al., 1996). Because of this, VA status assessment measures such as serum reitnol concentrations are biased in individuals having subclinical infection (Thurnham et al., 2003). In the public health literature concerned with VA status of a population, it is conventional to exclude individuals with acute inflammation from statistics. For the current study of retinol allocation among mothers in an ecological setting with high disease stress levels, however, individuals with inflammation may constitute an important segment of the population. For this reason, we used the sample inclusive of mothers exhibiting APR.
Variables
The Outcome Variable Milk-Retinol Allocation Index
In order to quantify the concept of allocation, we constructed an original milk-retinol allocation index variable (MRA), using the formula shown below.
This formula used milk retinol concentration (ug/umol) as the numerator and the sum of milk retinol (ug/umol) and serum retinol (ug/umol) as the denominator. The denominator is based on the logic that it should represent the total pool of retinol resource the mother is endowed with that had the potential for secretion into milk. The numerator represents the retinol actually secreted into milk. The formula therefore represents the fraction of retinol allocated to milk out of the pool of maternal retinol that had the immediate potential to be allocated to the milk (i.e., not sequestered in the liver storage). A higher MRA value indicates having a higher degree of retinol allocation to milk for a given level of maternal circulating retinol. Natural-log transformation was applied to satisfy the normality assumption.
Milk Retinol Concentration
The milk retinol concentration values were based on HPLC analysis at the Mayo Medical Laboratories (Rochester, Minnesota). The lower limit of detection was 20 ug/L (0.07 μmol/L). Of the 171 women 58 had milk retinol levels below this limit, and were assigned the value of 19 μg/L (0.066 μmol/L; this method has been utilized in Fujita, 2008 and Fujita et al., 2011). We analyzed the data both inclusive and exclusive of the sub-range specimens rather than just excluding them from the study. The reason is that the sub-range specimens are likely to represent an important segment of the population occupying the lowest range of the milk VA variation spectrum, i.e. experiencing a life with limited retinol.
Serum Retinol Concentration
Serum retinol values were based on the fasting venous blood specimens collected via needle and syringe by a local nurse and the serum portion was separated via centrifugation and frozen in a liquid nitrogen tank within 4 hours of blood collection. HPLC analysis was conducted in the Clinical Nutrition Research Unit laboratory of the Harborview Medical Center (Seattle, WA) using thawed specimens.
Dietary Vitamin A Intake
Human beings can obtain VA from pre-formed retinol exclusively from foods of animal origin or from its precursor, pro-VA carotenoids (e.g. β-carotene), widely available in both plant and animal sources. The latter has a substantially lower retinol value per weight than the former, for only a fraction of the ingested β-carotene has the potential to be absorbed and converted into retinol in the human intestine. In many ecological settings, however, foods rich in retinol content are rare or expensive, and β-carotene is considered an important dietary source of VA especially for the poor. Dietary intake of retinol and β-carotene were estimated from a 24-hour dietary recall interview with the mother. The recalled food items and their weights (calculated from estimated volumes using a conversion table based on the local utensils) were entered into the software Vitamin A Calculator (Erhardt, 2003) which computed the retinol and β-carotene intake values in μg.
Postpartum Vitamin A Supplementation
At the time of data collection, an intervention program to combat the public health problem of VAD had been newly implemented in the area. Local program providers, including the Food for the Hungry International of Marsabit and Catholic clinics, were distributing a single, high-dose maternal postpartum oral supplementation capsule (200,000 IU per the WHO recommendation and made available by the Micronutrient Initiative) to women within 6 weeks postpartum. A local nurse affiliated with Food for the Hungry International estimated that the program managed to reach about a half of all mothers in the area. Because it has been shown that this supplementation increases the maternal serum retinol and to a lesser degree milk retinol (Rice et al., 1999; Roy et al., 1997; Stoltzfuls et al, 1993), we included a variable, high-dose vitamin A supplementation (y=1/n=0), to adjust for the possible impact of this supplementation. This variable was obtained from the mother who was asked to recall if she had received a vitamin A supplementation capsule within some weeks postpartum.
Vitamin A Deficiency
RDR is based on the percentage change in the serum retinol concentrations between the fasting morning venous blood sample and the second venous blood sample collected 5 hours after an oral dose of VA (post-dose blood). The RDR test is based on the observation that individuals having deficient hepatic VA reserves tend to respond with a substantial, sustained increase in their serum retinol within a few hours of the oral dose (Mobarhan et al., 1981). Because the dose-response may also be influenced by other factors such as protein-energy malnutrition, RDR is conventionally utilized as a qualitative indicator of liver VA status, deficient vs. sufficient, using the cutoff value of 14% (Mobarhan et al., 1981) or more conservatively 20% (Amedee-Manesme et al., 1984). Maternal hepatic VAD was determined using the RDR>14% cutoff (Mobarhan et al., 1981). We adjusted for the effect of VAD (deficient=1/sufficient=0) because VAD is known to cause systemic changes in an individual’s biological functions, including the rates of absorption and bioconversion of carotenoids (Bender, 2003; Castenmiller and West, 1998) and the concentrations of the breast milk retinol. The mean breast milk retinol concentrations for VAD populations are substantially lower than those in VA sufficient populations (WHO, 1996). Other biological functions systematically compromised by VAD include the visual system, the immune system, and the reproductive system (McLaren and Frigg, 2001).
Statistical Analysis
Statistical analyses were conducted using Stata v13.1. The descriptive statistics were computed for all the variables, and the correlations and associations between the variables were assessed. We applied regression models, using the outcome MRA (natural log transformed). The models were adjusted for maternal VA nutriture, including retinol intake, β-carotene intake, postpartum high-dose VA supplementation status, and liver VAD. Maternal age, parity, postpartum time, breastmilk fat (using Creamatocrit % as an indicator), arm fat area (AFA), APR, and land ownership (y=1/n=0) were also adjusted for. The factor of community membership was controlled as a random effect. We analyzed the model for both the entire sample (n=171) and the sub-sample of mothers having milk retinol concentration values within the HPLC assay range (n=113).
Postpartum time (duration of lactation) and milkfat were included in the regression models because they are known to correlate with milk retinol concentrations (Ettyang et al., 2004; Fujita, 2008; Fujita et al., 2011; Gross et al., 1998; Haskell & Brown, 1999; HKI, 1999; Jelliffe & Jelliffe, 1978; Newman, 1993; WHO 1996). AFA was used to adjust for the possible effect of energy deficiency. Protein-energy deficiency can diminish the level of retinol-binding protein synthesis (Jahoor et al., 1996 in a pig model) and therefore can alter the mobilization of maternal VA between the liver, blood, and milk. AFA is an indicator for the subcutaneous fat contribution to the upper arm, and is derived from the mid-upper arm circumference (MAC) and triceps skin-fold thickness (TSF) as follows: AFA = [(MAC2)/4* π ] – [(MAC-(TSF*π))2/4*π ] (Frisancho, 1990). Community membership was also adjusted for because the data come from mothers residing in three communities having different ecological settings and economic bases.
Results
Sample characteristics are shown in Table 1. The overall mean ± SD parity was 3.7 ± 2 births, ranging from 1 to 12. Maternal age was 28 ± 7 years, ranging from 18 to 46 years. The mean postpartum time was 238 ± 135 days, and breastfeeding frequency (the typical frequency during a 24-hour period reported by the mother) was 9 ± 4 times per day. The mean AFA was 16 ± 2 cm2. The mean fasting serum retinol was 1.49 ± 0.44 μmol/L, ranging from 0.68 to 3.04. The mean RDR was −0.43 ± 0.16. Seventeen mothers (10%) had VAD (i.e. low liver retinol reserve; RDR>14%). Twenty-five mothers (15%) reported having been administered an oral dose of postpartum vitamin A supplementation within several weeks of giving birth to the current infant.
Table 1.
Sample Characteristics (mean ± SD)
| Variable | Total (n=171) | Karare (n=117) | Korr (n=22) | Kituruni (n=32) | F a | P |
|---|---|---|---|---|---|---|
| Age | 28.3 ± 6.9 | 28.3 ± 6.9 | 28.1 ± 7.1 | 30.0 ± 5.3 | 1.00 | .378 |
| Parity | 3.7 ± 2.3 | 3.7 ± 2.3 | 3.6 ± 2.3 | 4.3 ± 2.3 | 1.02 | .363 |
| Postpartum Time (day) | 238 ± 135 | 237 ± 135 | 253 ± 136 | 170 ± 107 | 3.76 | .025 |
| Triceps Skinfold Thickness (mm) | 14.6 ± 5.9 | 14.6 ± 5.9 | 14.8 ± 6.4 | 13.5 ± 4.7 | 0.46 | .635 |
| Mid-Upper Arm Circumference (cm) | 24.3 ± 2.4 | 24.3 ± 2.4 | 24.1 ± 2.3 | 24.9 ± 3.3 | 1.18 | .310 |
| Arm Fat Area (cm2) | 16.2 ± 7.3 | 16.2 ± 7.3 | 16.3 ± 7.6 | 15.8 ± 7.3 | 0.05 | .955 |
| Dietary Retinol | 48.9 ± 58.3 | 48.9 ± 58.3 | 55.8 ± 63.1 | 26.2 ± 29.0 | 3.0 | .052 |
| Dietary β-carotene | 922 ± 757 | 922 ± 757 | 925 ± 687 | 620 ± 433 | 2.9 | .056 |
| Breastfeeding Frequency | 9 ± 4 | 9 ± 4 | 8 ± 3 | 12 ± 3 | 46.7 | .000 |
| Creamatocrit (%) | 4.2 ± 2.4 | 4.2 ± 2.4 | 4.3 ± 2.4 | 4.1 ± 2.3 | 0.54 | .585 |
| Breast Milk Retinol (μmol/L) | 0.10 ± 0.04 | 0.10 ± 0.04 | 0.09 ± 0.04 | 0.10 ± 0.03 | 2.42 | .092e |
| Serum Retinol (μmol/L) | 1.49 ± 0.44 | 1.49 ± 0.44 | 1.52 ± 0.47 | 1.48 ± 0.33 | 1.44 | .239 |
| RDR | 0.42 ± 0.16 | 0.43 ± 0.16 | 0.42 ± 0.18 | 0.38 ± 0.13 | 1.39 | .252 |
| Milk-Retinol Allocation | 0.06 ± 0.02 | 0.06 ± 0.02 | 0.07 ± 0.03 | 0.08 ± 0.03 | 4.44 | .013e |
| Milk-Retinol Allocation (ln) | −2.82 ± 0.38 | −2.88 ± 0.38 | −2.75 ± 0.35 | −2.64 ± 0.34 | 5.54 | .005e |
| Vitamin A Deficiencyb | 17 (10%) | 11 (9%) | 2 (9%) | 4 (13%) | --- | .922d |
| Vitamin A Supplementationc | 25 (15%) | 8 (7%) | 15(68%) | 2 (6%) | --- | .000d |
| Acute Phase Response | 17 (10%) | 12 (10%) | 2 (9%) | 3 (9%) | --- | 1.00d |
| Land Ownership | 111 (65%) | 74 (63%) | 10 (45%) | 27 (84%) | --- | .009d |
ANOVA F-test df = 2, 168
Relative-dose response value >14%
Supplemented with a single oral dose of vitamin A capsule (200,000 IU) after giving birth to the current infant.
Fisher’s Exact Test
Between community difference was not significant in the sub-sample (n=113) exclusive of mothers with sub-range milk retinol.
For the 113 women having milk retinol values within the HPLC detection range, the mean milk retinol concentration was 32 ± 10 μg/L (0.11 ± 0.03 μmol/L) with a range of 20–64 μg/L (0.07 – 0.22 μmol/L). After including the 58 women with the sub-range value of 19 μg/L, the mean milk retinol concentration was 28 ± 10 μg/L (0.10 μmol/L ± 0.04 μmol/L), with a range from 19 to 64 μg/L (0.066 to 0.22 μmol/L; n=171). These values indicate that these mothers had milk retinol levels much lower than what is considered necessary to meet infant requirements (1 μmol/L) and lower than the means previously reported from other populations (a range from 0.4 to 1.8 μmol/L; Stoltzfus, 1994).
The milk retinol variable inclusive of the sub-range specimens (n=171) was found to have biologically plausible correlations with multiple key variables, including serum retinol (r=.16, p=.037), milkfat (r=.29, p=.0001), maternal age (r=.16, p=.034), and parity (r=.14, p=.061). By contrast, when sub-range specimens were excluded, its correlations with age and parity became not significant (r=.14, p=.147 and r=.14, p=.152, respectively) although it still had significant correlations with serum retinol (r=.19, p=.041, n=113) and milkfat (r=.39, p<.0001).
The communities were significantly different with respect to MRA (log transformed, ANOVA F(2, 168)= 5.54, p=.005), breastfeeding frequency (F(2, 168)=46.7, p<.0001; Kituruni had the highest frequency), and postpartum time (F(2, 168)=3.76, p=.025; Kituruni had shortest time since birth). They were marginally different in terms of breast milk retinol (ANOVA F(2, 168)=2.42, p=.092), dietary retinol intake (F(2, 168)=3.0, p=.052), and β-carotene intake (F(2, 168)=2.9, p=.056). They were also different in terms of the frequency of mothers who had a high-dose postpartum vitamin A supplementation (Fisher’s Exact, p<.001; Korr 68%, Karare 7%, and Kituruni 6%), and land ownership (Fisher’s Exact, p<.009; Kituruni 84%, 63% Karare, 45% Korr.)
The pairwise correlation tests of the key variables are shown in the Table 2. The outcome variable MRA(ln) correlated positively with breast milk retinol (r=.69, p<.001, n=171), RDR (r=.15, p<.05) and creamatocrit (r=.21, p<.001), and negatively with serum retinol (r=−.57, p<.001). For n=171, parity was positively correlated with milk retinol (r=.14, p<.1), serum retinol (r=.33, p<.001), and RDR (r=−.18, p<.05). Maternal age and parity were highly positively correlated with each other (r=.78, p<.0001). For the sub-sample (n=113), MRA also correlated positively with breast milk retinol (r=.60, p<.001) and negatively with serum retinol (r=−.64, p<.001) but was not significantly correlated with RDR (r=.10, p=.286).
Table 2.
Pairwise Correlations of Variables (n=171)
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Breast Milk Retinol | ||||||||||||
| 2. Serum Retinol | r | 0.16b | ||||||||||
| 3. Relative-Dose Response | r | −0.12f | −0.33d | |||||||||
| 4. Milk-Retinol Allocation (ln) | r | 0.69d | −0.57d | 0.15b e | ||||||||
| 5. Age | r | 0.16b e | 0.31d | −0.14a | −0.07f | |||||||
| 6. Parity | r | 0.14a e | 0.33d | −0.18b | −0.10f | 0.78d | ||||||
| 7. Dietary Retinol | r | 0.01 | 0.13f | 0.09 | −0.05 | −0.03 | −0.08 | |||||
| 8. Dietary β-carotene | r | −0.03 | v0.12 | 0.05 | 0.08 | −0.06 | −0.06 | 0.06 | ||||
| 9. Postpartum Time | r | 0.13a e | 0.07 | 0.04a e | 0.04 | 0.01 | −0.11 | 0.08 | 0.04 | |||
| 10. Breastfeeding Freq. | r | 0.06 | −0.07 | 0.06 | 0.11 | 0.05 | 0.14a f | −0.18b | 0.05 | −0.20c | ||
| 11. Arm Fat Area | r | −0.01 | 0.09 | 0.06 | −0.06 | 0.05 | 0.01 | −0.09 | −0.05 | −0.09 | −0.04 | |
| 12. Creamatocrit | r | 0.29d | 0.02 | −0.01 | 0.21c | −0.05 | −0.05 | 0.06 | −0.03 | 0.18b | −0.06 | −0.03 |
P < 0.1,
P < 0.05,
P < 0.01,
P < 0.001
Correlation was not significant in the sub-sample (n=113) exclusive of sub-range milk retinol specimens.
Correlation was marginally significant only in the sub-sample (n=113) exclusive of sub-range milk retinol specimens.
The regression model (Table 3, Model A, n=171) indicated milkfat (β=.037, p=.001), VAD (β=.238, p=.009), APR (β=.186, p=.042), and land ownership (β=.165, p=.006) positively predicted the MRA index. Community membership in Kituruni (β=.209, p=.016) was associated with a higher value of the index than the other two communities. The model exclusive of sub-range milk retinol mothers (Table 3, Model B, n=113) indicated milkfat (β=.045, p=.002) and APR (β=.212, p=.047) as significant positive predictors while VAD and Kituruni were non-significant positive predictors (β=.123, p=.214, β=.117, p=.232, respectively).
Table 3.
Regression Models for Milk-Retinol Allocation Index predicted by maternal characteristics
| Predictor | Model A (n=171)a | Model B (n=113)b | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| β | S.E. | Beta | P | β | S.E. | Beta | P | |
| Parity | −0.015 | 0.019 | −0.770 | 0.441 | −0.014 | 0.023 | −0.600 | 0.548 |
| Retinol Intake | −0.001 | 0.000 | −1.070 | 0.285 | 0.000 | 0.001 | −0.850 | 0.396 |
| β-carotene Intake | 0.000 | 0.000 | 0.780 | 0.433 | 0.000 | 0.000 | 0.830 | 0.404 |
| Vitamin A Deficiency | 0.238 | 0.091 | 2.600 | 0.009 | 0.123 | 0.099 | 1.240 | 0.214 |
| Vitamin A Supplementation | 0.051 | 0.096 | 0.530 | 0.597 | −0.100 | 0.110 | −0.910 | 0.361 |
| Age | 0.001 | 0.006 | 0.170 | 0.867 | −0.001 | 0.007 | −0.140 | 0.891 |
| Postpartum Time | 0.000 | 0.000 | 0.060 | 0.951 | 0.000 | 0.000 | −0.240 | 0.808 |
| Breastfeeding Frequency | −0.001 | 0.009 | −0.160 | 0.870 | −0.006 | 0.010 | −0.590 | 0.553 |
| Arm Fat Area | −0.003 | 0.004 | −0.900 | 0.367 | −0.005 | 0.005 | −0.940 | 0.346 |
| Creamatocrit | 0.037 | 0.011 | 3.270 | 0.001 | 0.045 | 0.014 | 3.150 | 0.002 |
| Korr | 0.148 | 0.108 | 1.370 | 0.171 | 0.183 | 0.125 | 1.470 | 0.142 |
| Kituruni | 0.209 | 0.087 | 2.400 | 0.016 | 0.117 | 0.098 | 1.200 | 0.232 |
| Karare (ref.) | ||||||||
| Land Ownership | 0.165 | 0.060 | 2.730 | 0.006 | 0.072 | 0.076 | 0.940 | 0.348 |
| Acute Phase Response | 0.186 | 0.091 | 2.030 | 0.042 | 0.212 | 0.107 | 1.990 | 0.047 |
| Constant | −3.099 | 0.184 | −16.830 | 0.000 | −2.777 | 0.242 | −11.480 | 0.000 |
All mothers; n=171, Wald χ2(14)=46.41, P<0.0001
Exclusive of sub-range milk retinol specimens; n=113, Wald χ2(14)=23.59, P=0.05
To help interpret the effects of VAD and APR on the outcome, the group differences in milk retinol and serum retinol were assessed using t-tests (Table 4). The observed mean serum retinol for VAD mothers was significantly lower than VA sufficient mothers (t(169)=3.53, p=.001), while the mean breast milk retinol concentrations were equivalent between these groups (t=−.27, p=.786). Similarly, the observed mean serum retinol for APR mothers was significantly lower than mothers without APR (t(169)=2.35, p=.02), while the mean breast milk retinol concentrations were not different between groups (t=0.51, p=.610). The sub-sample of mothers exclusive of sub-range milk retinol values showed similar patterns where the mean serum retinol values were significantly different by VAD/APR groups while the means for breast milk retinol were not significantly different between groups.
Table 4.
Comparison of Observed Values by Vitamin A Status and Inflammatory Status
| Non-VADa (n=154) | VAD (n=17) | Non-APRb (n=154) | APR (n=17) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Mean | SE | Mean | SE | t | p | Mean | SE | Mean | SE | t | p | |
| Breast Milk Retinol (μmol) | 0.10 | 0.00 | 0.10 | 0.01 | −0.27 | 0.786 | 0.10 | 0.00 | 0.09 | 0.01 | 0.51 | 0.610 |
| Serum Retinol (μmol) | 1.53 | 0.04 | 1.14 | 0.06 | 3.53 | 0.001 | 1.51 | 0.04 | 1.25 | 0.09 | 2.35 | 0.020 |
| Milk-Retinol Allocation (ln) | −2.84 | 0.03 | −2.57 | 0.08 | −2.89 | 0.004 | −2.83 | 0.03 | −2.68 | 0.11 | −1.59 | 0.113 |
Vitamin A Deficient;
Acute Phase Response
Discussion
This study was based on a dataset from an endemically VAD region of Kenya where VA is a limited resource. By utilizing a derived index MRA, representing the degree of maternal milk-retinol allocation priority, this study explored the factors that may relate to the maternal allocation of retinol to the milk.
As with many other high fertility populations, age and parity were highly correlated with each other in this sample. Both age and parity were also positively correlated with the outcome variable MRA (for the whole sample). After adjusting for the effect of maternal age and other covariates in the multiple regression models, we found that the effect of parity was non-significant. This suggests that maternal parity – the number of live births previously given – had marginal or significant correlation with milk retinol, serum retinol, and liver retinol, respectively, but did not have a relationship with our indicator of milk-retinol allocation.
The importance of parity for the study of the evolutionary theory has been clearly established (e.g. Shell-Duncan and Yung, 2004; Tracer, 1991; Miller, 2010). The effect of parity on the maternal energy status or allocation has been confusing, however. While some studies associated advancing parity with depletion in maternal energy status, others found the opposite or no associations (as reviewed in Shell-Duncan, 2004). Focusing directly on maternal reproduction and parity, a meta-analysis of pregnancy outcome risks in women of different parity found that first time mothers have increased odds of low birth weight and reduction in birth weight compared to higher parity mothers (Shah et al., 2010).
In the breast milk research, the traditional view is that human lactation performance is buffered from maternal condition-related fluctuations (Sellen, 2007). Still, parity has been reported in many studies as one of the major factors of the concentrations of many human milk components, including retinol (Liyanage et al., 2008; Newman, 1993), carotenoids (in the colostrum, Patton, 1990), iron (Silvestre et al., 2000), and copper (Arnaud and Favier, 1995). Yet, in this literature as well, some studies report parity to have no relationship with human milk retinol (Panpanich et al., 2002) or copper (Feely et al., 1983). Similarly, parity has been a confusing variable in the study of the antimicrobial proteins in human milk. For example, many studies report secretory immunoglobulin A (the production and the secretion of which are affected by maternal VA status; Mora et al., 2006; Stephensen, 2001) to be linearly or non-linearly correlated with parity (Hennart et al., 1991; Miller, 2011, Prentice et al., 1983), yet others report no relationship between parity and the antibody (Filteau et al., 1999).
It has been suggested that the possible reasons for this confusion may relate to the differences in the subsistence modes or the levels of economic development between populations, where maternal activity and dietary characteristics can vary substantially within and between populations (Shell-Duncan and Yung, 2004). This in turn may increase the difficulty of phenotypic correlation which can negate the fundamental assumption of an evolutionary model that resource is limited for all individuals because some populations or individuals within populations may have abundant resources and therefore do not have to allocate between competing biological domains. The current study joins the previous studies reporting the lack of association between parity and an indicator of the maternal priority shift in reproductive investment or life-history strategies, and echoes the challenge in studying life-history strategies among human beings in ecological settings even after adjusting for a range of possible confounders.
The positive effect of VAD on MRA (for the whole sample) indicates that milk retinol is relatively buffered from the negative impact of serum retinol deficiency. The comparison of between-group differences between milk retinol and serum-retinol appears to corroborate this interpretation. While serum retinol of VAD mothers was significantly lower than VA sufficient mothers, breast milk retinol was comparable between these groups. This idea of buffered milk quality is consistent with the previously mentioned general view on human milk that human lactation performance is reasonably buffered from maternal condition-related variations. The possibility of maternal buffering associated with VAD found here, however, may be an artifact of the truncated milk retinol concentration data distribution because a large segment of mothers had concentrations below the assay range. That the effect of VAD is not significant in the second model for the sub-sample mothers exclusive of sub-range milk retinol specimens provides partial support for this interpretation.
The positive effect of APR on MRA may also be explained by the differential impact of maternal inflammation on breast milk retinol relative to serum retinol. In our data, the mean serum retinol for mothers having APR was significantly lower than the non-APR group while the mean breast milk retinol was not different between the groups. It is well established that APR will suppress the serum retinol concentrations as explained above (Rosales et al., 1996; Thurnham et al., 2003). This seems not to be the case for milk retinol although only a few studies have thus far examined the effect of maternal inflammation on milk retinol. A rare example is the study of milk retinol in Malawi, reporting that milk retinol concentrations are unaffected by APR (Dancheck et al., 2005). Once again, our finding is consistent with the idea that the breast milk retinol may be relatively buffered from maternal systematic inflammation.
That breast milk retinol may be buffered from the adverse impact of the maternal deficiency or acute inflammation may also be interpreted from an evolutionary perspective. It has been suggested that if the environment is such that the extrinsic mortality is high and life for adults is risky, then it does not pay to invest heavily in somatic maintenance at the expense of reproduction (Stearns, 2005). From this perspective, the buffered milk retinol could be regarded as the uncompromised maternal investment in current reproduction in the face of VAD and APR which may signal a relatively high extrinsic mortality risk for the mothers. It should also be noted that the prioritized milk-retinol allocation or the buffered milk retinol among some of these mothers (e.g. VAD) does not necessarily mean that these mothers were sacrificing their survival. In the life-history perspective, we should expect mothers to meet their own survival needs first then invest in reproduction. Yet, under certain conditions lactating mothers may shift the investment priority toward current reproductive effort over their somatic capital to a limited degree so that they do not jeopardize their survival but delay or potentially sacrifice their future reproduction.
Previous studies in resource limited settings have shown the possible link between adult undernourishment and increased current reproductive effort, possibly at the expense of future reproduction or somatic maintenance. Undernourished mothers tend to have longer periods of postpartum amenorrhea than mothers consuming nutritionally enhanced diets (Lunn et al., 1980, 1984) or mothers having superior nutritional status (Wood, 1994). Prolonged postpartum amenorrhea is an indicator of the delayed resumption of the ovulatory cycle and therefore delayed transition from current to future reproduction. However, this may be too simplistic an interpretation, for the adaptive value of ovarian suppression is extremely difficult to demonstrate empirically (Wood, 1994). In addition, APR may be conceptualized as an indicator of heightened somatic maintenance effort because immune responses such as APR entail a high cost of energy and other biological resources (McDade, 2005). A more nuanced explanation that considers the subtle balancing of scarce retinol between competing biological domains and subdomains may be necessary.
Community membership in Kituruni was associated with the highest value of MRA. Based on the data, Kituruni had mothers with younger infants than the other communities. Consistent with this difference, mothers breastfed their infants more frequently (12 times/d) than mothers in other communities (8–9 times/d). At the same time, Kituruni had a higher proportion of household land ownership (84%) than others. Although these characteristics were controlled in the model, Kituruni still had a significantly higher MRA than the other communities. It is possible that other unobserved community-based variables, such as the access to drinking water, soil productivity, or socioeconomic status, are related to MRA.
It has been suggested that the lactation hormone prolactin may be a signal to enhance the mobilization of VA from the liver toward the blood circulation and the mammary in breastfeeding women (Cumming and Briggs, 1983; Gal and Parkinson, 1972). To our knowledge, this possibility has not yet been empirically tested except for a small pilot study (Fujita et al., 2014). Because maternal serum prolactin concentrations are positively correlated with breastfeeding frequency (Freeman et al., 2000), it is possible that the substantially high breastfeeding frequency in Kituruni may be in part physiologically attributable to the greater milk-retinol allocation among these mothers via elevated serum prolactin. This possibility should be investigated in a future study.
We also found that the family’s land ownership had a positive effect on MRA. It is likely that there are some more proximate variables that connect land ownership to MRA. A future study should explore this possibility.
We acknowledge important caveats of our dataset. Related to the sampling strategy, our original study exclusively recruited mothers who were apparently healthy, without visible signs of acute infections. While this exclusion was necessary to the validity of biomarkers for MRA, we may have excluded an important subset of mothers. Marginally nourished mothers in VAD endemic regions such as northern Kenya may include a sizable subset of individuals having clinical symptoms of acute infection at any given time because undernourishment can compromise the integrity of protective barriers against pathogens. If this subset is significantly different from the sampled mothers, our findings may not be generalizable to the whole population.
As described in the variables section, the milk retinol values in our data were overall very low. Milk retinol concentrations in this sample may be very low because the VA levels of the mothers in this study were truly low due to low dietary intake among these mothers who were experiencing severe food insecurity due to prolonged drought when the data were collected. It is also possible that there was a methodological reason. The milk sampling procedure utilized may, at least in part, have contributed to the low milk retinol. Being a fat-soluble vitamin, retinol is located in the milkfat and therefore milk retinol levels are dependent on milkfat levels. It is known that human milk has low milkfat levels at the start of nursing (i.e. foremilk) than toward the end (hindmilk) of each nursing episode (Miller et al., 2013). It is also known that the fullness of the breast from which the milk is sampled has an inverse relation with milkfat content. Namely, we know the fuller the breast or the longer time the breast was not nursed, the lower the milkfat content (Stoltzfus & Underwood, 1995). The current study was based on the foremilk specimens from a breast that was not nursed overnight. This sampling approach allowed minimizing of the source of variation in the concentration of retinol in breast milk due to the timing within each nursing episode or time of the day, but it may have resulted in sampling of unusually low concentration values. It is also possible that these low values may be attributed to other methodological shortcomings such as the assay limitation of the Mayo Medical Laboratories’ HPLC or the storage effect prior to laboratory analysis (see Fujita et al., 2011 for a discussion).
We should emphasize that results from this study are tentative, given the possibility that the low milk retinol is due to some sort of methodological issue, possibly in the laboratory analysis. Even if there was no issue, the extremely low milk retinol makes it difficult to generalize the results of this study. Additionally, for the 58 women (34%) having a milk retinol concentration below the HPLC detection range (20 ug/L), we assigned the value of 19 ug/L. This assignment of a uniform value may have collapsed the sample variance in milk retinol and may have served to underestimate the variance in MRA. This may have therefore limited the statistical models’ ability to detect variation in patterns potentially important for understanding the maternal allocation of retinol to milk, particularly in the larger model inclusive of mothers having milk samples in the lowest range of the milk retinol variation spectrum.
The dietary vitamin A intake data were based on a single 24-hour dietary recall interview per mother instead of multiple recalls which would better reflect day-to-day dietary intake variation (Gibson, 1990). This may have led to an underestimation of the ranges in retinol and β-carotene intake values, which may in turn have affected the statistical significance of their effects on the outcome.
Given these caveats, findings from this study are considered preliminary. However, this study is of high value methodologically and theoretically because, to our knowledge, it is the first attempt to quantify the concept of milk-retinol allocation using data collected from a group of breastfeeding mothers in an ecological setting. As such, findings from this study provide an important exploratory understanding of the principle of retinol distribution within the maternal system and the allocation to milk. This may be of use to human biologists interested in reproductive ecology and public health researchers striving to combat the public health problem of VAD.
Acknowledgments
Funding Sources: Michigan State University Faculty Initiative Grant (MF), NSF BCS-0622358 (MF), Wenner-Gren Foundation Gr.7460 (MF), the Micronutrient Initiative (MF), NICHD research infrastructure grant, R24 HD042828 (EB).
Drs. Bettina Shell-Duncan, Kathleen O’Connor, Jonathan Gorstein of the University of Washington and Dr. Eric Roth of the University of Victoria provided invaluable guidance for the original dissertation research by MF which laid the foundation of the current study. Professor Elizabeth Ngugi of the University of Nairobi, and Drs. David Mwaniki and Yeri Kombe as well as Mr. Philip Ndemwa of the Center for Public Health Research, Kenya Medical Research Institute facilitated the data collection. Dr. Daniel Sellen of the University of Toronto and Dr. Tina Moffat of McMaster University provided inspirational comments at the 2014 Canadian Association for Physical Anthropology meeting where a preliminary version of this study was presented. Nerli Paredes Ruvalcaba and Alexis Rife of the Biomarker Laboratory for Anthropological Research of Michigan State University edited and proofread earlier drafts. Partial support for this research came from a Michigan State University College of Social Science Faculty Initiative Fund grant to MF, and a Eunice Kennedy Shriver National Institute of Child Health and Human Development research infrastructure grant, R24 HD042828, to the Center for Studies in Demography & Ecology at the University of Washington. We are deeply appreciative of the two anonymous reviewers and the Editor-In-Chief for constructive critiques and suggestions.
Footnotes
Conflict of Interest: None
Author Contributions
MF conceived the study and collected the data. EB provided necessary intellectual and logistical support. YL provided critical comments on variables and data analysis. MF and YL analyzed the data. MF drafted the manuscript, and EB and YL edited the manuscript for intellectual content.
References Cited
- Amédée-Manesme O, Anderson D, Olson JA. Relation of the relative dose response to liver concentrations of vitamin A in generally well-nourished surgical patients. Am J Clin Nutr. 1984 Jun;39(6):898–902. doi: 10.1093/ajcn/39.6.898. [DOI] [PubMed] [Google Scholar]
- Arnaud J, Favier A. Copper, iron, manganese and zinc contents in human colostrum and transitory milk of French women. Sci Total Environ. 1995 Jan 6;159(1):9–15. doi: 10.1016/0048-9697(94)04314-d. [DOI] [PubMed] [Google Scholar]
- Bender DA. Nutritional biochemistry of the vitamins. 2. Cambridge: Cambridge University Press; 2003. [Google Scholar]
- Brindle E, Fujita M, Shofer J, O’Connor KA. Serum, plasma, and dried blood spot high sensitivity C-reactive protein enzyme immunoassay for population research. Journal of Immunological Methods. 2010;362:112–120. doi: 10.1016/j.jim.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castenmiller JJ, West CE. Bioavailability and bioconversion of carotenoids. Annu Rev Nutr. 1998;18:19–38. doi: 10.1146/annurev.nutr.18.1.19. [DOI] [PubMed] [Google Scholar]
- Cumming FJ, Briggs MH. Changes in plasma vitamin A in lactating and non-lactating oral contraceptive users. Br J Obstet Gynaecol. 1983;90(1):73–7. doi: 10.1111/j.1471-0528.1983.tb06750.x. [DOI] [PubMed] [Google Scholar]
- Dancheck B, Nussenblatt V, Ricks MO, Kumwenda N, Neville MC, Moncrief DT, Taha TE, Semba RD. Breast milk retinol concentrations are not associated with systemic inflammation among breast-feeding women in Malawi. J Nutr. 2005 Feb;135(2):223–6. doi: 10.1093/jn/135.2.223. [DOI] [PubMed] [Google Scholar]
- Erhardt SIGHT AND LIFE vitamin A intake calculation program. [Accessed October 20, 2011];A free program made available by SIGHT AND LIFE. 2003 Available at: http://www.nutrisurvey.de/vac/vac.htm.
- Ettyang G, Oloo A, van Marken Lichtenbelt W, Saris W. Consumption of vitamin A by breastfeeding children in rural Kenya. Food Nutr Bull. 2004;25(3):256–63. doi: 10.1177/156482650402500305. [DOI] [PubMed] [Google Scholar]
- Feeley RM, Eitenmiller RR, Jones JB, Jr, Barnhart H. Copper, iron, and zinc contents of human milk at early stages of lactation. Am J Clin Nutr. 1983 Mar;37(3):443–8. doi: 10.1093/ajcn/37.3.443. [DOI] [PubMed] [Google Scholar]
- Fratkin E. Ariaal pastoralists of Kenya: Surviving drought and development in Africa’s arid lands. Boston: Allyn & Bacon; 1998. [Google Scholar]
- Fratkin E, Roth EA, Nathan M. Pastoral sedentarization and its effects on children’s diet, health, and growth among Rendille of northern Kenya. Hum Ecol. 2004;32:531–558. [Google Scholar]
- Freeman ME, Kanyicska B, Lerant A, Gyorgy Nagy. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1524–1585. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- Frisancho AR. Anthropometric standards for the assessment of growth and nutritional status. Ann Arbor: The University of Michigan Press; 1990. pp. 1–30. [Google Scholar]
- Filteau SM, Rice AL, Ball JJ, Chakraborty J, Stoltzfus R, de Francisco A, Willumsen JF. Breast milk immune factors in Bangladeshi women supplemented postpartum with retinol or beta-carotene. Am J Clin Nutr. 1999;69(5):953–8. doi: 10.1093/ajcn/69.5.953. Epub 1999/05/08. [DOI] [PubMed] [Google Scholar]
- Fujita M. Maternal vitamin A deficiency in a settled Ariaal community in northern Kenya: a direction for future research. Journal of Development Alternative and Area Studies. 2006;25(3):88–100. [Google Scholar]
- Fujita M. Doctoral Dissertation. University of Washington; Seattle: 2008. An epidemiological and evolutionary investigation of mother-offspring Vitamin A transfer. [Google Scholar]
- Fujita M, Shell-Duncan B, Ndemwa P, Brindle E, Lo Y, Kombe Y, O’Connor KA. Vitamin A dynamics in breastmilk and liver stores: a life history perspective. American Journal of Human Biology. 2011;23:664–673. doi: 10.1002/ajhb.21195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Lo Y, Baranski JR. Dietary diversity score is a useful indicator of vitamin A status of adult women in northern Kenya. American Journal of Human Biology. 2012;24:829–834. doi: 10.1002/ajhb.22327. [DOI] [PubMed] [Google Scholar]
- Fujita M, Lo Y, Baranski JR, Brindle E. In endemically vitamin A deficient northern Kenya, undernourished mothers allocate a higher proportion of blood vitamin A to breastmilk than better-nourished mothers, with effects moderated by the lactation hormone prolactin (Abstract). Podium presentation at the 42nd Annual Meeting of the Canadian Association for Physical Anthropology; Fredericton, Canada. November; 2014. http://www.unb.ca/conferences/capa/programand%20abstracts.pdf. [Google Scholar]
- Fujita M, Brindle E, Lo Y, Castro P, Cameroamortegui F. Nutrient intakes associated with elevated serum C-reactive protein concentrations in normal to underweight breastfeeding women in Northern Kenya. American Journal of Human Biology. 2014;26:796–802. doi: 10.1002/ajhb.22600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal I, Parkinson CE. Variation in the pattern of maternal serum vitamin A and carotenoids during human reproduction. Int J Vitam Nutr Res. 1972;42(4):565–75. [PubMed] [Google Scholar]
- Gebre-Medhin M, Vahlquist A, Hofvander Y, Uppsall L, Vahlquist B. Breast milk composition in Ethiopian and Swedish mothers. I. Vitamin A and beta-carotene. Am J Clin Nutr. 1976;29(4):441–51. doi: 10.1093/ajcn/29.4.441. [DOI] [PubMed] [Google Scholar]
- Gibson RS. Principles of nutritional assessment. New York: Oxford University Press; 1990. [Google Scholar]
- Gross R, Hansel H, Schultink W, Shrimpton R, Matulessi P, Gross G, Tagliaferri E, Sastroamdijojo S. Moderate zinc and vitamin A deficiency in breast milk of mothers from East-Jakarta. Eur J Clin Nutr. 1998;52(12):884–90. doi: 10.1038/sj.ejcn.1600660. [DOI] [PubMed] [Google Scholar]
- Haskell MJ, Brown KH. Maternal vitamin A nutriture and the vitamin A content of human milk. J Mammary Gland Biol Neoplasia. 1999;4(3):243–57. doi: 10.1023/a:1018745812512. [DOI] [PubMed] [Google Scholar]
- Hennart PF, Brasseur DJ, Delogne-Desnoeck JB, Dramaix MM, Robyn CE. Lysozyme, lactoferrin, and secretory immunoglobulin A content in breast milk: influence of duration of lactation, nutrition status, prolactin status, and parity of mother. Am J Clin Nutr. 1991 Jan;53(1):32–9. doi: 10.1093/ajcn/53.1.32. [DOI] [PubMed] [Google Scholar]
- Jahoor F, Bhattiprolu S, Del Rosario M, Burrin D, Wykes L, Frazer M. Chronic protein deficiency differentially affects the kinetics of plasma proteins in young pigs. J Nutr. 1996 May;126(5):1489–95. doi: 10.1093/jn/126.5.1489. [DOI] [PubMed] [Google Scholar]
- Jelliffe DB, Jelliffe EF. The volume and composition of human milk in poorly nourished communities: A review. Am J Clin Nutr. 1978;31(3):492–515. doi: 10.1093/ajcn/31.3.492. [DOI] [PubMed] [Google Scholar]
- Lombardi J. Comparative Vertebrate Reproduction. Boston: Kluwer Academic Publishers; 1998. Life Histories; pp. 353–373. [Google Scholar]
- Lunn PG, Austin S, Prentice AM, Whitehead RG. The effect of improved nutrition on plasma prolactin concentrations and postpartum infertility in lactating Gambian women. Am J Clin Nutr. 1984 Feb;39(2):227–35. doi: 10.1093/ajcn/39.2.227. [DOI] [PubMed] [Google Scholar]
- Lunn PG, Prentice AM, Austin S, Whitehead RG. Influence of maternal diet on plasma-prolactin levels during lactation. Lancet. 1980 Mar 22;1(8169):623–5. doi: 10.1016/s0140-6736(80)91119-8. [DOI] [PubMed] [Google Scholar]
- Liyanage C, Hettiarachchi M, Mangalajeewa P, Malawipathirana S. Adequacy of vitamin A and fat in the breast milk of lactating women in south Sri Lanka. Public Health Nutr. 2008 Jul;11(7):747–50. doi: 10.1017/S1368980008001857. Epub 2008 Feb 29. [DOI] [PubMed] [Google Scholar]
- McDade TW. The ecologies of human immune function. Annu Rev Anthropol. 2005;34:495–521. [Google Scholar]
- McLaren DS, Frigg M. Sight and life manual on vitamin A deficiency disorders (VADD) Basel: Task Force SIGHT AND LIFE; 2001. [Google Scholar]
- Mobarhan S, Russell RM, Underwood BA, Wallingford J, Mathieson RD, Al-Midani H. Evaluation of the relative dose response test for vitamin A nutriture in cirrhotics. Am J Clin Nutr. 1981;34:2264–2270. doi: 10.1093/ajcn/34.10.2264. [DOI] [PubMed] [Google Scholar]
- Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314(5802):1157–60. doi: 10.1126/science.1132742. Epub 2006/11/18. [DOI] [PubMed] [Google Scholar]
- Miller EM. Maternal hemoglobin depletion in a settled northern Kenyan pastoral population. Am J Hum Biol. 2010;22:768–774. doi: 10.1002/ajhb.21078. [DOI] [PubMed] [Google Scholar]
- Miller EM. Breastfeeding and immunity in Ariaal mothers and infants. Ann Arbor: University of Michigan; 2011. [Google Scholar]
- Miller EM, Aiello M, Fujita M, Hinde K, Milligan L, Quinn EA. Field and laboratory methods in human milk research. Am J Hum Biol. 2013;25:1–11. doi: 10.1002/ajhb.22334. [DOI] [PubMed] [Google Scholar]
- Micronutrient Initiative and UNICEF. Vitamin and mineral deficiency reports: a global progress report. The Micronutrient Initiative; Ottawa: 2004. [Google Scholar]
- Newman V. Vitamin A and breastfeeding: a comparison of data from developed and developing countries. San Diego: Well-start International; 1993. [Google Scholar]
- Panpanich R, Vitsupakorn K, Harper G, Brabin B. Serum and breast-milk vitamin A in women during lactation in rural Chiang Mai, Thailand. Ann Trop Paediatr. 2002;22(4):321–4. doi: 10.1179/027249302125001976. [DOI] [PubMed] [Google Scholar]
- Patton S, Canfield LM, Huston GE, Ferris AM, Jensen RG. Carotenoids of human colostrum. Lipids. 1990 Mar;25(3):159–65. doi: 10.1007/BF02544331. [DOI] [PubMed] [Google Scholar]
- Prentice A, Prentice AM, Cole TJ, Whitehead RG. Determinants of variations in breast milk protective factor concentrations of rural Gambian mothers. Arch Dis Child. 1983 Jul;58(7):518–22. doi: 10.1136/adc.58.7.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice AL, Stoltzfus RJ, de Francisco A, Chakraborty J, Kjolhede CL, Wahed MA. Maternal vitamin A or beta-carotene supplementation in lactating Bangladeshi women benefits mothers and infants but does not prevent subclinical deficiency. J Nutr. 1999;129(2):356–65. doi: 10.1093/jn/129.2.356. [DOI] [PubMed] [Google Scholar]
- Rosales FJ, Ritter SJ, Zolfaghari R, Smith JE, Ross AC. Effects of acute inflammation on plasma retinol, retinol-binding protein, and its mRNA in the liver and kidneys of vitamin A-sufficient rats. J Lipid Res 1996. 1996;37:962–71. [PubMed] [Google Scholar]
- Roy SK, Islam A, Molla A, Akramuzzaman SM, Jahan F, Fuchs G. Impact of a single megadose of vitamin A at delivery on breastmilk of mothers and morbidity of their infants. Eur J Clin Nutr. 1997;51(5):302–7. doi: 10.1038/sj.ejcn.1600398. [DOI] [PubMed] [Google Scholar]
- Sellen DW. Evolution of infant and young child feeding: implications for contemporary public health. Annu Rev Nutr. 2007;27:123–48. doi: 10.1146/annurev.nutr.25.050304.092557. [DOI] [PubMed] [Google Scholar]
- Shah PS Knowledge Synthesis Group on Determinants of LBW/PT births. Parity and low birth weight and preterm birth: a systematic review and meta-analyses. Acta Obstet Gynecol Scand. 2010 Jul;89(7):862–75. doi: 10.3109/00016349.2010.486827. [DOI] [PubMed] [Google Scholar]
- Shell-Duncan, Yung The maternal depletion transition in northern Kenya: te effects of settlement, development and disparity) Soc Sci Med. 2004;58:2487–2498. doi: 10.1016/j.socscimed.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Silvestre MD, Lagarda MJ, Farré R, Martínez-Costa C, Brines J, Molina A, Clemente G. A study of factors that may influence the determination of copper, iron, and zinc in human milk during sampling and in sample individuals. Biol Trace Elem Res. 2000 Sep;76(3):217–27. doi: 10.1385/BTER:76:3:217. [DOI] [PubMed] [Google Scholar]
- StataCorp LP. Stata statistical software, version 13.1. College Station Texas; StataCorp LP: 1985–2013. [Google Scholar]
- Stearns SC. Issues in Evolutionary Medicine. Am J Hum Biol. 2005;17:131–140. doi: 10.1002/ajhb.20105. [DOI] [PubMed] [Google Scholar]
- Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr. 2001;21:167–92. doi: 10.1146/annurev.nutr.21.1.167. Epub 2001/05/26. [DOI] [PubMed] [Google Scholar]
- Stoltzfus RJ. SCN News. Vol. 11. United Nation Subcommittee on Nutrition; 1994. [Accessed on December 7, 2015]. Vitamin A deficiency in the mother-infant dyad; pp. 25–27. at: http://www.unsystem.org/SCN/archives/scnnews11/ch10.htm. [PubMed] [Google Scholar]
- Stoltzfus RJ, Humphrey JH. Vitamin A and the nursing mother-infant dyad: evidence for intervention. Adv Exp Med Biol. 2002;503:39–47. doi: 10.1007/978-1-4615-0559-4_4. [DOI] [PubMed] [Google Scholar]
- Stoltzfus RJ, Miller KW, Hakimi M, Rasmussen KM. Conjunctival impression cytology as an indicator of VA status in lactating Indonesian women. Am J Clin Nutr. 1993;58(2):167–73. doi: 10.1093/ajcn/58.2.167. [DOI] [PubMed] [Google Scholar]
- Stoltzfus RJ, Underwood BA. Breast-milk vitamin A as an indicator of the vitamin A status of women and infants. Bull World Health Organ. 1995;73(5):703–11. [PMC free article] [PubMed] [Google Scholar]
- Thurnham DI, McCabe GP, Northrop-Clewes CA, Nestel P. Effects of subclinical infection on plasma retinol concentrations and assessment of prevalence of vitamin A deficiency: meta-analysis. Lancet. 2003;362:2052–8. doi: 10.1016/s0140-6736(03)15099-4. [DOI] [PubMed] [Google Scholar]
- Tracer DP. Fertility-related changes in maternal body composition among the Au of Papua New Guinea. Am J Phys Anthro. 1991;85:393–405. doi: 10.1002/ajpa.1330850404. [DOI] [PubMed] [Google Scholar]
- Williams CD. Fifty years ago. Archives of diseases in childhood 1933. A nutritional disease of childhood associated with a maize diet. Arch Dis Child. 1983;58(7):550–60. doi: 10.1136/adc.58.7.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Indicators for assessing vitamin A deficiency and their application in monitoring and evaluating intervention programmes. WHO; Geneva: 1996. [Google Scholar]
- WHO. Guideline: vitamin A supplementation in postpartum women. World health Organization; 2011. [PubMed] [Google Scholar]
- WHO. e-Library of Evidence for Nutrition Actions (eLENA) World Health Organization; 2014. Vitamin A supplementation in postpartum women. Update. Available at: http://www.who.int/elena/titles/vitamina_postpartum/en/ [Google Scholar]
- Wood JW. Dynamics of human reproduction: Biology, biometry, demography. New York: Aldine de Gruyter; 1994. [Google Scholar]