Abstract
Background
To examine swallowing-related lower cranial nerve palsy (LCNP) in oropharyngeal cancer (OPC) survivors after intensity modulated radiotherapy (IMRT).
Methods
Patients treated with definitive IMRT (66–72 Gy) were pooled from trial databases. Prospective analyses on parent trials included videofluoroscopy, clinical LCNP examination, and questionnaires pre- and 6-, 12-, and 24-months post-IMRT. Time-to-event and incidence of LCNP was estimated with competing risk methods. Literature review (1977–2015) summarized published LCNP outcomes.
Results
Three of 59 OPC survivors with a minimum 2-year follow-up developed hypoglossal palsy ipsilateral to the index tumor (median latency: 6.7 years, range: 4.6–7.6). At a median of 5.7 years, cumulative incidence of LCNP was 5%. LCNP preceded progressive dysphagia in all cases. Published studies found median incidence of radiation-associated LCNP was 10.5% after NPC, but no OPC-specific estimate.
Conclusions
While uncommon, the potential for late LCNP preceding swallowing deterioration highlights the importance of long-term functional surveillance in OPC survivorship.
Keywords: Dysphagia, cranial neuropathy, hypoglossal nerve, radiotherapy, oropharynx cancer
INTRODUCTION
The epidemiology of oropharyngeal cancer (OPC) has changed dramatically in recent decades as the incidence of human papillomavirus (HPV)-associated disease has increased and tobacco-associated disease has decreased.1,2 In this same time, survival rates of OPC steadily improved and organ preservation regimens of radiation and chemoradiation replaced radical surgical methods as the primary modality for treating OPC. Projections suggest that the number of OPC survivors will continually rise over the next 2 decades,2 and the majority of these survivors will have received curative doses of radiotherapy in excess of 65-Gy. Consequently, an ever growing number of patients with HPV-associated OPC have the potential for long-term cure surviving decades after their index cancer, mandating an unprecedented focus on late effects of therapy for OPC.
Radiation-associated cranial neuropathy is a rare but functionally devastating late effect of head and neck radiotherapy. Cranial nerves are widely regarded as relatively radio-resistant structures. However, over the decades, small cohort studies have examined lower cranial nerve palsy (LCNP) among head and neck cancer survivors,3–6 principally among patients with nasopharyngeal cancers, suggesting a long latency, the potential for progressing polyneuropathies, and implicating various central and peripheral mechanisms of denervation. The potential for radiation associated LCNP in non-NPC head and neck cancer survivors has rarely been examined in published reports. Yet, with exceptional numbers of OPC survivors achieving long-term disease control, recent case reports highlight the potential for de novo LCNP as a previously unexpected late effect of even modern conformal IMRT for OPC.7,8 In this report, we examine incidence, latency, and patterns of delayed LCNP after oropharyngeal IMRT with particular interest in implications on long-term swallowing function.
MATERIALS AND METHODS
Study Design and Eligibility
A pooled dataset was analyzed from 2 institutional single-arm organ preservation trials for locoregionally advanced stage head and neck cancer. Trial databases were sampled to include patients treated with definitive IMRT and systemic therapy for stage III-IV squamous cell carcinoma of the oropharynx with minimum 1-year disease free survival after enrollment. Among 64 oropharyngeal cancer patients enrolled, we excluded patients treated with 3D conformal technique (n=2), less than 1 year disease-free follow-up (n=2), and also excluded a single patient who discontinued radiotherapy against medical advice after 41 Gy in 19 fractions. Protocols were approved by the local Institutional Review Board and all patients provided informed consent for trial participation.
Treatment
All included patients received definitive IMRT with systemic therapy. Fifty five patients (93%) were treated with a split field technique (IMRT delivered to primary tumor and upper neck, while levels III and IV were treated with an anterior portal and lower larynx shielding). Thirty six patients were treated on an induction chemotherapy PCC trial,9,10 and the remaining 23 on a trial of adaptive-IMRT.11 Trial details and clinical reports have been published elsewhere, and are briefly reviewed below.
Induction trial
After a loading dose of cetuximab 400 mg/m2 intravenously, patients received 6 weekly cycles of cetuximab 250 mg/m2 and paclitaxel 135 mg/m2 followed by carboplatin area under the curve 2 followed by risk based local therapy. Definitive radiotherapy commenced 2 to 3 weeks after induction therapy. Target volumes and local therapy assignments were based on initial staging (not response to induction): radiation as a single modality for T1-T2 and concurrent chemoradiation for T3-T4 stage OPC. Gross disease and margin were administered a dose of 66 Gy in 30 fractions for T1 disease and 72 Gy in 40 to 42 fractions with a concomitant boost fractionation schedule for patients with T2–4 tumors. All radiation schedules were planned for 6 weeks of therapy. Neck dissection was recommended for residual adenopathy after completion of chemoradiotherapy. Among 47 included in the initial clinical report, complete and partial response to induction was 19% and 77% respectively. The 1 and 3 -year locoregional control was 94% and 87%, respectively.9
Adaptive IMRT trial
An image guided radiotherapy and adaptive re-planning paradigm was used for IMRT delivery among patients enrolled on this trial. Baseline IMRT planning was conducted according to institutional standards as detailed previously. Adaptive replanning was performed for all patients on trial at least once based on daily CT-on-rails images. Systemic therapy was delivered in all patients, most commonly single agent weekly cisplatin. Among 22 patients included in the initial clinical report, 2-year locoregional control was 95%.11
Functional assessments
Prospective functional assessment methods were identical on each trial and included a modified barium swallow (MBS) study, swallow specific clinical cranial nerve examination, and swallow-specific questionnaires at baseline, and 6-, 12-, and 24-months after end of radiotherapy. Modified barium swallow studies were conducted according to a standard protocol (2 trials each of: 5-mL, 10-mL, 20-mL, cup sips Varibar thin liquid, Varibar pudding, and barium coated cracker) at 30 frames per second. MBS results were scored according to the validated DIGEST tool that assigns a CTCAE compatible severity grade to pharyngeal stage dysphagia (1=mild, 2=moderate, 3=severe, 4=life threatening).12 Questionnaires included the patient-reported MD Anderson Dysphagia Inventory (MDADI)13 and the clinician administered Performance Status Scale of Head and Neck Cancer (PSSHN).14 MDADI is a validated, widely used 20-item PRO measure of swallowing-related quality of life (20=worst score, 100=best score). PSSHN Normalcy of Diet subscale is a validated semi-structured interview measure of oral intake (0=NPO, 100=Full, unrestricted oral diet). LCNP were assessed by clinical cranial nerve examination at swallowing studies and head and neck cancer surveillance visits. Given our a priori focus on denervation as a driver of late dysphagia, nerves IX, X, and XII were selected for this report for their particular importance in pharyngeal swallow function. Motor functions for swallowing were considered (IX: pharyngeal shortening/constriction, X: glottic closure, velar elevation, pharyngeal constriction, XII: tongue mobility). EMG and neurology consultations were not standard. All included patients had normal pre-therapy and post-radiation clinical cranial nerve examination. All patients presenting with delayed LCNP were referred for late functional assessment with comparable swallowing evaluation methods to the earlier functional assessments on trial (MBS and swallow-related questionnaires).
Statistical methods
Descriptive statistics were calculated and graphically plotted. Chi-square test or Fisher exact test were used to test differences of categorical variables and Wilcoxon rank-sum test or Kruskal-Wallis were used to assess between group differences for continuous variables. Survival outcomes were estimated by the Kaplan-Meier method with log-rank tests to examine between group differences. Times were censored at the last event-free contact. Cumulative incidence rates of late LCNP events were calculated with death or new disease (locoregional recurrence, distant metastasis, or second primary malignancy) as a competing risk. Effects of T-classification and tumor site on cumulative incidence of late events were evaluated in the univariate setting using Gray's test. SAS version 9.2 and S-Plus version 8.04 were used for analyses.
Literature review methods
A supporting literature review was conducted to examine the body of published work on radiation-associated LCNP. The primary search for the literature review was conducted using the electronic MEDLINE database (data source: OVID) between June 2013 and July 2013. A second search was conducted through September 2015. The search was limited to human subject research in peer-reviewed journal articles. Medical subject heading (MeSH) terms were used to identify papers for common to 3 groups of terms: 1) radiotherapy, 2) pharyngeal neoplasms or oropharyngeal neoplasms or nasopharyngeal neoplasms or hypopharyngeal neoplasms or laryngeal neoplasms or head and neck neoplasms, and 3) cranial nerve diseases or vagus nerve diseases or glossopharyngeal nerve diseases or hypoglossal nerve diseases. Abstracts were screened according to the following exclusion criteria: 1) neuropathies of non-radiotherapy source (e.g., tumor-associated, surgery-induced, or unclear source), 2) review articles, 3) non-English language, or 4) animal studies. The full-text of 27 articles published between 1977 and 2015 reporting LCNP as a result of definitive radiotherapy or chemoradiotherapy for head and neck cancers (any site) was reviewed with standard elements extracted on a study-specific form. Results were summarized in descriptive format.
RESULTS
Patient characteristics
Fifty-nine oropharyngeal cancer patients treated with definitive IMRT and systemic therapy were included. The median age at trial enrollment was 53, and 81% were male. All had stage III/IV disease. TNM classification, tumor site, and therapeutic combinations are detailed in Table 1. Median overall survival was not yet reached; 5- and 10-year overall survival rates were 98.2% and 87.3%, respectively (Figure 1).
Table 1.
Patient Characteristics
| No. (%) pfs |
|
|---|---|
| Sex | |
| Male | 43 (81%) |
| Female | 11(19%) |
| Age | |
| Median (range) | 53 (35–78) |
| Smoking status | |
| Current | 12 (20%) |
| Former | 22 (37%) |
| Never | 25 (42%) |
| T classification | |
| X/1 | 17 (29%) |
| 2 | 22 (37%) |
| 3 | 15 (25%) |
| 4 | 5 (9%) |
| N classification | |
| 0 | 3 (5%) |
| 1 | 2 (3%) |
| 2 | 48 (81%) |
| 3 | 6 (10%) |
| Tumor subsite | |
| Base of tongue | 37 (63%) |
| Tonsil | 22 (37%) |
| Chemotherapy | |
| Neoadjuvant/adjuvant | 22 (38%) |
| Concurrent | 22 (38%) |
| Induction + concurrent | 15 (25%) |
| Neck dissection | |
| No | 40 (68%) |
| Yes | 19 (32%) |
FIGURE 1. Kaplan-Meier curve of overall survival.
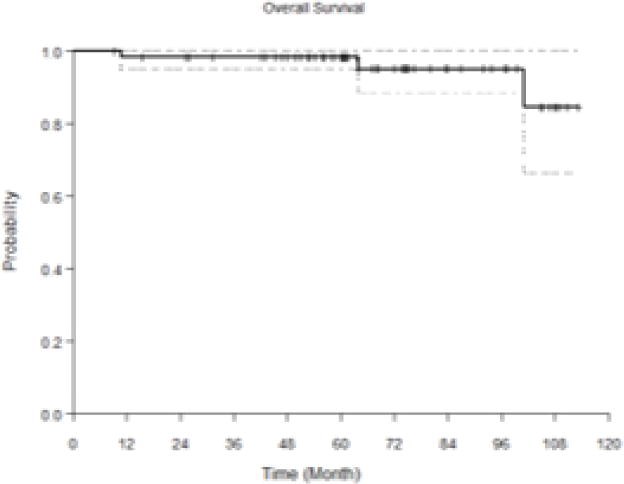
Median overall survival was not yet reached; 5- and 10-year overall survivor rates were 98.2% and 87.3% in this pooled cohort.
Cranial neuropathies
Three patients developed delayed hypoglossal palsy ipsilateral to the index oropharyngeal tumor with a median latency of 6.7 years (range: 4.6 – 7.6, Table 2). At a median follow up of 10 years, 5% of patients enrolled had developed lower cranial neuropathy. Cumulative incidence of cranial neuropathy is illustrated in Figure 2. LCNP cumulative incidence rates estimated from competing risk analysis were 6-year: 2.1% (95% CI: 0.2–10%), 7-year: 6.1% (95% CI: 0.9%–19%), and 8-year: 11% (95%: 2.4%–28%). LCNP preceded swallow deterioration in all patients relative to post-RT recovery, both by patient-report (per MDADI) and clinician-rating (per MBS) as illustrated in Figure 3.
Table 2.
LCNP Cases (n = 3)
| Age, Sex, Smoke | Site, Stage | Chemo | Latency (yrs) | LCNP |
|---|---|---|---|---|
| 68, M, former | T4N2b Tonsil | Induction + concurrent | 7.6 | XII (ipsi) |
| 43, M, former | T2N3 Tonsil | Induction + concurrent | 6.7 | XII (ipsi) |
| 51, M, former | T2N2b EOT | Concurrent | 4.6 | XII (ipsi) |
LCNP, lower cranial nerve palsy. Three patients developed delayed hypoglossal palsy ipsilateral to the index oropharyngeal tumor with a median latency of 6.7 years (range : 4.6–7.6 years).
FIGURE 2. Cumulative incidence of LCNP.
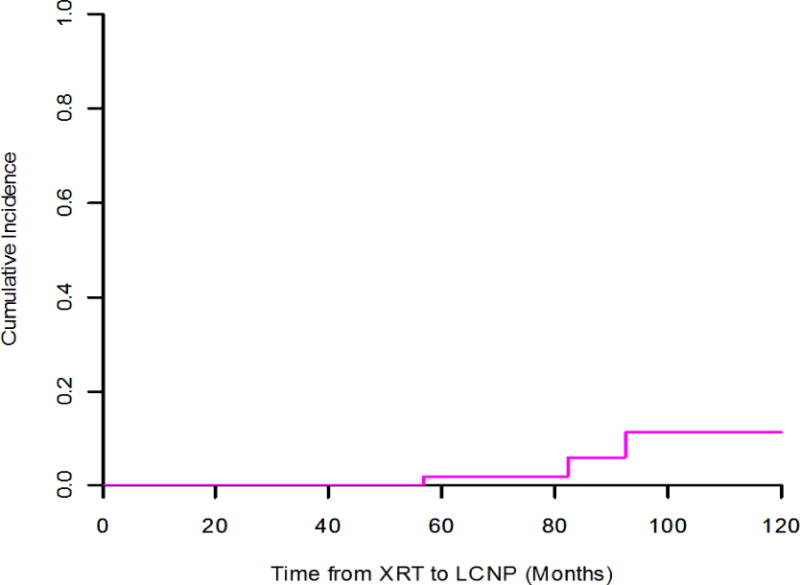
At a median follow-up of 5.7 years, cumulative incidence of delayed lower cranial neuropathy was 5%; 5- and 7-year LCNP rates were 2.1% and 6.1%, respectively.
FIGURE 3. LCNP case trajectories.
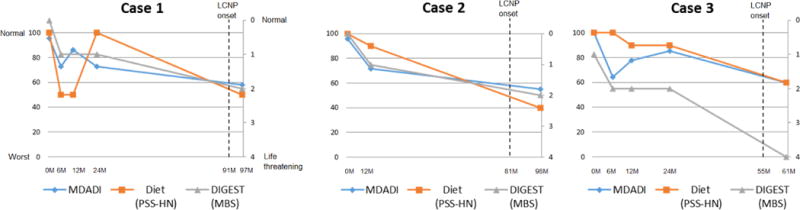
Measures of swallowing function longitudinally plotted for each LCNP case using modified barium swallow (MBS) outcomes (Dynamic Imaging Grade of Swallowing Toxicity, DIGEST), perceived dysphagia per MD Anderson Dysphagia Inventory (MDADI) questionnaire, and standardized diet rating per Performance Status Scale of Head and Neck Cancer (PSSHN). Delayed LCNP preceded progressive late dysphagia (per fluoroscopy, diet, and self-report) in all 3 cases.
Literature review
27 papers reporting on 2,775 patients were included in the literature review. Study designs of the 27 papers included 12 case reports, 6 case series, and 9 retrospective cohorts. No prospective studies were identified. Subjects included 97.1 % (2,694/2,775) with NPC, 2.3 % (63/2,775) with OPC, 0.2% (5/2,775) with hypopharynx cancer, 0.1% (5/2,775) with laryngeal cancer, 0.1% (3/2,775) with oral cavity cancer, and 0.2% (5/2,775) with other sites head and neck cancer. Radiation delivery varied among the patients examined in this literature review, with the majority of patients receiving a total dose around 70 Gy, often with a parapharyngeal boost,5,7,15–18 and chemoradiotherapy.7,15–17,19–21 Incidence rates of radiation-associated LCNP were reported in nine retrospective cohorts comprising 2,565 patients with NPC and 12 patients with non-NPC sites of HNC. The median incidence rate reported of patients with any radiation-associated LCNP was 10.5% with a range from 3.7 to 25.6% as shown in Figure 4.16,22 Hypoglossal nerve palsy was the most frequently reported radiation-associated LCNP. The median latency of onset measures for any LCNP ranged from 2.9 to 7.9 years.4,23 The upper limit was reported at 34 years,24 and the lower limit at 1 year.25 Case reports and case series examining radiation-associated LCNP are summarized in supplementary tables. Incidence reports from cohort studies and latency data are summarized in Table 3 and supplementary tables, respectively.
FIGURE 4. LCNP literature review.
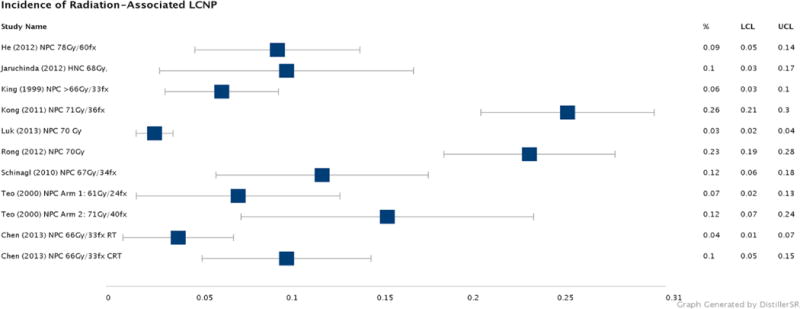
Incidence rates of LCNP after head and neck radiotherapy in published cohort studies are summarized by Forest Plot method. Median incidence rate of LCNP was 10.5% in long-term survivorship of NPC. While most cohorts observed incidence rates under 12%, two cohorts with long follow-up of survivors (minimum of 3.8 years) found more than 20% incidence of LCNP at median of 11.4Y follow-up. No OPC-specific incidence studies were identified in published data.
Table 3.
Incidence of Radiation-Associated Lower Cranial Neuropathies in Published Cohorts
| Lower Cranial Neuropathies | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Study | n | Site of Disease | Treatment | Boost (+/−) | IX | X | XII | Any | Multiple |
|
| |||||||||
| Retrospective Cohorts | |||||||||
| He [31] | 158 | NP | Accelerated fractionation, total dose of 78 Gy/60 fx/6 weeks | + | − | 7% | 7% | 11% | 4% |
| Jaruchinda [4] | 70 | NP, OP, HP, OC, O | 1.8–2 Gy/fx/day total dose 44–50 Gy for N0, total dose 66–70 Gy for N1 & N2, 1 pt with RICNP received CRT | − | − | 10% | − | 10% | − |
| King [5] | 234 | NP | ≥ 66 Gy, 2 Gy/fx/day or 1.6 Gy/2 fx/day Boost |
+ | − | 1.71% | 5.98% | 5.98% | 1.71% |
| Kong[23] | 317 | NP | Technique A: median dose 71 Gy Technique B: median dose 61 Gy Boost |
+ | − | − | − | 25.6% (total from Technique A and B) | − |
| Luk [17] | 965 | NP | 70 Gy, boost 16.7%, CRT 58.3% | − | − | − | 3.1% | 3.7% | − |
| Rong [22] | 307 | NP | 60-80 Gy 1 fx/day | − | 19% | 12% | 23% | 20% | |
| Schinagl [16] | 117 | NP | 2Gy/fx/day, or 70Gy + 7 Gy boost, or endocavitary boost dose of 12 Gy, or neoadjuvant chemotherapy | + | 1% | − | 10% | 20.5% | 4.3% |
| Teo [18] | 159 | NP | Arm I: conventional 2D 61Gy/24 fx Arm II: 2D hyperfractionation 71Gy 2/40 + 14 Gy boost |
+ | − | − | − | Arm I: 8.7% Arm II: 13.0% Total LCNP: 11.0% |
− |
| Chen [32] | 316 | NP | >66 Gy ± weekly cisplatin | − | − | − | − | RT: 2.53% CRT: 6.33% |
− |
DISCUSSION
Radiation-associated LCNP is commonly recognized as rare late effect of radiotherapy for NPC, but infrequently reported in non-NPC survivors. With ever growing numbers of OPC survivors, case reports are emerging that suggest that clinicians now increasingly encounter LCNP in long-term survivorship after curative radiotherapy or chemoradiotherapy for OPC.1,7,8 Results of this cohort analysis suggest that LCNP is indeed a potential late effect of oropharyngeal IMRT, detected in 3 of 59 survivors in this study. LCNP appears to be associated with both clinician- and patient-detected deterioration in swallowing function and associated decrements in QOL in long-term survivorship.
The source of injury in radiation-associated LCNP is debated. In our review of the literature, authors speculated as to various sources of injury in patients who develop radiation-associated LCNP but the underlying mechanisms are not well studied. When survivors present with de novo LCNP, it is first necessary to rule out tumor invasion before radiation can be attributed as the source of nerve palsy. A retrospective study investigating hypoglossal nerve palsy in NPC survivors found that recurrent tumor accounted for only 11.8% (2/17) of patients first seen with XII CNP after RT, and radiation was the cause of denervation in the majority of patients.5 Others have suggested that upper cranial nerve palsy is more often a result of tumor invasion, whereas LCNP is typically a result of radiation-injury.23 The debate of peripheral or central pathology causing radiation-associated damage can be considered only once tumor recurrence is excluded as the source of LCNP, as was the case in all patients examined in this cohort analysis.
In published literature, radiation-associated LCNP was most often hypothesized to be a result of compressive fibrosis and/or direct ischemic nerve damage.25 For instance, necropsy in a single LCNP case revealed notable peripheral fibrosis surround the affected nerve with myelin loss and atrophy, but comparably no micro- or macroscopic fibrosis in a control patient without LCNP.4 The authors concluded that the probable location of source injury was peripheral due to fibrotic tissue pressure, vascular interference, and morphology of neural vascular vessels.4 Another NPC case series supporting this notion of compressive fibrosis on the nerve tracts based this on the observation of “marked neck fibrosis” in 63% (12/19) of LCNP cases evaluated.6 While some suggest permanent depletion of Schwann cells or nerve ischemia may contribute to functional injury,26 many suggest that soft tissue fibrosis may be a substantive and more prevalent factor leading to radiation-associated LCNP than other sources. It is, however, also possible that radiation-associated LCNP could be caused by central injury to the brainstem nuclei as a result of the higher dose the bulbar region receives in some conformal methods such as IMRT that spread low dose bath circumferentially leading to higher brainstem dose relative to older parallel beam methods.27
In NPC, the most common organ-at-risk for nerve injury is typically considered the carotid sheath in the lower nasopharyngeal region.5 A retrospective cohort analyzing the potential risk factors of developing radiation-associated LCNP suggested that the possible exposure of 70 Gy or above to the carotid space as a potential source location of injury due cranial nerves’ passage along the vessel, and we have similarly found a candidate dose-threshold of mean 62-Gy to the superior pharyngeal constrictor region discriminating OPC survivors with LCNP from non-LCNP controls.17,28 Several other studies also attributed fibrosis of the neurovascular bundle in the parapharyngeal region as the source location for radiation-associated LCNP.18,22 Historically, Berger & Bataini (1977) suggested that the location of injury can be determined by the number of nerves affected, suggesting that an isolated XII nerve palsy is presumably related to submandibular damage, isolated X nerve is related to carotid sheath damage, and any combination of X, XI, and XII nerves reflects skull base pathology.
Treatment-related factors predisposing to LCNP reviewed in the extant (predominantly NPC) literature are not easily transferable to our OPC cohort. Nonetheless, predisposing factors were discussed in more than a dozen studies, dominated by speculations regarding therapeutic radiation dose, radiation field, radiation boost, and individual susceptibility and radio-sensitivity. Total radiation dose was the most commonly reported predisposing factor of LCNP. Dose thresholds around 70 Gy were commonly conjectured,23,29 alongside suggestions of greater risk of nerve damage with RT boost techniques. It was also suggested that the interval between radiotherapy and appearance of palsy may be inversely related to total dose.3 Radiation field is obviously an additional factor that predisposes patients to LCNP, but rarely discussed in published work to date outside of, again, occasional mention of higher risk in those who require therapeutic doses along the carotid sheath, parapharyngeal space, or treatment of large subdigastric lymph nodes or retropharyngeal nodes.5 Effects of concomitant or adjuvant chemotoxicity on LCNP were conflicting with some authors reporting no demonstrable impact17 and others reporting multiple (4 of 63) patients developing a CNP within a few days after administration of cisplatin.30 With a rare late event such as LCNP, appropriately powered risk studies are exceedingly challenging to conduct and have not been examined to date. At this time, predisposing factors (treatment or patient related) and source(s) of injury are quite poorly understood, providing little rational basis for risk reduction strategies in current clinical practice.
Herein, we report the latency and incidence of radiation-associated LCNP among a small cohort of 59 long-term OPC survivors treated with similar IMRT methods and systemic therapy. Our initial interest in LCNP in OPC was spawned from a line of work in late radiation-associated dysphagia (“late-RAD”) in which we noticed a preponderance of clinically detectable LCNP as the presenting symptom in late-RAD cases who suffer from progressive, treatment refractory dysphagia in long-term survivorship. For this reason, our report focused on swallowing related LCNP including IX, X, and XII. Extensions of this work may need to consider XI as we have clinically seen a tendency for brachial plexus dysfunction co-existing in survivors who present with late-RAD. We acknowledge the limitations of a retrospective analysis of prospective trial data, foremost among these the lack of electromyographic data, neurography, or DTI to better characterize the cranial nerve dysfunction. There is also the potential that we have under-reported the incidence of late effects because late cranial nerve testing and swallowing evaluations (after 2 years’ survivorship) were only conducted by symptomatic referral. Nonetheless, these data highlight the potential for a rare, but functionally critical late effect in a growing, young survivorship pool of HNC survivors. Future work must seek to examine risk in larger cohorts for more precise risk estimates, and better characterization of actionable risk factors.
CONCLUSIONS
Radiation-associated LCNP is known as a rare, but potentially severe late effect in NPC survivors. This report represents the first cohort analysis examining radiation-associated LCNP in OPC survivorship among patients treated with definitive IMRT with systemic therapy. At a median follow-up of 5.7 years, cumulative incidence of delayed lower cranial neuropathy was 5% and delayed LCNP preceded progressive dysphagia in all cases. While rare, the potential for late onset cranial neuropathies precipitating swallowing deterioration highlights the importance of long-term functional surveillance in OPC survivorship.
Supplementary Material
Acknowledgments
Funding: This work was completed with support of the MD Anderson Oropharynx Program Patient-Reported Outcomes/Function Core. Dr. Hutcheson receives grant support from the MD Anderson Institutional Research Grant Program and the National Cancer Institute (R03 CA188162). Drs. Lai, Fuller, and Hutcheson receive funding support from the National Institute for Dental and Craniofacial Research (R01 DE025248 and 1R56DE025248-01). Dr. Fuller received/receives grant and/or salary support from: the National Institutes of Health/National Cancer Institute’s Paul Calabresi Clinical Oncology Award Program (K12 CA088084-06) and Clinician Scientist Loan Repayment Program (L30 CA136381-02); the SWOG/Hope Foundation Dr. Charles A. Coltman, Jr., Fellowship in Clinical Trials; a General Electric Healthcare/MD Anderson Center for Advanced Biomedical Imaging In-Kind Award; an Elekta AB/MD Anderson Department of Radiation Oncology Seed Grant; the Center for Radiation Oncology Research at MD Anderson Cancer Center; and the MD Anderson Institutional Research Grant Program. This work was supported in part infrastructure support by National Institutes of Health Cancer Center Support (Core) Grant CA016672 to The University of Texas MD Anderson Cancer Center. These listed funders/supporters played no role in the study design, collection, analysis, interpretation of data, manuscript writing, or decision to submit the report for publication.
Footnotes
CONFLICT OF INTEREST DISCLOSURES: The authors made no disclosures.
References
- 1.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 2.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger PS, Bataini JP. Radiation-induced cranial nerve palsy. Cancer. 1977;40(1):152–155. doi: 10.1002/1097-0142(197707)40:1<152::aid-cncr2820400125>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 4.Jaruchinda P, Jindavijak S, Singhavarach N. Radiation-related vocal fold palsy in patients with head and neck carcinoma. J Med Assoc Thai. 2012;95(Suppl 5):S23–28. [PubMed] [Google Scholar]
- 5.King AD, Leung SF, Teo P, Lam WW, Chan YL, Metreweli C. Hypoglossal nerve palsy in nasopharyngeal carcinoma. Head Neck. 1999;21(7):614–619. doi: 10.1002/(sici)1097-0347(199910)21:7<614::aid-hed5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 6.Lin YS, Jen YM, Lin JC. Radiation-related cranial nerve palsy in patients with nasopharyngeal carcinoma. Cancer. 2002;95(2):404–409. doi: 10.1002/cncr.10668. [DOI] [PubMed] [Google Scholar]
- 7.Huang AT, Song S, Dominguez LM, Nguyen J, Goldman RA, Reiter ER. Delayed lower cranial neuropathies following primary radiotherapy for oropharyngeal squamous cell carcinoma. Laryngoscope. 2013;123(5):1207–1209. doi: 10.1002/lary.23938. [DOI] [PubMed] [Google Scholar]
- 8.Hutcheson KA, Yuk MM, Holsinger FC, Gunn GB, Lewin JS. Late radiation-associated dysphagia (late-RAD) with lower cranial neuropathy in long-term oropharyngeal cancer survivors: Video case reports. Head Neck. 2015;37(4):E56–62. doi: 10.1002/hed.23840. [DOI] [PubMed] [Google Scholar]
- 9.Kies MS, Holsinger FC, Lee JJ, et al. Induction chemotherapy and cetuximab for locally advanced squamous cell carcinoma of the head and neck: results from a phase II prospective trial. J Clin Oncol. 2010;28(1):8–14. doi: 10.1200/JCO.2009.23.0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutcheson KA, Lewin JS, Holsinger FC, et al. Long-term functional and survival outcomes after induction chemotherapy and risk-based definitive therapy for locally advanced squamous cell carcinoma of the head and neck. Head Neck. 2014;36(4):474–480. doi: 10.1002/hed.23330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz DL, Garden AS, Thomas J, et al. Adaptive radiotherapy for head-and-neck cancer: initial clinical outcomes from a prospective trial. Int J Radiat Oncol Biol Phys. 2012;83(3):986–993. doi: 10.1016/j.ijrobp.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutcheson KA, Barrow MP, Barringer DA, et al. Dynamic Imaging Grade of Swallowing Toxicity (DIGEST): Scale development and validation. Cancer. 2016 doi: 10.1002/cncr.30283. [e-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen AY, Frankowski R, Bishop-Leone J, et al. The development and validation of a dysphagia-specific quality-of-life questionnaire for patients with head and neck cancer: the M. D. Anderson dysphagia inventory. Arch Otolaryngol Head Neck Surg. 2001;127(7):870–876. [PubMed] [Google Scholar]
- 14.List MA, Ritter-Sterr C, Lansky SB. A performance status scale for head and neck cancer patients. Cancer. 1990;66(3):564–569. doi: 10.1002/1097-0142(19900801)66:3<564::aid-cncr2820660326>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 15.Lau DP, Lo YL, Wee J, Tan NG, Low WK. Vocal fold paralysis following radiotherapy for nasopharyngeal carcinoma: laryngeal electromyography findings. J Voice. 2003;17(1):82–87. doi: 10.1016/s0892-1997(03)00028-6. [DOI] [PubMed] [Google Scholar]
- 16.Schinagl DAX, Marres HAM, Kappelle AC, et al. External beam radiotherapy with endocavitary boost for nasopharyngeal cancer: treatment results and late toxicity after extended follow-up. Int J Radiat Oncol Biol Phys. 2010;78(3):689–695. doi: 10.1016/j.ijrobp.2009.08.072. [DOI] [PubMed] [Google Scholar]
- 17.Luk YS, Shum JSF, Sze HCK, Chan LLK, Ng WT, Lee AWM. Predictive factors and radiological features of radiation-induced cranial nerve palsy in patients with nasopharyngeal carcinoma following radical radiotherapy. Oral Oncol. 2013;49(1):49–54. doi: 10.1016/j.oraloncology.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Teo PM, Leung SF, Chan AT, et al. Final report of a randomized trial on altered-fractionated radiotherapy in nasopharyngeal carcinoma prematurely terminated by significant increase in neurologic complications. Int J Radiat Oncol Biol Phys. 2000;48(5):1311–1322. doi: 10.1016/s0360-3016(00)00786-0. [DOI] [PubMed] [Google Scholar]
- 19.Kang MY, Holland JM, Stevens KR., Jr Cranial neuropathy following curative chemotherapy and radiotherapy for carcinoma of the nasopharynx. J Laryngol Otol. 2000;114(4):308–310. doi: 10.1258/0022215001905436. [DOI] [PubMed] [Google Scholar]
- 20.Chaudhry MR, Akhtar S. Bilateral vocal cord paralysis following radiation therapy for nasopharyngeal carcinoma. ORL J Otorhinolaryngol Relat. 1995;57(1):48–49. doi: 10.1159/000276707. [DOI] [PubMed] [Google Scholar]
- 21.Dejaeger E, Goethals P. Deglutition disorder as a late sequel of radiotherapy for a pharyngeal tumor. Am J Gastroenterol. 1995;90(3):493–495. [PubMed] [Google Scholar]
- 22.Rong X, Tang Y, Chen M, Lu K, Peng Y. Radiation-induced cranial neuropathy in patients with nasopharyngeal carcinoma. Strahlenther Onkol. 2012;188(3):282–286. doi: 10.1007/s00066-011-0047-2. [DOI] [PubMed] [Google Scholar]
- 23.Kong L, Lu JJ, Liss AL, et al. Radiation-induced cranial nerve palsy: a cross-sectional study of nasopharyngeal cancer patients after definitive radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79(5):1421–1427. doi: 10.1016/j.ijrobp.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Stern Y, Marshak G, Shpitzer T, Segal K, Feinmesser R. Vocal cord palsy: possible late complication of radiotherapy for head and neck cancer. Ann Otol Rhinol Laryngol. 1995;104(4 Pt 1):294–296. doi: 10.1177/000348949510400407. [DOI] [PubMed] [Google Scholar]
- 25.Rison RA, Beydoun SR. Delayed cervicobulbar neuronopathy and myokymia after head and neck radiotherapy for nasopharyngeal carcinoma: a case report. J Clin Neuromuscul Dis. 2011;12(3):147–152. doi: 10.1097/CND.0b013e31820d4f20. [DOI] [PubMed] [Google Scholar]
- 26.Takimoto T, Saito Y, Suzuki M, Nishimura T. Radiation-induced cranial nerve palsy: hypoglossal nerve and vocal cord palsies. J Laryngol Otol. 1991;105(1):44–45. doi: 10.1017/s0022215100114793. [DOI] [PubMed] [Google Scholar]
- 27.Mohamed ASR, Lai SY, Murri M, et al. Dose-volume correlates of osteoradionecrosis of the mandible in oropharynx patients receiving intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2015;96(Supplement):S220–221. [Google Scholar]
- 28.Awan MJ, Mohamed AS, Lewin JS, et al. Late radiation-associated dysphagia (late-RAD) with lower cranial neuropathy after oropharyngeal radiotherapy: a preliminary dosimetric comparison. Oral Oncol. 2014;50(8):746–752. doi: 10.1016/j.oraloncology.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang V, Liao KK, Ju TH, Lin KP, Wang SJ, Wu ZA. Myokymia and neuromyotonia of the tongue: a case report of complication of irradiation. Zhonghua Yi Xue Za Zhi (Taipei) 1993;52(6):413–415. [PubMed] [Google Scholar]
- 30.Frustaci S, Barzan L, Comoretto R, Tumolo S, Lo Re G, Monfardini S. Local neurotoxicity after intra-arterial cisplatin in head and neck cancer. Cancer Treat Rep. 1987;71(3):257–259. [PubMed] [Google Scholar]
- 31.He X, Ye M, Guo X, et al. Treatment outcome of patients with stages I-II nasopharyngeal carcinoma after late course accelerated hyperfractionation radiotherapy alone. Oral Oncol. 2012;48(10):1058–1063. doi: 10.1016/j.oraloncology.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Sun Y, Liang SB, et al. Progress report of a randomized trial comparing long-term survival and late toxicity of concurrent chemoradiotherapy with adjuvant chemotherapy versus radiotherapy alone in patients with stage III to IVB nasopharyngeal carcinoma from endemic regions of China. Cancer. 2013;119(12):2230–2238. doi: 10.1002/cncr.28049. [DOI] [PubMed] [Google Scholar]
- 33.Prepageran N, Raman R. Delayed complication of radiotherapy: laryngeal fibrosis and bilateral vocal cord immobility. Med J Malaysia. 2005;60(3):377–378. [PubMed] [Google Scholar]
- 34.Shapiro BE, Rordorf G, Schwamm L, Preston DC. Delayed radiation-induced bulbar palsy. Neurolog. 1996;46(6):1604–1606. doi: 10.1212/wnl.46.6.1604. [DOI] [PubMed] [Google Scholar]
- 35.Shin HY, Park HJ, Choi YC, Kim SM. Clinical and electromyographic features of radiation-induced lower cranial neuropathy. Clin Neurophysiol. 2013;124(3):598–602. doi: 10.1016/j.clinph.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 36.Hsieh YL, Chang MH, Wang CC. Laryngeal electromyography findings of vocal fold immobility in patients after radiotherapy for nasopharyngeal carcinoma. Head Neck. 2014;36(6):867–872. doi: 10.1002/hed.23388. [DOI] [PubMed] [Google Scholar]
- 37.King AD, Ahuja A, Leung SF, Chan YL, Lam WW, Metreweli C. MR features of the denervated tongue in radiation induced neuropathy. Br J Radiol. 1999;72(856):349–353. doi: 10.1259/bjr.72.856.10474495. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


