Abstract
The investigation of callous-unemotional (CU) traits has been central to contemporary research on child behavior problems, and served as the impetus for inclusion of a specifier for conduct disorder in the latest edition of the official psychiatric diagnostic system. Here, we report results from two studies that evaluated the construct validity of callousness as assessed in adults, by testing for affiliated deficits in behavioral and neural processing of fearful faces, as have been shown in youthful samples. We hypothesized that scores on an established measure of callousness would predict reduced recognition accuracy and diminished electocortical reactivity for fearful faces in adult participants. In Study 1, 66 undergraduate participants performed an emotion recognition task in which they viewed affective faces of different types and indicated the emotion expressed by each. In Study 2, electrocortical data were collected from 254 adult twins during viewing of fearful and neutral face stimuli, and scored for event-related response components. Analyses of Study 1 data revealed that higher callousness was associated with decreased recognition accuracy for fearful faces specifically. In Study 2, callousness was associated with reduced amplitude of both N170 and P200 responses to fearful faces. Current findings demonstrate for the first time that callousness in adults is associated with both behavioral and physiological deficits in the processing of fearful faces. These findings support the validity of the CU construct with adults and highlight the possibility of a multi-domain measurement framework for continued study of this important clinical construct.
Keywords: callousness, psychopathy, face processing, EEG
Research over the past two decades has provided compelling evidence for the importance of affective features of psychopathy, termed callous-unemotional (CU) traits in the youth psychopathy literature, as an appreciably heritable characteristic (Viding, Jones, Frick, Moffitt, & Plomin, 2008) that distinguishes a subset of children with severe behavior problems who exhibit distinct cognitive and affective characteristics. Children with high levels of CU traits also display a particularly severe, aggressive, and stable pattern of antisocial behavior across the lifetime compared to other antisocial youth (Frick, Ray, Thornton, & Kahn, 2014). As an indication of the perceived etiological and clinical importance of CU traits, a “Limited Prosocial Emotions” specifier reflecting this construct was added to the criteria for conduct disorder in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5; American Psychiatric Association [APA], 2013). While CU traits have been studied extensively in child and adolescent samples (Frick et al., 2014; Viding & Kimonis, in press), the expression of CU traits in adulthood is less well understood, with research having been limited mainly to the affective facet of the Psychopathy Checklist—Revised (PCL-R; Hare, 2003), a measure that may not correspond entirely with CU traits. To address this gap, recent work has undertaken to formally delineate features corresponding to CU traits in models and measures of adult psychopathy (Patrick, Fowles, & Krueger, 2009; Strickland, Drislane, Lucy, Krueger, & Patrick, 2013; Drislane & Patrick, in press), and examine the stability and course of CU traits from childhood through to adulthood (Viding & Kimonis, in press).
Further research is needed to substantiate hypothesized links between CU traits as assessed in young participants and measures that purport to index the construct in adults. In particular, research is needed to evaluate whether adult self-report measures of callousness show behavioral and biological correlates paralleling those reported for CU traits in younger samples. While the majority of juvenile psychopathy research has taken a “top-down” approach, extending adult conceptions to adolescents, it is important to also consider the developmental trajectory of these traits from early childhood through to adulthood (Lynam, 1996). Initial evidence from studies assessing CU traits during adulthood provides support for similar physiological reactivity deficits among children and adults in relation to emotional stimuli (Fanti, Panayiotou, Kyranides, & Avramides, 2016). However, no prior work has examined brain event-related potential correlates of callousness in adult participants. The current work was undertaken to address this research need, and in the process contribute to a multi-domain measurement framework for clarifying the nature and bases of callous-unemotional tendencies as a distinct subdomain of psychopathy.
Adult Counterparts to Callous-Unemotional Traits
Classic historic writings on psychopathy as it presents in adult criminal offender samples highlighted emotional insensitivity and predatory-exploitative tendencies as central features. Hare's (2003) interview-based PCL-R, developed to index psychopathy in adult criminal populations, contains items pertaining to callousness, lack of remorse, and shallow affect that covary to form a “deficient affective experience” subdimension (Cooke & Michie, 2001). Self-report inventories of psychopathy also include coverage of these affective features. For example, the well-validated Psychopathic Personality Inventory (Lilienfeld & Widows, 2005) includes a Coldheartedness subscale that reflects tendencies distinct from impulsive-disinhibitory proclivities (Drislane, Patrick, & Arsal, 2014). Even antisocial personality disorder (ASPD) as represented up through the current-fifth edition of the DSM (APA, 2013), although lacking in coverage of core psychopathic features (e.g., Hare, Hart, & Harpur, 1991), includes an adult “lack of remorse” criterion that encompasses tendencies toward guiltlessness, callous indifference, and rationalization of harmful behavior.
Drawing in part on these lines of evidence, along with the child CU-traits literature and writings on the relevance of the broad trait of antagonism to adult psychopathy (e.g., Lynam & Derefinko, 2005; Widiger & Lynam, 1998), Patrick and colleagues identified callous-exploitativeness – conceptualized as disaffiliated agency or “meanness”– as a core facet of psychopathy in their 2009 triarchic model. In addition to CU traits, another referent for the callousness construct in the triarchic model was structural analytic work on the externalizing spectrum of adult psychopathology (Krueger, Markon, Patrick, Benning, & Kramer, 2007) that identified a callous-aggression subdimension (subfactor), distinct from general disinhibitory tendencies, involving presence versus absence of empathy, relational and destructive aggression, and excitement seeking. Subsequent work (Krueger et al., 2007; Drislane et al., 2014; Sellbom & Phillips, 2013) has demonstrated that this callous-aggression subfactor correlates selectively (relative to disinhibition and boldness) with the Inventory of Callous-Unemotional Traits (ICU; Kimonis, Branch, Hagman, Graham, & Miller, 2013), a widely-used measure of the CU construct in research on children and adolescents (Frick & Ray, 2015; Viding & Kimonis, in press). Other work has shown that the callous-aggression subfactor of the externalizing spectrum model correlates robustly and selectively with (a) the affective facet of the PCL-R (Venables & Patrick, 2012), (b) the Coldheartedness subscale of the PPI (Drislane et al., 2014), and (c) the broad trait of antagonism as indexed by reversed scores on the agreeableness dimension of Costa and McCrae's (1992) NEO Personality Inventory-Revised (Poy, Segarra, Esteller, Lopez, & Molto, 2014). For simplicity, and to establish a common vernacular for referencing this trait construct, we use the term ‘callousness’ in the current paper.
Critically, assessing callousness in trait-dimensional terms distinct from other facets of psychopathy is complementary with two prominent modern approaches to the study of psychopathology: the alternative dimensional system for characterizing personality pathology in DSM-5 and the National Institute of Mental Health's (NIMH) Research Domain Criteria (RDoC) initiative (Kozak & Cuthbert, 2016). The new dimensional-trait system for personality pathology in Section III of DSM-5 (APA, 2013), “Emerging Measures and Models,” provides a trait-based conceptualization of adult ASPD that features strong representation of traits from the domain of Antagonism (i.e., Callousness, Deceitfulness, Manipulativeness) along with traits from the domains of Disinhibition and Negative Affect. Recent research (Strickland et al., 2013) has shown that traits from the Antagonism domain, especially Callousness, correlate substantially with the callous-aggression subfactor of the adult externalizing model as indexed by the Meanness subscale of the Triarchic Psychopathy Measure (TriPM; Drislane et al., 2015; see also Patrick, Kramer, Krueger, & Markon, 2013).
The other recent major development in psychopathology research is the NIMH-RDoC framework, which calls for investigation of psychological disorders in terms of biologically-oriented ‘process’ constructs such as acute threat (‘fear’), response inhibition, and affiliation/attachment (Kozak & Cuthbert, 2016; Patrick & Hajcak, 2016). Recent writings have sought to connect symptomatic facets of psychopathy, including the callous-unemotionality facet, to constructs in the RDoC framework (e.g., Patrick & Drislane, 2015). As discussed in the next two subsections, an underlying objective of the work reported here was to contribute to positioning callousness within the RDoC framework by identifying neural and behavioral performance correlates of this construct in adult participants. Specifically, the current research examined whether adults identified as high in callousness show deficits in fear recognition and affective-brain response that have been reported in youth who score high on CU traits (Dawel, O'Kearney, McKone, & Palermo, 2012).
Callous-Unemotionality and Affective Face Processing
Behavioral, physiological, and neuroimaging studies have demonstrated abnormalities in affective processing among children high in CU traits. Recent meta-analytic work has demonstrated that children with CU tendencies show pervasive deficits in accurately recognizing emotional stimuli, with the largest effect size evident for fear (Dawel et al., 2012; see also Blair, Colledge, Murray, & Mitchell, 2001; Marsh & Blair, 2008). Blair (1995) hypothesized that deficient processing of distress cues contributes to psychopathic tendencies by inhibiting normal social learning and interfering with moral development. In line with this view, high-CU children show reduced attention to the eye region of the face when processing emotional faces, and this impairment relates in turn to deficient fear recognition and low empathic tendencies in such children (Dadds, Jambrak, Pasalich, Hawes, & Brennan, 2011). Of note, these deficits appear specific to CU traits in youth, being unrelated to narcissistic or impulsive personality features.
These behavioral findings dovetail with findings from neuroimaging research on affective face processing in subgroups of youth with conduct problems distinguished by the presence versus absence of callous-unemotional traits. In particular, functional magnetic resonance imaging (fMRI) studies have reliably demonstrated that youth high in CU traits exhibit diminished amygdala reactivity to fearful face stimuli relative to youth low on CU traits or psychopathy (Jones, Laurens, Herba, Barker, & Viding, 2009; Marsh et al., 2008). Additionally, youth high on CU traits show reduced amygdala response (along with diminished reactivity of ventromedial prefrontal cortex) to visual images of aversive stimuli other than fear faces (e.g., attacking animals, aimed weapons) relative to control youth (Hwang et al., 2016). These converging lines of evidence support the idea that children and adolescents high on CU traits exhibit deficits in reactivity to emotionally salient cues, particularly fearful facial expressions.
An alternative methodology for examining brain reactivity to emotional face stimuli is through use of cortical event-related potentials (ERPs). Three ERP components, the N170, P200, and late positive potential (LPP) responses, have been shown to differ for fearful compared to neutral face stimuli in healthy adults. Jiang and colleagues (2009) demonstrated that the N170, a negative deflection in the waveform peaking around 170 ms after stimulus presentation and maximal at temporal-parietal scalp sites, is associated with face detection and categorization in adults. Paulmann and Pell (2009) identified the P200, a positive-going waveform deflection occurring approximately 200 ms after face presentation, following the N170 and maximal at parietal scalp sites, as reflecting encoding of emotional content in facial expressions. Research has also demonstrated that the LPP response, a later onset component that is maximal at the midline, is enhanced for visual affective stimuli, including faces (Schupp et al., 2000). Work by Shannon, Patrick, Venables, and He (2013) utilizing a sample of adult twins showed that N170, P200, and LPP response amplitudes were enhanced for fearful versus neutral faces, indicating that each indexes aspects of emotional processing of face stimuli. Utilizing twin-correlational analyses, investigators also showed that variations in the amplitude of the two earlier ERP responses were highly heritable, particularly P200 amplitude. In light of these converging findings, these distinct components of brain reactivity to face stimuli may be of value for studying affective processing deviations in high-callous individuals of differing ages, as a complement to the use of fMRI measurement.
Current Study Aims and Hypotheses
Based on evidence for deficient recognition accuracy and reduced brain reactivity for fearful faces in youth with high levels of CU traits, the current work was undertaken to test for analogous effects in adults scoring high in callousness. One major aim was to extend the well-established finding of reduced fear-face recognition in high-CU children to adults, using the callous-aggression subdimension of the externalizing spectrum model to operationalize callousness. Specifically, in Study 1, we hypothesized that adults high in callousness would exhibit a selective deficit in identifying fearful faces, relative to other affective facial expressions (cf. Marsh and Blair, 2008), when completing an affect recognition task. Beyond seeking to replicate this known behavioral effect from the child CU literature in an adult-aged sample, we also evaluated the specificity of this effect by testing for correlations with the disinhibition and boldness facets of psychopathy in addition to the callousness facet.
Study 2 was undertaken to build on previously reported findings of diminished fMRI-brain reactivity to fearful faces in youth high on CU traits by testing for corresponding reductions in ERP-brain responses to faces of this type in adults scoring high in callousness. Based on prior work demonstrating sensitivity of the N170 and P200 ERP components to the affective content of face stimuli, we hypothesized that callousness would be associated with decreased amplitude of N170, P200, and LPP brain responses to fearful faces specifically (i.e., in contrast with neutral faces). As in Study 1, we also evaluated the specificity of predicted effects for these ERP variables by examining relationships with disinhibition and boldness facets of psychopathy along with callousness.
Study 1
Method
Participants and procedures
Participants were 66 undergraduate students (M age = 19.6 years, SD = 1.79; 43 females) recruited from psychology classes at Florida State University. The racial/ethnic composition of the sample was: 79.1% Caucasian, 9% African American, 4.5% Asian Indian, and 7.4% more than one race; 20.9% of participants were Hispanic. Individuals participated in a single session in which they completed a computerized facial discrimination task (Marsh, Yu, Pine, & Blair, 2010; see below) along with self-report scale measures of psychopathic traits and demographic characteristics. Study procedures were approved by the Florida State University Institutional Review Board and all participants provided informed written consent prior to testing.
Trait measures
Callousness and disinhibition
Callousness and impulsive-disinhibitory tendencies were assessed using the Meanness and Disinhibition scales, respectively, of the Triarchic Psychopathy Measure (TriPM; Drislane et al., 2014). Items of the TriPM are answered using a 4-point Likert scale that ranges from 0 (mostly false) to 3 (mostly true). This measure is keyed such that higher scores reflect higher pathological tendencies. The TriPM Meanness subscale consists of 19 items from the Externalizing Spectrum Inventory (ESI; Krueger et al., 2007) that index the inventory's callous-aggression subfactor (Patrick, Kramer, et al., 2013). Paralleling the composition of the ESI's Callous Aggression subfactor, the TriPM Meanness scale includes 10 items that index presence versus absence of empathy, and 9 other items from the ESI's Relational Aggression, Destructive Aggression, Excitement Seeking, and Honesty subscales. The TriPM Disinhibition subscale consists of 20 items from the ESI selected to index its general externalizing-proneness factor (Patrick, Kramer, et al., 2013). Scores on these two TriPM scales demonstrate strong convergent and discriminant validity with corresponding facet scales of other psychopathy inventories and relevant normal-range personality traits (Drislane et al., 2014; Drislane & Patrick, in press). Within the Study 1 sample, internal consistency reliabilities were high for both scales (Cronbach's α's = .93 and .87, for Meanness and Disinhibition respectively).
Boldness
Fearless-dominant (bold) tendencies were measured using a subset of items (n = 20) from the brief-form version of the Multidimensional Personality Questionnaire (MPQ-BF; Patrick, Curtin, & Tellegen, 2002) that were selected to form a Boldness scale (Brislin, Drislane, Smith, Edens, & Patrick, 2015). Items are answered using a two-point response format (true/false for single-statement items, A/B for dual-statement, forced-choice items), and keyed such that higher scale scores reflect greater levels of boldness. Prior work has demonstrated strong convergent and discriminant validity for this scale in relation to other measures of boldness, fear/fearlessness, and relevant clinical-symptom variables across various samples (Brislin et al., 2015). Internal consistency (α) for the MPQ-Boldness scale in the current study sample was .76.
Laboratory measure: Emotion recognition task
The Emotion Recognition Task is a computer-based task designed to index facial recognition ability. Faces from the Ekman stimulus set depicting six basic emotions (anger, disgust, fear, happiness, sadness, surprise) were used. Each face was digitally adjusted (morphed) in emotional intensity from neutral to full expression in 10% increments, such that participants viewed the same person displaying the same emotion 10 times, in graded intensities of expression. The face images were counterbalanced in terms of presentation and there were 360 images total (i.e., six different presentations of each of the six emotions). Each face stimulus was presented for 500 ms, after which the participant was given unlimited time to choose which of the six emotions was expressed by the face. Accuracy scores (percent trials correct) were calculated for each emotion category, aggregating across differing intensities of expression.
Data analyses
Correlational analyses were used to evaluate associations between psychopathy facet scores and recognition accuracy scores for each affective face category. Within the current study sample (as in past work; e.g., Drislane et al., 2014), Meanness scale scores, used to index callousness, were correlated moderately with Disinhibition scale scores and modestly with Boldness scale scores (rs = .54 and .27, respectively), whereas Disinhibition and Boldness were uncorrelated (r = -.05). To control for the overlap between Meanness and the other trait measures, multiple regression analyses were performed in which scores for all three facets were included together as predictors of recognition accuracy for each face category; this provided for determination of the unique contribution of each facet to prediction of recognition accuracy. Statistical effects were evaluated using alpha levels of p < .05 and p < .005 in an effort to balance Type I and Type II error.
Results
Associations between the trait measures and recognition accuracy for emotional faces of each type are presented in Table 1. At the zero-order (simple bivariate) level, both Meanness and Disinhibition scores were negatively associated with accuracy of identification for faces expressing fear. However, when all three trait measures were entered as concurrent predictors in a regression model, Meanness emerged as the only significant, unique predictor of fear accuracy, accounting for variance in fear-face recognition above and beyond Disinhibition. Similarly, both Meanness and Disinhibition were negatively associated with recognition of happy faces at the zero-order level; however, neither remained significant in the regression analysis utilizing all three scales as predictors. Disinhibition showed a negative relationship with accurate recognition of surprised faces at both the zero-order level and when entered with the other facet scales into a regression model. No significant associations with recognition accuracy for angry faces or sad faces were evident for any of the psychopathy facets, either at the zero-order level or in the context of regression modeling.
Table 1. Facial Recognition Task: Correlations between Psychopathy Facet Scores and Accuracy for Faces of Differing Types.
| Face Type | MPQ Boldness r (β) | TriPM Meanness r (β) | TriPM Disinhibition r (β) | Multiple R/R2 |
|---|---|---|---|---|
| Disgust | .06 (.07) | -.14 (-.09) | -.19 (-.14) | .20/.04 |
| Sadness | .17 (.09) | .17 (.25) | -.07 (-.20) | .27/.07 |
| Fear | .01 (.11) | -.39** (-.37*) | -.29* (.08) | .41/.17* |
| Anger | -.21 (-.15) | -.15 (-.18) | .04 (.12) | .25/.06 |
| Surprise | -.20 (-.23) | -.22 (.02) | -.31* (-.34*) | .38/.15* |
| Happiness | .14 (.21) | -.29* (-.28) | -.29* (-.13) | .38/.15* |
Note. N for all correlations = 63. r = Pearson correlation coefficient; β = standardized beta coefficient from regression model incorporating scores for the three psychopathy facets as predictors.
p<.05,
p<.005.
Discussion
Findings from this study serve to connect the emerging literature on callous tendencies in adults to the extensive existing literature on CU traits in youth (Frick et al., 2014) by demonstrating a key behavioral effect in relation to scores on an adult measure of callousness. Paralleling findings from studies of CU traits in children and adolescents (Marsh & Blair, 2008), self-reported levels of callousness as indexed by the Meanness scale of the TriPM showed a selective negative association (relative to other psychopathy facets) with accuracy of recognition for fearful faces. This provides additional evidence for the construct validity of the TriPM Meanness scale, and by extension other related scale measures such as the ICU (Drislane et al., 2014), beyond published work to date showing theory-consistent relations with measures in the domains of self-report and interview-based ratings.
In addition, the fact that this behavioral effect of impaired fear recognition was evident for callousness but unrelated to boldness or disinhibition when controlling for overlap among the three facets through use of regression has implications for understanding the basis of the effect. In particular, the construct of boldness, which connects empirically to the construct of dispositional fear/fearlessness (Brislin et al., 2015), is predictive of reduced physiological fear reactivity to aversive stimuli (Patrick & Bernat, 2009). The lack of reduced fear-face recognition in relation to high boldness suggests this characteristic of youth with high CU traits is reflective of a separate mechanism, such as a lack of sensitivity to the distress of others associated more with low empathy or impaired affective resonance (cf. Blair, 1995; Dadds et al., 2011). Additionally, in line with the RDoC initiative's call for multi-domain assessment of core process constructs in the study of psychological problems, these findings point to decreased recognition of fearful faces as a viable laboratory indicator of callousness across differing developmental stages. Of note, these findings are limited by the small sample size. Therefore, while significant effects were found specifically for fear faces, we cannot rule out the possibility that effects would have been evident for other face types in a larger participant sample, consistent with recent meta-analytic work (Dawel et al., 2012).
Building upon this evidence that behavioral deficits associated with callousness in childhood extend to adults, we next examined physiological reactivity to fearful faces in adult-aged participants assessed for callousness along with boldness and disinhibition. As noted earlier, CU traits in youthful participants have been shown to be reliably associated with diminished brain processing of fearful faces (as evidenced by reduced fMRI-assessed amygdala reactivity; Jones et al., 2009; Marsh et al., 2008). In an effort to determine whether callousness in adults is likewise related to diminished brain processing of fearful faces, we tested for associations between callous-aggression scores and distinct components of ERP brain response known be sensitive to the affective content of face stimuli.
Study 2
Method
Participants and procedures
Participants were 254 community members (M age = 29.4 years, SD = 4.8; 90 females) recruited from the Minneapolis-St. Paul urban area. The racial/ethnic composition of the sample was: 97.6% Caucasian, .8% African American, 1.6% Other/Unspecified race. Testing took place in a single session in which participants completed a computerized face-viewing task, during which electrocortical data were collected, along with self-report scales. Procedures for the study were approved by the Institutional Review Board of the University of Minnesota and all participants provided informed written consent prior to testing. Of those tested, one participant was excluded due to a medical condition that prevented him from being able to view the stimuli without assistance, 18 were excluded due to missing self-report data, one was excluded due to missing data for the N170 response, and 13 were excluded due to missing data for the P200 and LPP responses—resulting in Ns of 234, 222, and 222, respectively, for analyses of these three ERP response components.
Trait measures
Callousness and disinhibition
Callousness and disinhibitory tendencies were assessed using separate subsets of items from an abbreviated (100-item) version of the ESI administered to this study sample. The items used to index callousness consisted of available items (n = 25) from ESI scales, as follows, that loaded above .3 on the callous-aggression factor in the original report of the ESI structural model (Krueger et al., 2007): Empathy (reversed); Relational, Destructive, and Physical Aggression; Excitement Seeking; Rebelliousness; and Honesty (reversed). Within a separate mixed gender sample (Strickland et al., 2013), scores for this 25-item Callous-Aggression scale were highly correlated with scores for the 19-item TriPM Meanness scale used in Study 1 (r = .78).
Disinhibitory tendencies were indexed using 30 items from subscales of the ESI that demarcate its general externalizing (disinhibition) factor: Problematic Impulsivity, Planful control (reversed), Irresponsibility, Dependability (reversed), Impatient Urgency, Alienation, and Theft. Scores on this 30-item scale correlate very highly with the 20-item TriPM Disinhibition scale (r = .85, based on data from Strickland et al., 2013), and this scale has been validated in relation to self-report, diagnostic, and physiological criterion measures in prior work (e.g., Yancey, Venables, Hicks, & Patrick, 2013). The Callous-Aggression and Disinhibition scales were each scored such that higher scores were indicative of greater levels of pathology. Within the participant sample for Study 2, internal consistency reliabilities were high for both scales (Cronbach's α's = .89 and .88, respectively).
Boldness
The item-based Boldness subscale from the MPQ-BF (Brislin et al., 2015) was used to index fearless-dominant tendencies (see Method section, Study 1 for details regarding this scale measure). Within the sample for Study 2, this scale demonstrated high internal consistency (Cronbach's α = .89).
Stimulus delivery and recording procedure
Participants viewed face stimuli under two conditions, blocked into separate trials, while wearing stereoscopic glasses: standard viewing trials, in which the same face image was presented to both eyes, and ‘suppressed’ viewing trials, in which a face image was presented to one eye and masked by presentation of a 20-Hz Mondrian ‘noise’ pattern to the other eye (for further details, see Shannon et al., 2013). Data for the standard viewing trials provided for the strongest test of our hypothesis that callous tendencies would be associated with reduced ERP brain response to fear-face stimuli, and thus current analyses focused only on data for these trials.
The face stimuli consisted of fearful and neutral expressions, posed by different actors, from the NimStim face set. Participants were seated at a standard position (100 cm from the screen, 2.91 × 3.88 degree viewing angle) in front of a 19” CRT monitor (1024 × 768 pixels, 85 Hz refresh rate) on which face stimuli were displayed for 500 ms each under the control of a Psychophysics Toolbox software routine (Brainard, 1997). As described by Shannon et al. (2013), scrambled versions of the same fear and neutral faces were also presented, but data for the scrambled trials were not included in current analyses.
Physiological measurement and data processing
Electroencephalographic (EEG) activity was recorded using 64 scalp electrodes embedded in a NeuroScan Quik-Cap. Recording sites consisted of the standard 10-20 system locations along with additional intermediate positions. The raw EEG signal was continuously recorded at a rate of 1000 Hz and band-pass filtered online at 0.05-200 Hz using a Neuroscan Synamps system, referenced online to the CPz electrode. The filtered continuous EEG recording was epoched offline from 1000 ms before to 2000 ms after stimulus onset, and then averaged across trials within face condition. The average epoched signal was then baseline corrected by subtracting from each aggregate time point the mean amplitude of EEG activity across a 500-ms pre-stimulus interval. Trials with eyeblinks, eye movements, or muscle potentials exceeding 75 μV at any electrode were excluded from averaging. In cases where there were less than three epochs meeting the above criteria for an individual at the target electrode for a given response variable (n = 13 for the P200 and LPP, n = 1 for the N170), data from that electrode for that subject were removed from analysis.
Data analyses
Data from selected temporal-parietal and parietal recording sites (i.e., electrode site P8 for the N170 response, and site PZ for the P200 and LPP responses) were selected for analysis, consistent with previous studies examining face processing (e.g., Shannon et al., 2013; Anokhin & Golosheykin, 2010). To index effects with maximal robustness, activity recorded from the right temporal-parietal site (P8) was referenced to the midline site CPz, and activity from the midline parietal electrode site (PZ) was referenced to linked mastoids. ERP component peaks corresponding to the N170 (scoring window = 150-230 ms) and P200 (150-300 ms) responses, along with mean activity over the time interval of the LPP (400-980 ms), were quantified for face stimuli of each type (fearful, neutral).
As reported by Shannon et al. (2013), peak amplitudes for N170 and P200, and mean amplitude of the LPP, were enhanced for fearful as compared to neutral faces within the sample as a whole. To evaluate associations with psychopathy facets, we computed correlations for ESI Callous-Aggression, ESI Disinhibition, and MPQ Boldness scores with peak-amplitude scores for N170 and P200 and mean amplitude for LPP, for fearful and neutral faces separately. As in Study 1, to control for overlap between Meanness and the other two trait measures (rs = .71 and .17, respectively, with Disinhibition and Boldness), multiple regression analyses were performed in which scores for all three traits were included together as predictors of response variables – in this case, ERP component scores.
For purposes of clarifying predicted effects for Callous-Aggression, regression analyses of two additional types were performed for the earlier two ERP components (N170, P200). First, for each of these components, responses to face stimuli of the two types (fear, neutral) were examined as concurrent predictors of Callous-Aggression scores in order to evaluate the specificity of the predictive relationship for fearful faces. Second, scores for the N170 and P200 components for fear face stimuli were examined as concurrent predictors of Callous-Aggression scores, to determine if associations for P200 and N170 reflected unique or overlapping processes.
Analyses were also performed for N170, P200, and LPP in which covariation due to twin-pair membership was controlled for. These analyses utilized multilevel models with random intercepts to account for shared twin attributes that relate to ERP amplitude (cf. Goldstein, 1995). In line with prior published work (e.g., Patrick et al., 2006), Pearson correlations and standardized beta coefficients are reported from the standard analytic models, but p values for these coefficients are adjusted to reflect robust standard errors based on the multilevel model results.
Results
Within the sample as a whole, peak amplitude of the N170 response was reliably enhanced during viewing of fearful faces as compared to neutral faces (Ms = -5.01 and -4.11 μV, respectively, SDs = 2.86 and 2.65), F(1, 234) = 67.43, p < .001, η2partial = .24 (cf. Shannon et al., 2013). Enhanced reactivity to fearful versus neutral faces was also evident for P200 peak amplitude (Ms = 1.31 and .84μV, SDs = 2.01 and 1.88) and LPP mean activity (Ms = .68 and .26 μV, SDs = 1.18 and 1.28), Fs(1, 221) = 19.93 and 14.63, respectively, ps < .001, η2partial = .08 and .06.
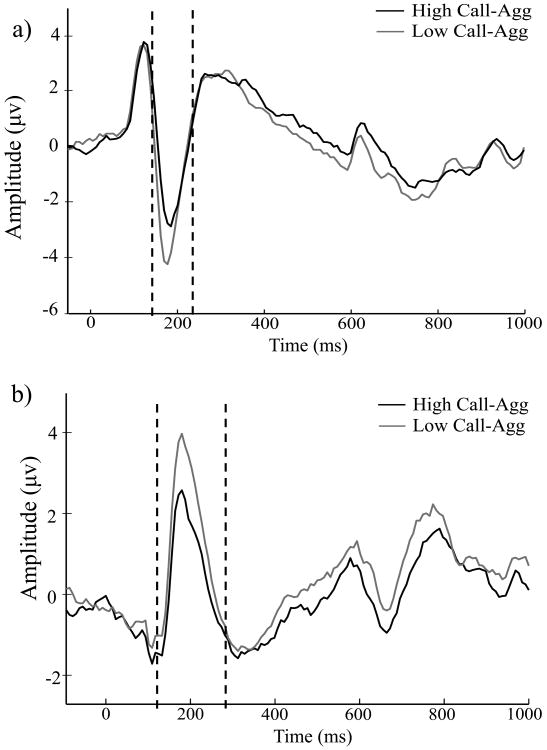
Results from simple bivariate (rs) and regression analyses (βs, Rs) examining main hypothesized relations of Callous-Aggression scores with ERP responses to fear and neutral face stimuli, along with relations for Disinhibition and Boldness scores, are presented in Table 2. Zero-order rs indicated significant reduction of N170 amplitude to fearful faces as a function of increasing levels of Callous-Aggression, and to a lesser degree Disinhibition. The regression model including scores on all three psychopathy facets as predictors of fear-face N170 response did not reveal a unique predictive effect for any one facet, although the largest predictive coefficient was evident for Callous-Aggression (β = .18, p = .14). N170 response to neutral faces also showed a significant association with Callous-Aggression scores at the zero-order level (see Table 2), but a follow-up regression analysis including N170 responses for both fearful and neutral faces as predictors of Callous-Aggression scores revealed a unique predictive association for fear-face response only, β =.22, p = .07 (β for neutral-face N170 = .01, p = . 92). The upper waveform plot in Figure 1 depicts mean N170 response to fearful faces for participants scoring high versus low on the Callous-Aggression scale.
Table 2. Face Viewing Task: Correlations between Psychopathy Facet Scores and Components of ERP Brain Response to Fearful and Neutral Faces.
| MPQ Boldness r (β) | ESI Callous-Aggression r (β) | ESI Disinhibition r (β) | Multiple R/R2 | |
|---|---|---|---|---|
| N170 | ||||
| Fearful Faces | .10(.07) | .22**(.18) | .17* (.04) | .23/.05* |
| Neutral Faces | .08 (.06) | .18* (.14) | .14 (.04) | .19/.03* |
| P200 | ||||
| Fearful Faces | -.03 (.02) | -.21** (-.30**) | -.10 (.12) | .23/.05* |
| Neutral Faces | -.03 (.01) | -.13(-.28**) | .00 (.20*) | .19/.05* |
| LPP | ||||
| Fearful Faces | .00 (-.01) | .09 (.05) | -.15* (-.19*) | .16/.03 |
| Neutral Faces | .07 (.08) | -.03 (-.04) | -.03 (.00) | .08/.01 |
Note: Ns = 234 for N170, and 222 for P200 and LPP (see main text).r = Pearson correlation coefficient; β; = standardized beta coefficient from regression model incorporating scores for the three psychopathy facets as predictors.
p<.05,
p<.005.
Figure 1.
ERP waveform plots. (a) N170: Waveform for ERP activity across fearful face trials at the right occipito-temporal electrode site (P8), showing mean N170 peak response (time window = 150-230 ms) for individuals scoring in the top (dark gray) versus bottom (light gray) quartiles on the Callous-Aggression scale. (b) P200: Waveform for ERP activity across fearful face trials at the midline electrode site (PZ), showing mean P200 peak response (time window = 150-300 ms), for participants scoring in the top (dark gray) versus bottom (light gray) quartiles on the Callous-Aggression scale.
For the P200, analyses revealed significantly reduced amplitude of response to fear faces in relation to the Callous-Aggression facet of psychopathy only, both at the zero-order level and in the context of regression (ps < .005). The association for neutral-face P200 response with Callous-Aggression was weaker and nonsignificant at the zero-order level, but emerged as significant in the regression model that included all three psychopathy facets as predictors. A regression analysis that included P200 responses for both fearful and neutral faces as predictors of Callous-Aggression scores revealed unique prediction for fear-face P200 only, β = .19, p = .02 (β for neutral-face P200 response = .07, p = . 88). The lower waveform plot in Figure 1 depicts mean P200 response to fearful faces for participants high as compared to low in Callous-Aggression scores.
To determine if the associations for these two early ERP components with Callous-Aggression reflected overlapping or distinctive processes, amplitude scores for both fear-face N170 and fear-face P200 were entered into a regression model predicting Callous-Aggression scores. Results indicated some reduction in the strength of associations for each (βs for N170 and P200 = .17 and -.15, respectively [partial rs = .15 and -.13]), but with some residual prediction maintained in each case (ps = .07 and .05, respectively). These results suggest some overlap along with some uniqueness in the processes indexed by these two early-ERP components as related to callous tendencies.
By contrast, the LPP component of response to fear faces was not associated with either Callous-Aggression or Boldness, but did show a negative relationship with Disinhibition scores at the zero-order level, which remained near-significant in the omnibus regression model, p = .06.
Discussion
Findings from this study demonstrate that callousness in adult-aged community participants is associated with deficits in early neural processing of fear faces. Further in line with the aims of this second study, we were also able to determine that neural response differences associated with callousness were specific to fearful faces above and beyond neutral faces, and extended only secondarily to the disinhibition facet of psychopathy.
Along with the evidence that current results provide for similarity of callousness-related deficits across different age groups, some additional aspects of these results warrant attention. First, the finding that the P200 component of fear-face responding emerged as the most robust, selective correlate of callous tendencies (i.e., showing significant relations with this psychopathy facet alone at both the zero-order level and in the regression analysis) is notable in view of prior work indicating that this component (a) indexes processing of the affective-expressive aspect of faces more so than processing of the ‘faceness’ of such stimuli (Paulmann & Pell, 2009), and (b) shows greater heritability, relative to either N170 or LPP, of fear-face specific variance (i.e., separate from that associated with neutral-face responding; Shannon et al., 2013). Considered along with work demonstrating prominent heritability for CU traits in younger samples (Viding et al., 2008), this raises the question of whether the observed association between fear-face P200 response and callousness in the current study might reflect some common heritable attribute. Although the current analysis sample consisted of monozygotic (MZ) and dizygotic (DZ) twins, the number of pairs of each type was too low to permit formal biometric decomposition of the role of genetic versus environmental influences (cf. Yancey et al., 2013) in the observed relationship between callousness scores and fear-face P200 response. Thus, follow-up utilizing larger twin participant samples will be needed to address this question.
Another notable point is that while N170 and P200 appeared to overlap somewhat in their associations with callousness, each evidenced predictive relations when entered together in a regression model. These findings suggest these two brain-response variables index somewhat different processes, with each showing a distinct, selective association with callousness. Based on what is known about the bases of the N170 and the P200 (e.g., Paulmann & Pell, 2009; Shannon et al., 2013), it can be hypothesized that callous tendencies are associated with reduced facilitation in detection of fearful expressions as face stimuli per se (relative to neutral expressions), and also with diminished responsiveness to the affective content of fearful expressions. Impairments of both types can be expected to interfere with social interchanges that call for awareness of and sensitivity to the feelings of others (Blair, 1995; Kimonis et al., 2008; Dadds et al., 2011).
Also of note is the finding that N170 and P200 responses to neutral face stimuli evidenced associations with callousness as well—but only as a function of corresponding relationships for fearful faces. That is, the correlations for neutral-face response were no longer significant when responses to faces of both types were examined together as predictors of callousness, whereas associations for fear-face response remained significant in the case of the P200 response and near-significant in the case of the N170 response. This finding is interesting in light of fMRI studies reporting heightened amygdala reactivity to neutral as well as negatively-valenced (fearful, angry) face stimuli in individuals with high levels of social anxiety (Phan, Fitzgerald, Nathan, & Tancer, 2006). The interpretation has been that high socially anxious individuals are more apt to interpret non-expressive faces as critical or otherwise threatening. Considering this, it is conceivable that reduced reactivity to neutral-face stimuli in the current study reflected, to some degree, callousness-related impairments in social perceptiveness/sensitivity that were indexed even more strongly by reduced fear-face reactivity.
Of further interest is the finding that later LPP responding was not related to callous-aggressive tendencies, but instead was related to the disinhibitory facet of psychopathy. If the face-stimulus LPP in the current study is viewed as a counterpart to the P3 response that occurs to rare or salient stimuli in visual processing paradigms of other types, then this finding for disinhibition can be seen as converging with work showing reduced P3 in relation to this facet of psychopathy in various other tasks (Patrick, Venables, et al., 2013). This points to the intriguing possibility that different brain responses within the same task might be used to index distinct processing deficits associated with callousness versus disinhibition. However, further work is needed to replicate the findings of differential relations for earlier (N170, P200) and later (LPP) components of face-stimulus with these two facets of psychopathy, and to test the affiliated hypothesis that earlier and later face-ERP components will show contrasting associations with P3 response assessed in other tasks.
Returning to the major aim of Study 2, the finding of a callousness-related reduction in ERP responding to fearful faces in this study extends findings from fMRI investigations demonstrating reduced amygdala reactivity to fear-face stimuli in adolescents high on CU traits. This finding provides additional support for the construct validity of callousness in adults as indexed by scores on the callous-aggression factor of the ESI, and other scale measures that converge with this operationalization (Kimonis et al., 2013; Drislane et al., 2014; Drislane & Patrick, in press). Additionally, this finding points to reduced early ERP brain-response to fear faces as a new neurophysiological indicator of callous-unemotionality, and suggests important directions for follow-up research. One is to evaluate whether reduced early ERP response to fear face stimuli predicts reduced fMRI-amygdala reactivity to faces of this type in participants assessed both ways, and whether variations in callous-unemotional tendencies mediate this relationship in whole or in part. Another important direction will be to evaluate whether children exhibiting conduct problems along with high CU traits show reduced early ERP responses to fear face stimuli, as suggested by research demonstrating emotional deficits for youth exhibiting this configuration of symptoms as opposed to conduct problems or CU traits alone (see Viding & Kimonis, in press). Just as theoretical and empirical work on CU traits in youth has informed conceptions of callous tendencies in adults, research with adults can in turn help to inform continuing work on this construct in younger participant samples.
General Discussion
Results of the two studies reported here corroborate and extend key findings from research on CU traits in children and adolescents by demonstrating impairments in both behavioral (recognition accuracy) and brain (N170 and P200 ERP amplitude) responses to fearful face stimuli in adult participants scoring high on a scale-report measure of callousness. The identification of reliable behavioral and brain indicators of callousness assessed through report-based methods indicates that this psychological construct has referents in different domains of measurement, and suggests the possibility of a multi-domain approach to conceptualizing, assessing, and understanding this important individual difference construct (Patrick & Drislane, 2015). Moreover, the identification of behavioral and brain measures that operate effectively as indicators of callous tendencies in both younger and older participants opens the door to a longitudinal-developmental analysis of CU traits as conceptualized in multi-domain terms.
A focus on assessing psychological constructs using variables from different domains of measurement (‘units of analysis’) is central to NIMH's RDoC framework, which seeks to reorient research on psychopathology toward new conceptions of mental disorders that link more closely to biological systems. For example, defining a clinical condition using symptom indicators along with brain response indicators, or behavioral indicators of a distinct brain process, can be expected to facilitate efforts to identify deviations in neural function associated with the condition (Yancey et al., 2013; Patrick, Venables, et al., 2013). Knowledge of biological and behavioral correlates of a condition can also be useful for research focusing on risk for clinical problems. For example, a liability index consisting of parental ratings of empathic tendencies, fear-face recognition accuracy, and ERP reactivity to fearful faces could prove useful for identifying young ndividuals with strong likelihood to develop antisocial-psychopathic behavior—and studying factors that interface with liability to either promote or prevent the emergence of such behavior.
Current findings also have implications for changes in the latest edition of the official diagnostic nomenclature. Specifically: (1) the criteria for conduct disorder in the main diagnostic section of DSM-5 (Section II) include a new “limited prosocial emotions” (LPE) specifier for designating a high-CU variant of this child behavior disorder, and (2) the dimensional model for personality pathology in Section III of DSM-5 includes a new trait-based conception of adult ASPD that allows for identification of variants with differing levels of antagonistic tendencies relative to disinhibitory tendencies. A potential limitation of the trait-based conception of ASPD is that it lacks a developmental referent in the form of a history of early conduct problems, which was seen as a strength of the traditional criterion-based diagnosis of ASPD that appears in Section II of DSM-5. However, findings from the two studies reported here, together with recent research(Strickland et al., 2013) showing that ASPD criterion-traits from the Antagonism domain of the adult Section III model effectively index callousness as operationalized in the current work (i.e.,as scores on the callous-aggression factor of the ESI), provide evidence for likely linkage between psychopathic proclivities indexed by these Section III APSD traits and those indexed by the LPE specifier for conduct disorder in Section II. Along this line, another valuable for avenue for futureresearch will be to examine the extent to which CU traits operationalized according to the LPE criteria at younger ages predict callous tendencies assessed using measures based around Section II antagonism traits in adulthood.
These possibilities highlight some notable limitations of the current work. One is that participants in Study 1 consisted exclusively of college students. Follow-up work with adults from the general community (as per Study 2) and clinical samples (e.g., mental health clinics, prisoners) spanning a broader range of both age and severity will be important for establishing the eneralizability of the face-recognition results. A further limitation is that recognition accuracy and brain response data were not available for the same set of participants, precluding analyses of relationships between the two. Collecting data of these (and other) types in a common participant sample represents an obvious and crucial next step for establishing a multi-domain conceptual-analytic framework for the callousness construct. The cross-sectional nature of the current work is a further limitation. Longitudinal designs will be needed to directly evaluate linkages between callousness as assessed by informant report at younger ages with callous tendencies as indexed by clinician ratings or self-report at later ages, and the extent to which behavioral and brain indicators of callousness covary with report- or rating-based assessments across time. Data of these types will be extremely useful for evaluating the developmental trajectory of callousness and how it is expressed in later psychopathy-related behaviors (cf. Lynam, 1996; Frick et al., 2014).
In sum, the current work provides valuable new evidence for convergence between CU traits as conceptualized and assessed in children or adolescents and newer operationalizations of callousness (e.g., TriPM Meanness, ESI Callous-Aggression) in adults. Findings from this work serve to highlight prospects for a multi-domain, longitudinal approach to the investigation of callousness that can advance our understanding of the nature and bases of this core dispositional construct and the role it plays in the emergence and persistence of severe antisocial behavior. Work of this kind can contribute to more effective procedures for identifying risk for serious antisocial behavior early in life, and to improved methods for preventing and treating such behavior and reducing the costly toll it exacts on society.
Acknowledgments
This work was supported by grants W911NF-14-1-0027 from the US Army, grants MH072850 and MH089727 from the National Institute of Mental Health, and the National Science Foundation Graduate Research Fellowship under Grant No. 952090. The views, opinions, and/or findings contained in this report are those of the authors and shall not be construed as an official Department of the Army position, policy, or decision, unless so designated by other documents.
Contributor Information
Sarah J. Brislin, Florida State University
James R. Yancey, Florida State University
Emily R. Perkins, Florida State University
Isabella M. Palumbo, Florida State University
Laura E. Drislane, Florida State University
Randall T. Salekin, University of Alabama – Tuscaloosa
Kostas A. Fanti, University of Cyprus
Eva R. Kimonis, University of New South Wales
Paul J. Frick, Louisiana State University, Australian Catholic University
James R. Blair R., Boys Town National Research Hospital
Christopher J. Patrick, Florida State University
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Washington, DC: Author; 2013. [Google Scholar]
- Anokhin A, Golosheykin S. Startle modulation by affective faces. Biol Psychology. 2010;83:37–40. doi: 10.1016/j.biopsycho.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR. A cognitive developmental approach to morality: Investigating the psychopath. Cognition. 1995;57(1):1–29. doi: 10.1016/0010-0277(95)00676-p. [DOI] [PubMed] [Google Scholar]
- Blair RJR, Colledge E, Murray L, Mitchell DGV. A selective impairment in the processing of sad and fearful expressions in children with psychopathic tendencies. journal of Abnormal Child Psychology. 2001;29(6):491–498. doi: 10.1023/a:1012225108281. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spatial Vision. 1997;10(4):433–436. [PubMed] [Google Scholar]
- Brislin SJ, Drislane LE, Smith ST, Edens JF, Patrick CJ. Development and validation of triarchic psychopathy scales from the Multidimensional Personality questionnaire. Psychological Assessment. 2015;27(3):838–851. doi: 10.1037/pas0000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke DJ, Michie C. Refining the construct of psychopathy: Towards a hierarchical model. Psychological Assessment. 2001;13(2):171–188. [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Normal personality assessment in clinical practice: The NEO Personality Inventory. Psychological Assessment. 1992;4(1):5–13. [Google Scholar]
- Dadds MR, Jambrak J, Pasalich D, Hawes DJ, Brennan J. Impaired attention to the eyes of attachment figures and the developmental origins of psychopathy. Journal of child Psychology and Psychiatry. 2011;52(3):238–245. doi: 10.1111/j.1469-7610.2010.02323.x. [DOI] [PubMed] [Google Scholar]
- Dawel A, O'Kearney R, McKone E, Palermo R. Not just fear and sadness: Meta-analytic evidence of pervasive emotion recognition deficits for facial and vocal expressions in psychopathy. Neuroscience & Biobehavioral Reviews. 2012;36(10):2288–2304. doi: 10.1016/j.neubiorev.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Drislane LE, Patrick CJ. Integrating alternative conceptions of psychopathic personality: A latent variable model of triarchic psychopathy constructs. Journal of personality Disorders. doi: 10.1521/pedi_2016_30_240. in press. [DOI] [PubMed] [Google Scholar]
- Drislane LE, Patrick CJ, Arsal G. Clarifying the content coverage of differing psychopathy inventories through reference to the Triarchic Psychopathy Measure. psychological Assessment. 2014;26(2):350–362. doi: 10.1037/a0035152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanti KA, Panayiotou G, Kyranides M, Avramides M. Startle modulation during violent films: Association with callous–unemotional traits and aggressive behavior. motivation and Emotion. 2016;40(2):321–333. [Google Scholar]
- Frick PJ, Ray JV, Thornton LC, Kahn RE. Can callous-unemotional traits enhance the understanding, diagnosis, and treatment of serious conduct problems in children and adolescents? A comprehensive review. Psychological Bulletin. 2014;140(1):1–57. doi: 10.1037/a0033076. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Ray JV. Evaluating callous-unemotional traits as a personality construct. journal of Personality. 2015;83(6):710–722. doi: 10.1111/jopy.12114. http://dx.doi.org/10.1111/jopy.12114. [DOI] [PubMed] [Google Scholar]
- Goldstein H. Hierarchical data modeling in the social sciences. Journal of Educational and Behavioral Statistics. 1995;20(2):201–204. [Google Scholar]
- Hare RD. Manual for the Revised Psychopathy Checklist. 2nd. Toronto: MHS; 2003. [Google Scholar]
- Hare RD, Hart SD, Harpur TJ. Psychopathy and the DSM-IV criteria for antisocial personality disorder. Journal of Abnormal Psychology. 1991;100(3):391–398. doi: 10.1037//0021-843x.100.3.391. [DOI] [PubMed] [Google Scholar]
- Hwang S, Nolan ZT, White SF, Williams WC, Sinclair S, Blair RJR. Dual neurocircuitry dysfunctions in disruptive behavior disorders: emotional responding and response inhibition. Psychological Medicine. 2016;46(7):1485–1496. doi: 10.1017/S0033291716000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Shannon RW, Vizueta N, Bernat EM, Patrick CJ, He S. Dynamics of processing invisible faces in the brain: Automatic neural encoding of facial expression information. NeuroImage. 2009;44(3):1171–1177. doi: 10.1016/j.neuroimage.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AP, Laurens KR, Herba CM, Barker GJ, Viding E. Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. American Journal of Psychiatry. 2009;166(1):95–102. doi: 10.1176/appi.ajp.2008.07071050. [DOI] [PubMed] [Google Scholar]
- Kimonis ER, Branch J, Hagman B, Graham N, Miller C. The psychometric properties of the Inventory of Callous–Unemotional Traits in an undergraduate sample. Psychological Assessment. 2013;25(1):84–93. doi: 10.1037/a0029024. [DOI] [PubMed] [Google Scholar]
- Kozak MJ, Cuthbert BN. The NIMH Research Domain Criteria initiative: Background, issues, and pragmatics. Psychophysiology. 2016;53(3):286–297. doi: 10.1111/psyp.12518. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer MD. Linking antisocial behavior, substance use, and personality: An integrative quantitative model of the adult externalizing spectrum. Journal of Abnormal Psychology. 2007;116(4):645–666. doi: 10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienfeld SO, Widows MR. Psychopathic Personality Inventory—Revised (PPI-R) professional manual. Odessa, FL: Psychological Assessment Resources; 2005. [DOI] [PubMed] [Google Scholar]
- Lynam DR. The early identification of chronic offenders: Who is the fledgling psychopath? Psychological Bulletin. 1996;120:209–234. doi: 10.1037/0033-2909.120.2.209. [DOI] [PubMed] [Google Scholar]
- Lynam DR, Derefinko K. Psychopathy and personality. In: Patrick CJ, editor. Handbook of Psychopathy. New York: Guilford Press; 2005. pp. 133–155. [Google Scholar]
- Marsh AA, Blair RJR. Deficits in facial affect recognition among antisocial populations: A meta-analysis. Neuroscience and Biobehavioral Reviews. 2008;32(3):454–465. doi: 10.1016/j.neubiorev.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DGV, Reid ME, Sims C, Kosson DS, et al. Blair RJR. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. American Journal of Psychiatry. 2008;165(6):712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Bernat EM. Neurobiology of psychopathy: A two-process theory. In: Berntson GG, Cacioppo JT, editors. Handbook of neuroscience for the behavioral sciences. New York: Wiley; 2009. pp. 1110–1131. [Google Scholar]
- Patrick CJ, Bernat E, Malone SM, Iacono WG, Krueger RF, McGue M. P300 amplitude as an indicator of externalizing in adolescent males. Psychophysiology. 2006;43:84–92. doi: 10.1111/j.1469-8986.2006.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Curtin JJ, Tellegen A. Development and validation of a brief form of the Multidimensional Personality Questionnaire. Psychological Assessment. 2002;14:150–163. doi: 10.1037//1040-3590.14.2.150. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Drislane LE. Triarchic model of psychopathy: Origins, operationalizations, and observed linkages with personality and general psychopathology. Journal of Personality. 2015;83(6):627–643. doi: 10.1111/jopy.12119. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Fowles DC, Krueger RF. Triarchic conceptualization of psychopathy: Developmental origins of disinhibition, boldness, and meanness. Development and Psychopathology. 2009;21(3):913–938. doi: 10.1017/S0954579409000492. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Hajcak G. RDoC: Translating promise into progress. Psychophysiology. 2016;53(3):415–424. doi: 10.1111/psyp.12612. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Kramer MD, Krueger RF, Markon KE. Optimizing efficiency of psychopathology assessment through quantitative modeling: Development of a brief form of the Externalizing Spectrum Inventory. Psychological Assessment. 2013;25(4):1332–1348. doi: 10.1037/a0034864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Venables NC, Yancey JR, Hicks BM, Nelson LD, Kramer MD. A construct-network approach to bridging diagnostic and physiological domains: application to assessment of externalizing psychopathology. Journal of Abnormal Psychology. 2013;122(3):902–916. doi: 10.1037/a0032807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulmann S, Pell MD. Facial expression decoding as a function of emotional meaning status: ERP evidence. NeuroReport. 2009;20(18):1603–1608. doi: 10.1097/WNR.0b013e3283320e3f. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biological Psychiatry. 2006;59(5):424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Poy R, Segarra P, Esteller A, Lopez R, Molto J. FFM description of the triarchic conceptualization of psychopathy in men and women. Psychological Assessment. 2014;26:69–76. doi: 10.1037/a0034642. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37(2):257–261. [PubMed] [Google Scholar]
- Shannon RW, Patrick CJ, Venables NC, He S. “Faceness” and affectivity: Evidence for genetic contributions to distinct components of electrocortical response to human faces. NeuroImage. 2013;83:609–615. doi: 10.1016/j.neuroimage.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland CM, Drislane LE, Lucy M, Krueger RF, Patrick CJ. Characterizing psychopathy using DSM-5 personality traits. Assessment. 2013;20(3):327–338. doi: 10.1177/1073191113486691. [DOI] [PubMed] [Google Scholar]
- Venables NC, Patrick CJ. Validity of the Externalizing Spectrum Inventory in a criminal offender sample: Relations with disinhibitory psychopathology, personality, and psychopathic features. Psychological Assessment. 2012;24(1):88–100. doi: 10.1037/a0024703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viding E, Jones AP, Frick PJ, Moffitt TE, Plomin R. Heritability of antisocial behaviour at 9: Do callous-unemotional traits matter? Developmental Science. 2008;11(1):17–22. doi: 10.1111/j.1467-7687.2007.00648.x. [DOI] [PubMed] [Google Scholar]
- Viding E, Kimonis ER. Callous-unemotional traits. In: Patrick CJ, editor. Handbook of psychopathy. 2nd. New York: Guilford Press; in press. [Google Scholar]
- Widiger TA, Lynam DR. Psychopathy and the five-factor model of personality. In: Millon T, Simonsen E, Birket-Smith M, Davis RD, editors. Psychopathy: Antisocial, criminal, and violent behavior. New York, NY: Guilford Press; 1998. pp. 171–187. [Google Scholar]
- Yancey JR, Venables N, Hicks B, Patrick CJ. Evidence for a heritable brain basis to deviance-promoting deficits in self-control. Journal of Criminal Justice. 2013;41:309–317. doi: 10.1016/j.jcrimjus.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]