Abstract
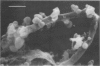
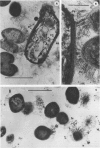
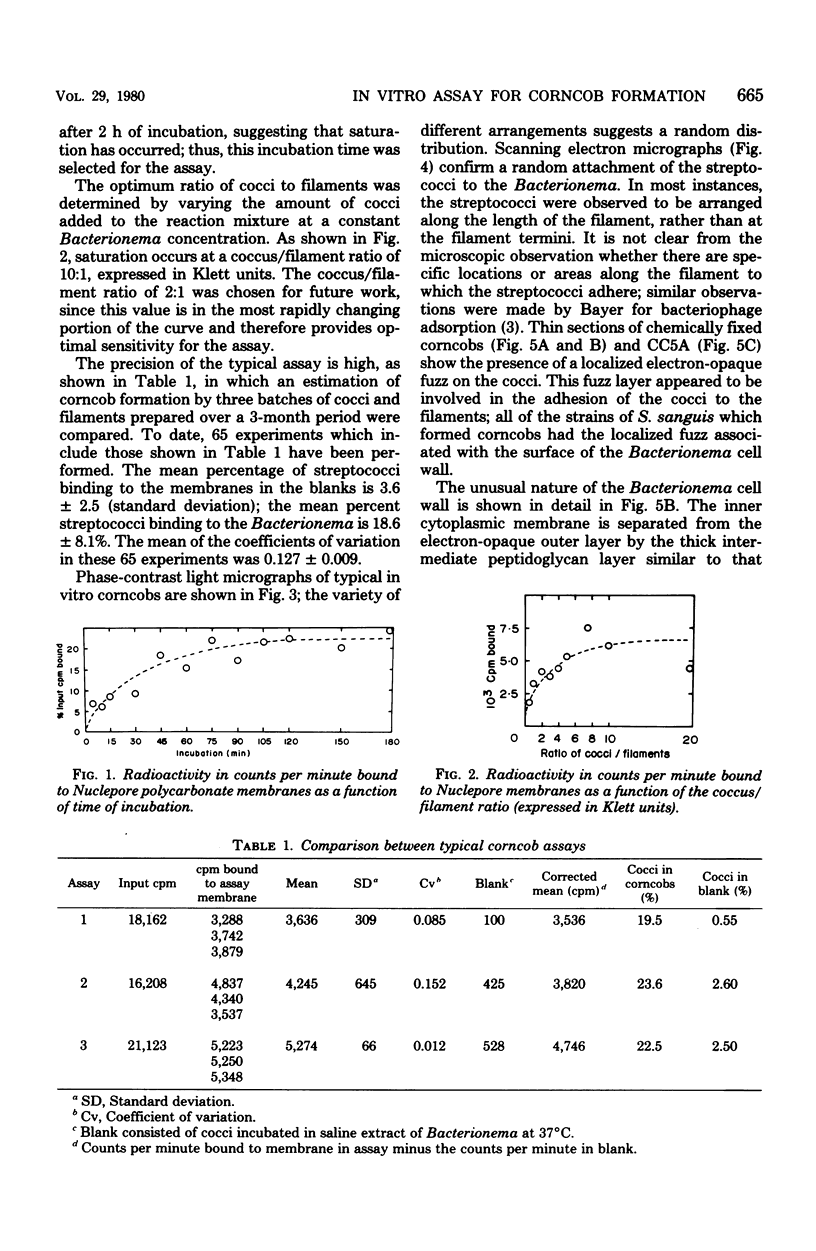
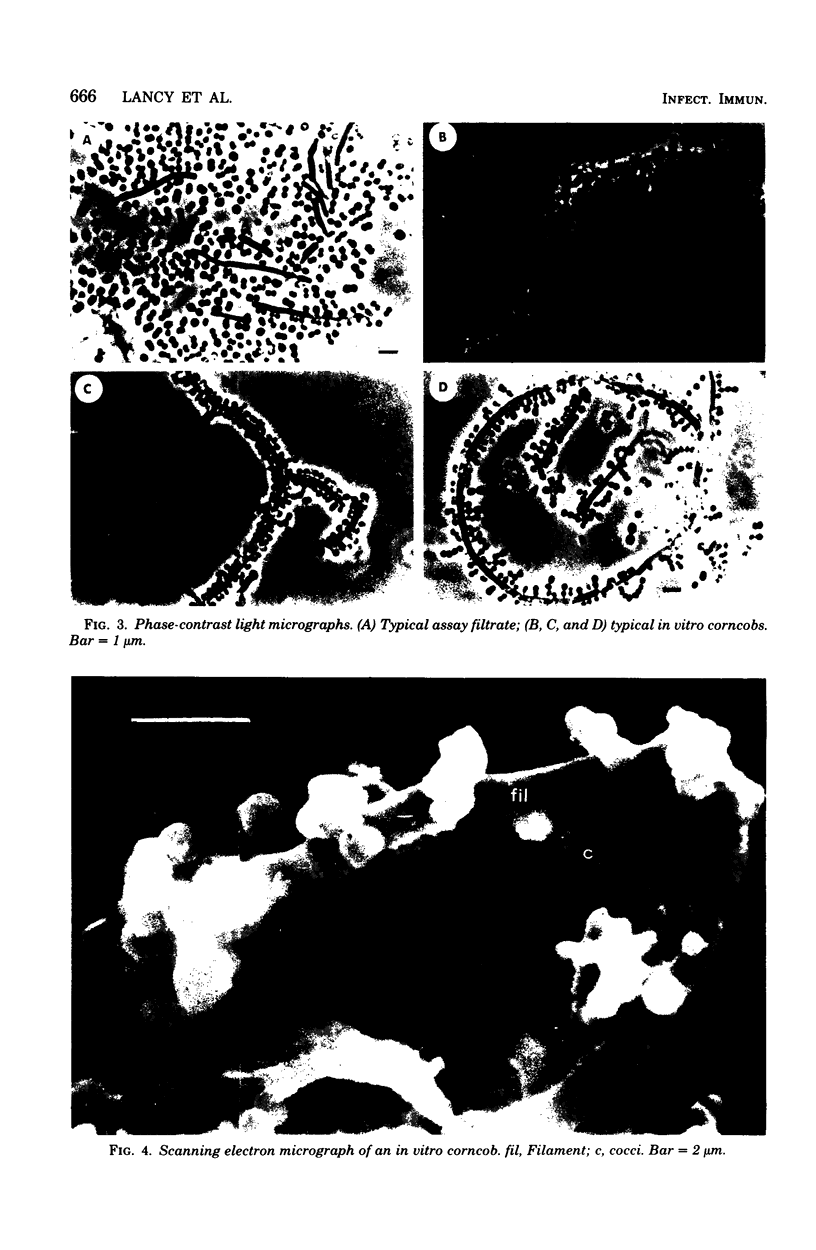
The interaction of Bacterionema matruchotii with strains of Streptococcus sanguis produces a structure which morphologically resembles a corncob. To determine the specific bacterial surface receptors involved in the interaction, we developed a quantitative assay. The assay consisted of mixing saline suspensions of [CH3-3H]thymidine-labeled streptococci and B. matruchotii, incubating at 37°C for 2 h, and filtering the mixture through a 5-μm polycarbonate membrane filter. The free cocci and filaments passed through the filter, but the corncobs were retained. Estimates of the number of corncobs formed were obtained by quantitating the radioactivity retained on the membranes relative to that of controls of streptococci alone. Although saturation of the Bacterionema occurred at a ratio of streptococci to Bacterionema of 10:1 (Klett units), a 2:1 ratio was chosen because of the increased sensitivity of the assay at this ratio. The percentage of streptococci binding at this ratio was 18.6 ± 8.1 (standard deviation). All five Bacterionema strains tested formed corncobs; in contrast, only three strains of S. sanguis were positive. These were serotype 1 strains which had localized surface “fuzz.” Although scanning electron microscopic observations revealed an almost random distribution of cocci along the filament surface, transmission electron microscopy revealed that the streptococci were attached to the Bacterionema by the surface fuzz. No differences in corncob formation were observed in sodium phosphate buffer, pH 6 to 8, at phosphate concentrations ranging from 0.005 to 0.05 M. Concentrations of NaCl or KCl up to 0.25 M did not affect corncob formation, and low concentrations of CaCl2 increased corncob formation slightly, whereas MgCl2, ethylenediaminetetraacetic acid, and citrate buffers reduced the number of streptococci binding to the filaments. These results suggest that divalent cations may play a role in this process.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelbaum B., Golub E., Holt S. C., Rosan B. In vitro studies of dental plaque formation: adsorption of oral streptococci to hydroxyaptite. Infect Immun. 1979 Aug;25(2):717–728. doi: 10.1128/iai.25.2.717-728.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum B., Rosan B. Antigens of Streptococcus sanguis: purification and characterization of the b antigen. Infect Immun. 1978 Sep;21(3):896–904. doi: 10.1128/iai.21.3.896-904.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E. Areas of adhesion between wall and membrane of Escherichia coli. J Gen Microbiol. 1968 Oct;53(3):395–404. doi: 10.1099/00221287-53-3-395. [DOI] [PubMed] [Google Scholar]
- Bourgeau G., McBride B. C. Dextran-mediated interbacterial aggregation between dextran-synthesizing streptococci and Actinomyces viscosus. Infect Immun. 1976 Apr;13(4):1228–1234. doi: 10.1128/iai.13.4.1228-1234.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Grahnén H., Jonsson G. Lactobacilli and streptococci in the mouth of children. Caries Res. 1975;9(5):333–339. doi: 10.1159/000260166. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Grahnén H., Jonsson G., Wikner S. Establishment of Streptococcus sanguis in the mouths of infants. Arch Oral Biol. 1970 Dec;15(12):1143–1148. doi: 10.1016/0003-9969(70)90005-1. [DOI] [PubMed] [Google Scholar]
- Carlsson J. Presence of various types of non-haemolytic streptococci in dental plaque and in other sites of the oral cavity in man. Odontol Revy. 1967;18(1):55–74. [PubMed] [Google Scholar]
- Ellen R. P., Balcerzak-Raczkowski I. B. Interbacterial aggregation of Actinomyces naeslundii and dental plaque streptococci. J Periodontal Res. 1977 Jan;12(1):11–20. doi: 10.1111/j.1600-0765.1977.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Ennever J., Streckfuss J. L., Takazoe I. Calcification of bacillary and streptococcal variants of Bacterionema matruchotii. J Dent Res. 1973 Mar-Apr;52(2):305–308. doi: 10.1177/00220345730520021901. [DOI] [PubMed] [Google Scholar]
- Facklam R. R. Physiological differentiation of viridans streptococci. J Clin Microbiol. 1977 Feb;5(2):184–201. doi: 10.1128/jcm.5.2.184-201.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Interbacterial aggregation of plaque bacteria. Arch Oral Biol. 1970 Dec;15(12):1397–1400. doi: 10.1016/0003-9969(70)90031-2. [DOI] [PubMed] [Google Scholar]
- Gilmour M. N., Turner G. Culture purity assessments and morphological dissociation in the pleomorphic microorganism Bacterionema matruchotii. Appl Microbiol. 1974 Jun;27(6):1134–1141. doi: 10.1128/am.27.6.1134-1141.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. J. A special relationship between spherical and filamentous microorganisms in mature human dental plaque. Arch Oral Biol. 1972 Mar;17(3):613–616. doi: 10.1016/0003-9969(72)90081-7. [DOI] [PubMed] [Google Scholar]
- Jones S. J. Natural plaque on tooth surfaces: a scanning electron microscopy study. Apex. 1971 Jun;5(3):93–98. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. H., Listgarten M. A., Rosan B. Immunoelectron microscopic identification and localization of Streptococcus sanguis with peroxidase-labeled antibody: localization of surface antigens in pure cultures. Infect Immun. 1975 Jan;11(1):193–199. doi: 10.1128/iai.11.1.193-199.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listgarten M. A., Mayo H. E., Tremblay R. Development of dental plaque on epoxy resin crowns in man. A light and electron microscopic study. J Periodontol. 1975 Jan;46(1):10–26. doi: 10.1902/jop.1975.46.1.10. [DOI] [PubMed] [Google Scholar]
- Listgarten M. A., Mayo H., Amsterdam M. Ultrastructure of the attachment device between coccal and filamentous microorganisms in "corn cob" formations of dental plaque. Arch Oral Biol. 1973 May;18(5):651–656. doi: 10.1016/0003-9969(73)90105-2. [DOI] [PubMed] [Google Scholar]
- McIntire F. C., Vatter A. E., Baros J., Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978 Sep;21(3):978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton C. Association bactérienne de types différents au sein de la plaque dentaire: la formation en épi de maäis. Etude morphologique par fluorescence au microscope en lumiére ultra-violette. J Biol Buccale. 1974 Sep;2(3):207–224. [PubMed] [Google Scholar]
- Mouton C., Reynolds H. S., Genco R. J. Characterization of tufted streptococci isolated from the "corn cob" configuration of human dental plaque. Infect Immun. 1980 Jan;27(1):235–245. doi: 10.1128/iai.27.1.235-245.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton C., Reynolds H., Genco R. J. Combined micromanipulation, culture and immunofluorescent techniques for isolation of the coccal organisms comprising the "corn cob" configuration of human dental plaque. J Biol Buccale. 1977 Dec;5(4):321–332. [PubMed] [Google Scholar]
- Newman H. N., McKay G. S. An unusual microbial configuration in human dental plaque. Microbios. 1973 Sep-Oct;8(30):117–128. [PubMed] [Google Scholar]
- Poirier T. P., Tonelli S. J., Holt S. C. Ultrastructure of gliding bacteria: scanning electron microscopy of Capnocytophaga sputigena, Capnocytophaga gingivalis, and Capnocytophaga ochracea. Infect Immun. 1979 Dec;26(3):1146–1158. doi: 10.1128/iai.26.3.1146-1158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz H. L. Microbial population shifts in developing human dental plaque. Arch Oral Biol. 1967 Dec;12(12):1561–1568. doi: 10.1016/0003-9969(67)90190-2. [DOI] [PubMed] [Google Scholar]
- Rosan B. Absence of glycerol teichoic acids in certain oral streptococci. Science. 1978 Sep 8;201(4359):918–920. doi: 10.1126/science.684416. [DOI] [PubMed] [Google Scholar]
- Rosan B. Antigens of Streptococcus sanguis. Infect Immun. 1973 Feb;7(2):205–211. doi: 10.1128/iai.7.2.205-211.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosan B., Lai C. H., Listgarten M. A. Streptococcus sanguis: a model in the application in immunochemical analysis for the in situ localization of bacteria in dental plaque. J Dent Res. 1976 Jan;55:A124–A141. doi: 10.1177/002203457605500105011. [DOI] [PubMed] [Google Scholar]
- Rosan B. Relationship of the cell wall composition of group H streptococci and Streptococcus sanguis to their serological properties. Infect Immun. 1976 Apr;13(4):1144–1153. doi: 10.1128/iai.13.4.1144-1153.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W., Streckfuss J., Vogel J., Ennever J. Struvite crystals in colonies of Bacterionema matruchotii and its variants. J Dent Res. 1971 May-Jun;50(3):777–777. doi: 10.1177/00220345710500033701. [DOI] [PubMed] [Google Scholar]
- Streckfuss J. L., Smith W. N. Isolation of Bacillary and Streptococcal Variants from Bacterionema matruchotii. J Bacteriol. 1970 Dec;104(3):1399–1400. doi: 10.1128/jb.104.3.1399-1400.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takazoe I., Ennever J. Ultrastructure of Bacterionema matruchotii. Bull Tokyo Dent Coll. 1969 May;10(2):45–60. [PubMed] [Google Scholar]
- Takazoe I., Matsukubo T., Katow T. Experimental formation of "corn cob" in vitro. J Dent Res. 1978 Feb;57(2):384–387. doi: 10.1177/00220345780570024101. [DOI] [PubMed] [Google Scholar]