Abstract
Purpose
Hepatic iron content (HIC) quantification via R2*-MRI using multi gradient echo (mGRE) imaging is compromised towards high HIC or at higher fields due to the rapid signal decay. Our study aims at presenting an optimized 2D UTE sequence for R2* quantification to overcome these limitations.
Methods
2D UTE imaging was realized via half pulse excitation and radial center-out sampling. The sequence includes CHESS pulses to reduce streaking artifacts from subcutaneous fat and spatial saturation (sSAT) bands to suppress out-of-slice signals. The sequence employs interleaved multi-echo readout trains to achieve dense temporal sampling of rapid signal decays. Evaluation at 1.5T and 3T was done in phantoms and clinical applicability demonstrated in five patients with biopsy-confirmed massively high HIC levels (>25 mg Fe/g dry weight liver tissue).
Results
In phantoms, the sSAT pulses were found to remove out-of-slice contamination, and R2* results were in excellent agreement to reference mGRE R2* results (slope of linear regression: 1.02/1.00 for 1.5/3T). UTE-based R2* quantification in patients with massive iron overload proved successful at both field strengths and was consistent with biopsy HIC values.
Conclusion
The UTE sequence provides a means to measure R2* in patients with massive iron overload both, at 1.5T and 3T.
Keywords: ultrashort echo time imaging, UTE, transfusional iron overload, half pulse excitation, T2* quantification, liver MRI
Introduction
Iron overload is a severe complication arising from multiple blood transfusions and increased intestinal absorption (1–3). Hematologic disorders such as β-thalassemia major and sickle cell anemia require frequent therapeutic blood transfusions to prevent disease complications (4,5). As there is no physiologic mechanism for iron excretion, repeated transfusions cause accumulation of iron in several organs, most notably in the liver (6,7), which can lead to substantial morbidity due to iron toxicity (8,9). Therefore, accurate assessment of the hepatic iron content (HIC) is essential for disease management and monitoring of treatment response to iron chelation therapy.
HIC has traditionally been monitored by analyzing the iron content of samples from liver biopsies. Non-invasive HIC quantification utilizing magnetic resonance imaging (MRI) has been successfully developed recently (10), eliminating the risks involved with liver biopsies (i.e., bleeding and pain) (11). One of these non-invasive MRI methods assesses HIC by quantifying the effective transverse relaxation rate R2* (= 1/T2*) of liver tissue via evaluation of the exponential signal decay seen in multi-echo gradient echo (mGRE) MRI. Previous biopsy-calibrated studies at 1.5 T have shown an excellent linear correlation between HIC and R2* with a high precision for HIC levels up to 20–25 mg Fe/g dry wt [ = mg of Fe per g of dry weight liver tissue] (12–15). For HIC levels > 25 mg Fe/g dry wt (termed as “massive iron overload” throughout this manuscript), R2*-based iron assessment suffers a lack in precision and might eventually even fail (10,13,14) as the employed mGRE techniques are intrinsically limited when detecting rapidly decaying MR signals. This aspect becomes even more pronounced at field strengths of 3 T and above, as R2* increases linearly with B0 (approximately twofold increase from 1.5 T to 3 T) (16–18). Conventional mGRE techniques start to become degraded when measuring signals with T2* times of 1 ms or less, corresponding to HIC levels above approximately 25 mg Fe/g dry wt at 1.5 T (13–15). This limitation of conventional mGRE imaging restricts the current, clinically accessible HIC range at 3 T to about 12.5 mg Fe/g dry wt and lower.
These limitations of mGRE-based HIC estimation could be overcome by using ultrashort echo time (UTE) imaging (19). UTE imaging allows for very short delays (≤ 100 μs) between signal excitation and data acquisition and enables the detection of tissues with very short transverse relaxation times as for example demonstrated in pulmonary and musculoskeletal applications (19–24). Here, UTE sequences have not only been used for basic imaging of such tissues but also to quantitatively characterize the short T2* properties of e.g. lung parenchyma, cortical bone, ultrashort T2 components in white matter, and iceball formation during cyroablation (21,24–30). Moreover, a study by Chappell et al. (31) on the feasibility of UTE imaging in hepatic diseases, such as cirrhosis, hemochromatosis, fibrosis, and hepatocellular carcinomas, already showed the general applicability of UTE-based T2* measurements in iron overload. However, this study did not aim at a specific investigation of UTE imaging in massive HIC settings as reflected by the reported mean T2* time of 7 ms (standard deviation 2.7 ms) for patients showing patterns of hemochromatosis. According to three independently performed, biopsy-calibrated R2*-HIC conversions (13–15), T2* times from 4 to 10 ms would translate into HIC values from about 2.3 to 6.6 mg/g dry wt which is still within a range that can be clinically assessed via mGRE imaging (10,13–15). Nevertheless, the study by Chappell et al. (31) demonstrates potential of UTE imaging in iron overload assessment.
Chappell et al. as well as other studies on UTE-based T2* assessment employ slice-selective 2D UTE imaging (24–31) which can be achieved via half-sinc RF pulse excitation in combination with center-out radial sampling (19,32,33). Half pulse excitation requires two acquisitions with respectively inverted slice selection gradients, but otherwise identical scan parameters which are combined in the complex domain to obtain the desired slice profile. The combination of two half-pulse acquisitions with inverted slice selection gradients should theoretically lead to a full cancellation of signals from out-of-slice locations (19,32,33). Unfortunately, this mechanism is sensitive to any system imperfections affecting the slice selection gradient such as eddy currents or gradient delays (34). Such imperfections can manifest in insufficient cancellation of unwanted out-of-slice signals (29,30,35–37), which hampers quantitative imaging as needed for R2*-based HIC assessment (24–30).
Here, we describe the technical implementation of a 2D UTE sequence that is specifically applicable for hepatic T2* quantitation whenever conventional mGRE techniques become imprecise due to T2* shortening: patients with high or massive HIC and at higher field strengths. We systemically studied confounding factors and integrated simple, readily available sequence modules to largely avoid unwanted effects hampering T2* quantitation in such situations. The sequence employs spatial saturation (sSAT) bands to reduce unwanted out-of-slice signal contributions and achieves dense temporal sampling of the rapidly decaying signal, as seen for massive HIC, via acquisition of interleaved echo trains without requiring breath holding. The sequence was tested and quantitatively evaluated in phantom experiments and in vivo.
Methods
Sequence Concept and Implementation
Our 2D UTE sequence uses half-sinc RF pulse excitation (pulse duration = 1.0 ms, time-bandwidth product = 2.0) together with center-out radial sampling (19,20,32,33) including data acquisition during ramp-up of the readout gradient. To minimize the delay between RF excitation and data acquisition, the half-sinc pulse shape is modified via rate selective excitation (VERSE) (38) to account for the time-varying gradient amplitude during ramp-down of the slice selection gradient. The two half pulse acquisitions with respectively inverted slice selection gradients are sampled consecutively (as inner averages) to directly combine the data in the complex domain.
Improved 2D UTE slice selectivity by suppression of unwanted out-of-slice signal contributions has been demonstrated via quadratic phase sSAT pulses (36). Similar to this concept, we added readily available, conventional truncated sinc-shaped sSAT pulses for out-of-slice signal suppression to the 2D UTE sequence. The sSAT pulses (pulse duration = 2.56 ms, time-bandwidth product = 8.0) were applied in each TR interval prior to half pulse excitation so that saturation bands were induced on both sides and in parallel to the UTE imaging slice.
As 2D UTE imaging employs radial sampling, the images are prone to streaking artifacts which can emerge from bright signal intensities in the periphery of the image (39). Such high peripheral signal intensities can arise from subcutaneous fat in mGRE imaging protocols especially when surface coils are used for optimized signal reception as done in abdominal imaging. To avoid high signal intensities from fat, fat suppression via chemically selective saturation (CHESS) (40) RF pulses was incorporated. The Gaussian CHESS pulses (duration = 10.24/5.12 ms, off-resonance = 187.5/375 Hz at 1.5 T/3 T) were also applied in each TR interval prior to the half pulses.
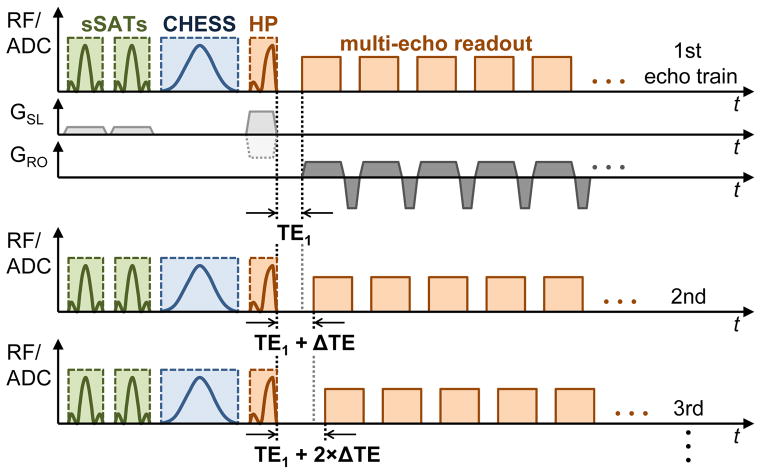
The sequence uses a multi-echo readout gradient with readout rewinding to acquire multiple echoes as needed for T2*/R2* quantification. All echoes are sampled as half-echoes including ramp sampling identical to the first (UTE) echo. As shown in other UTE applications, e.g. in cortical bone (25,26), dense echo sampling is required to accurately capture the rapid signal decay for tissues with very short T2* times. Therefore, additional UTE echoes need to be acquired which was accomplished by acquisition of additional multi-echo readout trains that are shifted by small echo time increments ΔTE relative to the first echo train. The radial sampling process of the UTE sequence is intrinsically insensitive to motion artifacts (41) and does not necessarily require breath holding as opposed to Cartesian acquisition schemes. Our UTE acquisition scheme exploits this fact to acquire additional, interleaved multi-echo trains in free breathing. A schematic of the free breathing interleaved multi-echo UTE (FB-imUTE) sequence is illustrated in Fig. 1.
Figure 1.
Schematic excitation and acquisition diagram of the free breathing interleaved multi-echo UTE (FB-imUTE) sequence, only important elements are shown: Prior to each half pulse (abbreviated as HP in diagram) excitation, CHESS pulses for fat suppression and sSAT pulses for out-of-slice signal suppression are applied. CHESS and sSAT pulses are followed by spoiler gradients (not shown) to dephase transverse magnetization. The sSAT bands are oriented in parallel to the imaging slice. The slice selection (GSL, light gray; dotted line indicates alternating polarity of slice selection gradients) and readout (GRO, dark gray) gradients are illustrated for the first echo train only. Each echo of the multi-echo readout train is acquired via center-out radial sampling including data acquisition during ramp-up of the readout gradients. The sequence acquires additional echo trains which are shifted relative to the previous echo train by a small echo time increment ΔTE to achieve dense temporal sampling even for fast T2* decay.
Images were reconstructed via inverse Fourier transformation after re-gridding of the radially collected data to a Cartesian grid using a Kaiser-Bessel kernel (42,43). The sequence and image reconstruction were implemented on 1.5 T and 3 T scanners (MAGNETOM Avanto and Trio, Siemens Healthcare, Erlangen, Germany). Prior to experimental validation in phantoms and initial in vivo testing, remaining image distortions, which can emerge in radial acquisitions due to temporal delays between readout gradients and data acquisition in the Analog-Digital-Converter (ADC) (44), were investigated and corrected in a series of pre-scans. The correction parameters and the sequence parameters affecting readout gradient and ADC timing (e.g., pixel bandwidth and number of data points per ADC) remained unchanged during all subsequent phantom and in vivo measurements, so that the pre-scans had to be executed only once for each scanner at the very beginning of this study (please refer to Figures S1 and S2 for details).
For all T2*-weighted, multi-echo image series (i.e., phantom and in vivo measurements) pixel-wise T2* fitting was done via a non-linear least square fit of the second moment of the gradient echo signal (45) implemented in MATLAB (MathWorks, Natick, MA). The second moment considers the expectation value of the squared magnitude signal 〈 〉 which is fitted to the equation , where S denotes the ideal, noise-free mono-exponentially decaying signal, and N reflects noise. In comparison to the expectation value of the plain magnitude signal 〈SM〉, the second moment fit has a much simpler form and was therefore implemented here (45).
Phantom Measurements
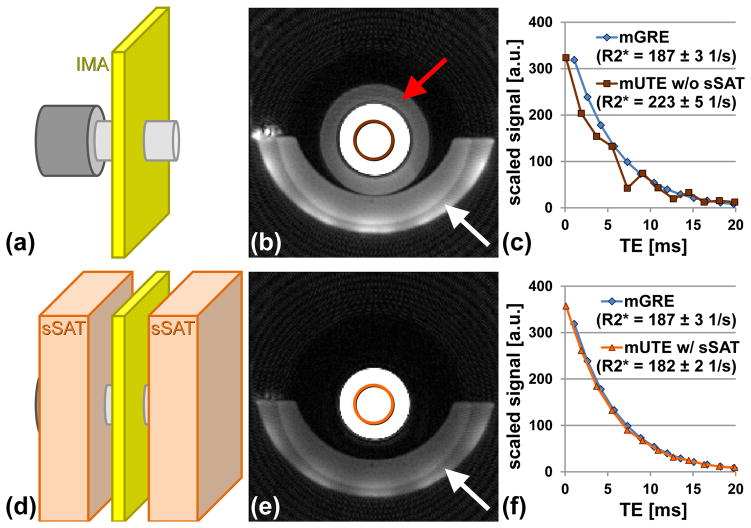
In a series of phantom measurements, the effect of out-of-slice signal contributions on T2*/R2* quantification was assessed. A cylindrical phantom bottle (V = 500 ml) filled with a 2%-Agar-water solution (Sigma-Aldrich, St. Louis, MO), that was doped with iron nanoparticles (BNF-Starch particles, diameter = 80 nm, micromod Partikeltechnologie GmbH, Rostock, Germany; Fe concentration = 13.75 μg/ml) was placed inside the system’s head coil in the iso-center of the magnet bore with the symmetry axis of the phantom bottle in parallel to B0. A second, larger cylindrical phantom bottle (V = 1000 ml) filled with 2%-Agar solution was positioned right behind the smaller bottle so that the larger bottle was located approximately 3 cm off the magnet’s iso-center (schematic illustration given in Fig. 2). Overall three multi-echo image series were acquired at the iso-center in axial slice orientation. Two image series were acquired with the 2D multi-echo UTE (mUTE) sequence without and with sSAT bands, respectively, and in addition one image series was measured with a conventional mGRE sequence serving as reference for T2*/R2 quantitation (14). The following image parameters were used for the 2D mUTE sequences without and with sSAT bands: TR/TE1 = 52.5/0.1 ms, 12 echoes, echo spacing (ESP) = 1.8 ms, field of view (FOV) = 250×250 mm2, slice thickness (SL) = 10 mm, 192 radial spokes, 192 points per spoke, pixel bandwidth (BW) = 780 Hz/px, flip angle (FA) = 20°; sSAT band parameters: sSAT band thickness = 100 mm, gap to imaging slice = 10 mm (i.e. equivalent to slice thickness); total acquisition time (TA) ≈ 20 s. No CHESS pulses and no additional ΔTE-shifted echo trains were applied in the mUTE acquisitions in these phantom measurements. The mGRE sequence was applied with the following parameters: TR/TE1 = 200/1.1 ms, 20 echoes, ESP = 1.6 ms, FOV = 250×250 mm2, SL = 10 mm, matrix = 128×128, BW = 1950 Hz/px, FA = 35°; TA ≈ 25 s.
Figure 2.
Effect of sSAT bands to suppress unwanted out-of-slice signals. (a,d) Schematic (dimensions not to scale) of experimental setup (IMA – imaging slice, sSAT – sSAT bands) with two phantom bottles (light and dark gray) placed one behind the other. Axial UTE images (b) without and (e) with sSATs. Substantial out-of-slice signals from the larger phantom positioned behind the smaller phantom bottle can be seen (red arrow). Signal below the phantom bottle (white arrows) arises from cushion used for positioning of the phantoms. T2* decay seen for averaged signal at the center (circles) of the phantom bottle for the mUTE (c) without and (f) with sSAT pulses. For comparison, the signal decay measured with a reference mGRE sequence is also shown (mGRE images not shown). Without sSATs, the out-of-slice signals lead to distortions of the signal decay hampering T2* analysis. Mean R2* values and standard deviation for each acquisition are given in plot legend.
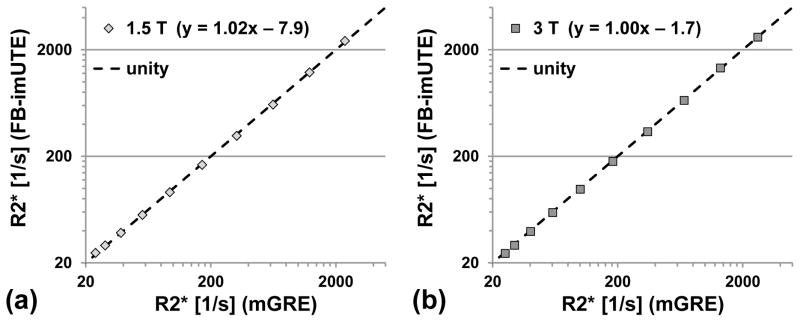
In a second series of phantoms measurements, the FB-imUTE sequence was evaluated for correct T2*/R2* quantitation using a mGRE sequence as reference. Ten cylindrical phantom bottles (V = 500 ml) were filled with 2%-Agar solution and doped with iron nanoparticles of different concentrations (BNF-Starch particles, diameter = 80 nm, micromod Partikeltechnologie GmbH, Rostock, Germany; range of Fe concentrations in phantoms = 0.4–220 μg/ml) to cover a wide range of T2*/R2* values reflecting low to massive HIC conditions. Axial images were acquired for each phantom individually (each phantom placed inside system’s head coil in the magnet’s iso-center) using the FB-imUTE sequence including CHESS and sSAT pulses and a mGRE protocol that was based on a previously published, biopsy-calibrated mGRE sequence for HIC assessment (14). The FB-imUTE sequence parameters were as described above except that 5 interleaved echo trains with ΔTE shifts of 0.25 ms were used (TA ≈ 1:40 min). The mGRE sequence parameters were also as described above except that signal averaging (number of averages = 5) was used to achieve a sufficiently high signal-to-noise ratio (SNR) even for phantoms with very short T2* times. Axially oriented multi-echo images (slice location in iso-center) were collected at 1.5 T and 3 T.
In vivo Testing
In vivo testing of the UTE sequence was done on 1.5 T and 3 T platforms in a total of 7 subjects with iron overload. Participants had a history of >12 cumulative packed red blood cell transfusions, and all were consented to participate in a prospective institutional review board approved study on iron overload assessment (www.clinicaltrials.gov #NCT01572922).
For all scans, subjects were placed on the patient table in supine position and positioned so that the liver was located in the iso-center of the magnet. Imaging slices were positioned at the location of the main portal vein and acquired in axial slice orientation with body array and spine array coils. Prior to the acquisition of any UTE and GRE images, the system’s ‘standard’ shim procedure was executed.
To study the reduction of streaking artifacts, two subjects were scanned using single breath hold (BH) multi-echo UTE acquisitions (BH-mUTE) including sSAT bands without and with CHESS pulses, respectively. The BH-mUTE images were acquired with sequence and sSAT parameters as described above (section Phantom Measurements) without additional ΔTE-shifted echo trains (TA ≈ 20 s).
The in vivo applicability of the FB-imUTE sequence for T2*/R2* quantification in the presence of massive iron overload was tested in five subjects with clinically anticipated massive HIC, four diagnosed with sickle cell disease and one with Diamond-Blackfan anemia. The lifetime cumulative number of packed red blood cell units was ≥ 90 for all five subjects. FB-imUTE images were acquired during regular breathing. The sequence parameters were as described in the section Phantom Measurements except that the FOV was adjusted individually for each subject (FOV = 400×400/420×420 mm2; TA ≈ 1:40 min). For comparison, the scan protocol also included BH-mUTE acquisitions without additional ΔTE-shifted echo trains but including sSAT bands and CHESS pulses (sequence parameters as given above, with FOV set identical to FB-imUTE; TA ≈ 20 s). For reference, subjects also received scans with a R2*-HIC biopsy-calibrated, single BH-mGRE sequence as previously described (14). The mGRE sequence parameters were also as described in the section Phantom Measurements except that a bipolar readout gradient scheme was employed to achieve a shorter ESP of 0.8 ms and a rectangular FOV (FOV = 340×276/320×260 mm2, matrix = 128×104; TA ≈ 21 s) was chosen.
For each acquisition, the mean hepatic R2* value was obtained from the mean value within a manually drawn whole liver region of interest (ROI) after pixel-wise T2*/R2* mapping as described above. Any unwanted structures such as blood vessels were excluded based on a histogram analysis (46).
Results
Phantom Testing
Unwanted out-of-slice signal contributions affect T2*/R2* extraction from 2D multi-echo UTE imaging as illustrated in Fig. 2. Without sSAT bands, substantial signal contributions from the larger phantom that was located outside the imaging slice are visible (Fig. 2b). Application of the sSAT pulses removes these unwanted signal contributions (Fig. 2e). In addition to the erroneous background signal intensities, the out-of-slice signal contributions also induce distortions in the measured signal decay which impacts the measured R2* values (R2* GRE vs. R2* UTE without sSAT = 187±3 1/s vs. 223±5 1/s; Fig. 2c). A smoother, mono-exponential signal decay without signal distortions was found in the UTE acquisition with sSAT pulses which substantially improves the R2* result (R2* GRE vs. R2* UTE with sSAT = 187±3 1/s vs. 182±2 1/s; Fig. 2f).
The systematic comparison of FB-imUTE and mGRE R2* results in phantoms with iron nanoparticles (approximate range of R2* values at 1.5/3 T = 20–2420/30–2640 1/s) shows that the proposed UTE sequence yields R2* values which are consistent with the results from the mGRE reference sequence (linear regression results at 1.5/3 T: y = 1.02x – 7.9/1.00x – 1.7; Fig 3). R2* quantitation improved at both field strengths for FB-imUTE relative to mGRE imaging with increasing iron concentration. For example, an approximately threefold reduction of the standard deviation (SD) of the R2* values was observed in the phantom with the highest iron concentration (SD of R2* values at 1.5 T and 3 T: ±296 1/s and ±167 1/s for mGRE, and ±101 1/s and ±55 1/s for FB-imUTE).
Figure 3.
Results of R2* measurements in phantoms doped with iron nanoparticles at different concentrations for (a) 1.5 T and (b) 3 T. R2* values were measured with the proposed FB-imUTE sequence and compared to a reference mGRE acquisition. Five averages were done for the mGRE sequence to achieve sufficient SNR for reliable R2* assessment in the phantoms with high iron particle concentration. Linear regression (results given in plot legend) shows that both sequences yield highly consistent R2* results for both field strengths across a large dynamic range.
In vivo Testing
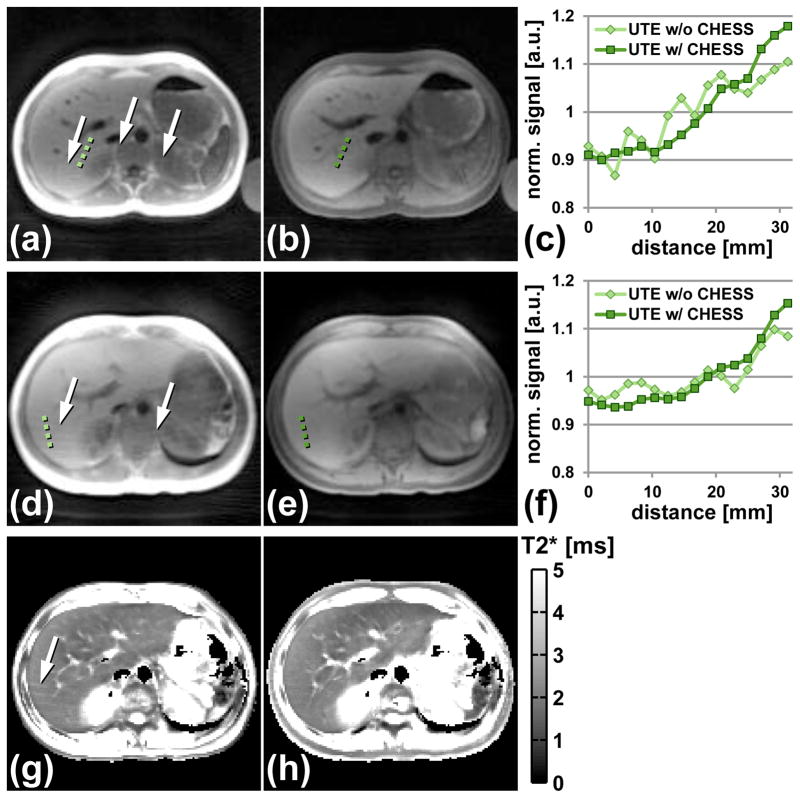
In vivo tests of the BH-mUTE protocols with and without CHESS pulses showed a reduction of streaking artifacts from bright peripheral subcutaneous fat signal by the application of CHESS (Fig. 4). Streaking artifacts are not only visible in the magnitude images (Fig. 4a/d) but also in the associated T2* maps leading to systematically larger T2* values compared to image regions where no streaking artifacts are present (Figs. 4g,h).
Figure 4.
Effect of CHESS pulses to suppress bright signal intensities in peripheral subcutaneous fat tissue. BH-mUTE images without CHESS at (a) 1.5 T and (d) 3 T show substantial streaking artifacts (white arrows). (b,e) Corresponding UTE images with CHESS pulses. Streaking artifacts are substantially reduced for both field strengths. The graphs in (c) and (f) reflect plots of normalized signal intensities along the green dotted lines given in (a,b) and (d,e) respectively. Without CHESS, the signal intensities show an oscillatory pattern which is almost absent in the UTE images with CHESS. The streaking artifacts also affect T2* mapping as shown exemplarily for the 3 T data where streaking artifacts can be seen in T2* maps (g) without CHESS in contrast to T2* maps (h) with CHESS.
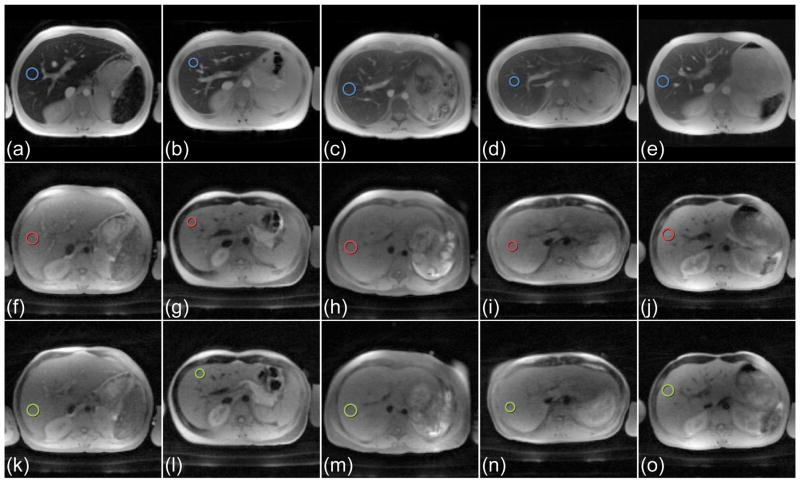
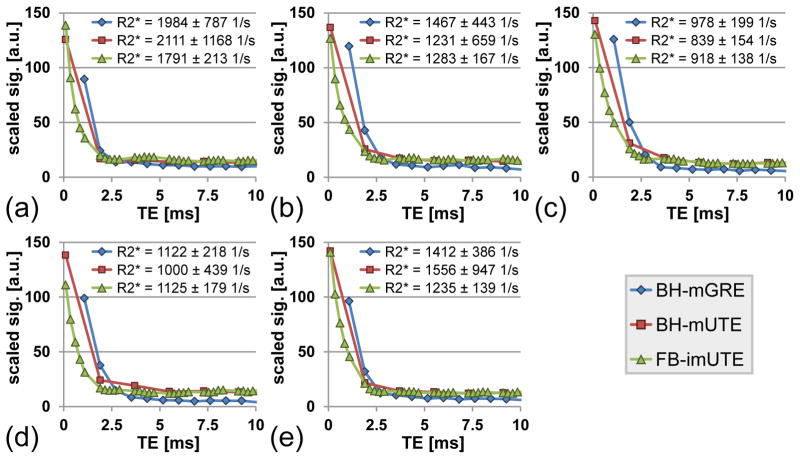
Figure 5 summarizes the 1.5 T results from the measurements with the BH-mGRE, the BH-mUTE, and the proposed FB-imUTE sequence in the five subjects with massive iron overload as confirmed by post-MRI liver biopsy with HIC values ranging from 25.4 to 35.4 mg Fe/g dry wt (mean HIC ± SD = 29.6±3.3 mg/g dry wt). Figure 6 depict signal-versus-TE plots as measured in circular hepatic ROIs with the BH-mGRE, BH-mUTE, and FB-imUTE sequences. In comparison to the BH-mGRE and BH-mUTE acquisitions, the FB-imUTE sequence did not yield a substantially higher SNR at the time point of TE1, but provided a denser temporal sampling of the rapid signal decay which improves T2*/R2* quantitation. For the FB-imUTE sequence, an approximately twofold smaller R2* SD was found in comparison to the BH-mGRE data. Figure 5 also highlights the motion-insensitivity of the FB-imUTE sequence: no additional artifacts from breathing motion can be seen in the free breathing UTE images (Fig. 5k–o) in comparison to the breath hold UTE images (Fig. 5f–j).
Figure 5.
In vivo imaging with the BH-mGRE, BH-mUTE, and FB-imUTE sequences in the five subjects with massive HIC at 1.5 T. Images acquired at first echo time with the (a–e) conventional BH-mGRE (TE1 = 1.1 ms), with the (f–j) BH-mUTE (TE1 = 0.1 ms), and with the (k–o) FB-imUTE (TE1 = 0.1 ms) sequence. The two UTE sequences were applied with sSAT bands and CHESS pulses as described. Barely any additional artifacts due to breathing motion can be seen in the free breathing acquisitions (k–o) in comparison to the breath hold acquisitions (a–j). Blue, red, and green circles indicate ROIs which were used to illustrate the signal decays given in Fig. 6.
Figure 6.
Signal decays as seen in hepatic ROIs (blue, red, and green circles in Fig. 5) measured with BH-mGRE, BH-mUTE and FB-imUTE sequences for the five subjects with massive HIC at 1.5 T. Note that signal intensities were re-scaled to an intensity range from 0–150 for comparison. Whole liver mean R2* results (mean ± standard deviation) of each subject are given in the plot legend for each sequence. With the FB-imUTE, additional UTE echoes are acquired which enables dense sampling of the rapid T2* decay. The dense temporal sampling of the FB-imUTE sequence improves the R2* assessment as can be seen from the reduced standard deviation.
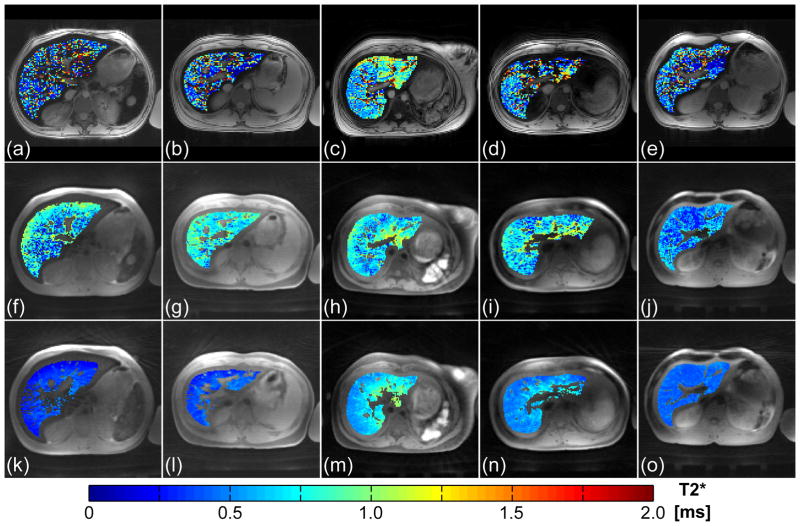
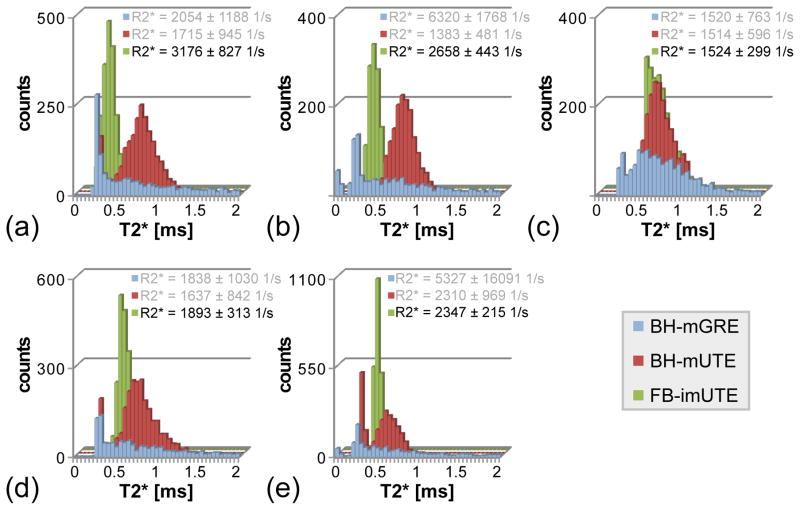
The results from the application of the FB-imUTE sequence at 3 T are shown in Figs. 7 and 8. As hepatic R2* values increase approximately linearly with B0 for patients with iron overload (16–18), the associated T2* decay for the five cases with massively high HIC is too fast to be captured sufficiently well with BH-mGRE acquisitions. T2* maps retrieved from the mGRE images (Fig. 7a–e) appear very noisy and the histogram plots of T2* values (Fig. 8) found within the liver parenchyma after exclusion of unwanted structures appear almost flat which prevents a reliable hepatic R2* assessment. Although the T2* maps extracted from the BH-mUTE images (Fig. 7f–j) appear less noisy, the precision of T2*/R2* assessment is also limited as can be seen from the histogram analysis (Fig. 8): the BH-mUTE T2* values are distributed over a wide range displaying a broader maximum at higher T2* values. In contrast, T2* maps extracted from the FB-imUTE acquisitions appear smooth within the entire liver parenchyma. For all five subjects, the T2* histogram plots show a well-defined, sharp maximum enabling a representative measurement of mean hepatic R2* values. Mean hepatic R2* values ranging from about 1500 to 3200 1/s were found with relative errors from about ±9 to ±26% (relative error = SD/mean) respectively.
Figure 7.
In vivo imaging with the BH-mGRE, BH-mUTE, and FB-imUTE sequences in the five subjects with massive HIC at 3 T. Images acquired at first echo time with the (a–e) conventional BH-mGRE (TE1 = 1.1 ms), with the (f–j) BH-mUTE (TE1 = 0.1 ms), and with the (k–o) FB-imUTE (TE1 = 0.1 ms) sequence. The colored overlay represents results from pixel-wise T2* fitting within the liver parenchyma after exclusion of unwanted structures such as blood vessels.
Figure 8.
Histogram plots of the T2* values found at 3 T in the liver parenchyma after exclusion of unwanted structures for the different sequences. For the two BH sequences, the histogram distribution does not display a clear maximum so that mean R2* quantitation is hampered whereas the FB-imUTE data exhibits a well-defined maximum allowing for a meaningful assessment of mean hepatic R2*. Mean R2* values (mean ± standard deviation) of each subject are given in the plot legend for all acquisitions, but only the FB-imUTE sequence gives a trustworthy result.
Discussion
We present the development and implementation of a 2D UTE imaging sequence for hepatic T2*/R2* assessment in massive hepatic iron overload at 1.5 T and 3 T. In such conditions, established mGRE techniques for R2*-based HIC estimation are limited or might even fail due to the rapidly decaying signals (10,13,14). The FB-imUTE sequence overcomes this limitation by providing dense temporal sampling of the fast signal decay at multiple ultrashort echo times which is paramount for correct T2*/R2* assessment. The sequence was equipped with sSAT bands for an improved slice selectivity of the half pulse excitation process and with CHESS pulses for a reduction of streaking artifacts. Otherwise both effects, improper slice selectivity and streaking, would interfere with quantitative imaging.
CHESS pulses were required to minimize streaking artifacts arising from bright peripheral subcutaneous fat signal. The streaking artifacts can be explained by the point-spread-function (47) of radial sampling so that localized, high signal levels in the periphery of the image partly spread their intensities over the entire image (39,48,49). The streaking artifacts did not only emerge in the magnitude images but also translated into erroneous T2*/R2* maps (Fig. 4). In comparison to other approaches (48–50), the use of CHESS pulses is appealing as a simple means to reduce streaking for our application. The CHESS pulses have the incidental benefit of reducing fat-water oscillations in the measured signal decay, which otherwise would have to be accounted for by appropriate signal modeling (51,52).
The sSAT pulses were implemented to suppress unwanted signal contributions from imperfect cancellation of out-of-slice signals which otherwise distort the measured signal decay and severely hamper T2*/R2* quantitation (Fig. 2). The out-of-slice contributions not only superimpose the in-slice signal manifesting as a simple offset in the measured signal decay but introduce modulations in the signal decay which distorts T2*/R2* estimation. The observed temporal modulations are consistent with other studies (30,37) in which the authors also report out-of-slice-induced variations in the signal-time course of half pulse 2D UTE imaging. They can be explained by in- and out-of-phase effects of the out-of-slice contributions to the in-slice signal (37).
The impact of improper slice selectivity of half pulse excitation due to eddy currents has been reported in other studies on 2D UTE-based T2* assessment (24–30), and several mechanisms were employed for correction. For example, 2D non-slice-selective approaches, which substitute the half pulses with short non-selective rectangular pulses and thus eliminate the need for slice selection gradients, have been successfully used to study T2* of smaller samples (25). However, such strategies might be disadvantageous in in vivo imaging of the liver as the obtained signal represents an integrated signal over the entire excitation volume. In 2D slice-selective UTE imaging, bipolar slice selection gradients were implemented to reduce unwanted effects from eddy currents (24,26–28), and more advanced concepts to apply eddy current compensated slice selection gradients were presented (29,30). Our strategy to minimize unwanted out-of-slice effects via application of sSAT pulses follows the concept of Josan et al. using quadratic phase pulses to yield an improved half pulse selectivity (36). Although such pulses offer a sharp saturation profile, they typically require more time than conventional sinc-shaped sSAT pulses and a more complex implementation. As can be seen from our phantom work (Figs. 2 and 3), the implementation of sSAT bands enables accurate T2* quantitation, and might therefore serve as a simple but effective approach to avoid distortions in slice selectivity.
Both, CHESS and sSATs pulses, might affect the T2*/R2* estimation. Previous studies have shown an effect of fat suppression on the measured T2*/R2* values in patients with transfusional iron overload (53,54) which most likely arises from saturation of off-resonant signal components within the broadened line profile associated with short T2* times. Similarly, the sSAT pulses might also impact the observed T2*/R2* values due to magnetization-transfer effects through saturation of signal from protons bound to the broad macro-molecular pool (37,55). However, no R2* differences were found in phantoms for mGRE acquisitions and UTE acquisitions employing CHESS and sSAT pulses (Fig. 3). Only minor R2* differences were seen at 1.5 T for the BH-mGRE and the FB-imUTE sequence for our subjects with massive HIC (Figs. 5 and 6), which should be validated in a larger patient cohort.
Our in vivo R2* results suggest that a multi-echo acquisition that only contains a single UTE image at the beginning of the echo train might not be sufficient to substantially improve R2* assessment over established mGRE protocols in massive iron overload: at 1.5 T, the BH-mUTE sequence, which collects all data within a single breath hold but only acquires a single UTE image at TE1 = 0.1 ms, did not yield a smaller SD of hepatic R2* values in comparison with a biopsy-calibrated BH-mGRE protocol. Contrary to the BH-mUTE sequence, the FB-imUTE sequence – although acquiring the data in free breathing – records multiple images with short TEs (TE1 < 1 ms) via ΔTE-shifted, interleaved echo trains which provides dense temporal sampling of the fast decays. This greatly improves the R2* quantitation via non-linear least square fitting as seen from the smaller SD of R2* values. The potential of the FB-imUTE sequence for R2* quantification in massive iron overload is shown in the in vivo measurements at 3 T: only the FB-imUTE data proved successful to reliably quantify mean hepatic R2* values of up to 3200 1/s as can be appreciated from the histogram plots of FB-imUTE T2* values exhibiting well-defined maxima for all five subjects (Fig. 8). The previously established BH-mGRE protocol and the BH-mUTE sequence (‘single’ UTE) do not yield a proper hepatic R2* assessment. Although the T2* maps derived from the BH-mUTE data appear less noisy compared to the mGRE results, the histogram plots show that the T2* values are also widely distributed with a broader peak at longer T2* times in comparison to the FB-imUTE (Fig. 8). The wide distribution of BH-mUTE-T2* values could be explained by inaccurate T2* fitting from BH-mUTE data due to a combination of limited SNR and insufficient temporal sampling of the rapid signal decay. The noisy and widely distributed BH data produce mean R2* values (cf. plot legend of Fig. 8), which do not appear as meaningful measures of mean hepatic R2* at 3 T. In contrast, the FB-imUTE sequence with its narrow T2* distributions seems to enable correct assessment of mean hepatic R2* even at 3 T. This is further supported by the fact that only the FB-imUTE data reflect an approximately two-fold R2* increase from 1.5 T to 3 T for all five cases (mean R2* ratio = 1.8; please refer to Supporting Table S1 for details) which is in excellent agreement with previous in vivo studies (16,17) as well as theoretical predictions (18).
Because of a potential bias between mGRE and FB-imUTE T2*/R2* measurements (due to the employed sSAT and CHESS pulses), R2* values obtained with FB-imUTE might not be directly used with existing R2*-HIC calibrations which have been exclusively based on mGRE acquisitions (12–15). A systematic comparison of mGRE and FB-imUTE acquisitions in a larger patient cohort could help provide a T2*/R2* correlation. However, the FB-imUTE sequence specifically aims at T2*/R2* quantitation in massive iron levels where existing R2*-HIC calibrations have shown limited precision (12–15), so that a new biopsy calibration study will be needed to independently measure reference HIC values and to establish a R2*-HIC calibration at such high HIC levels.
Besides R2*-based methods for HIC assessment, other approaches, e.g. via liver-to-muscle signal intensity ratio (56) or T2/R2 quantitation (57) have been investigated. Liver-to-muscle techniques are based on GRE acquisitions as well (56) so that their precision is also intrinsically limited in massive iron overload or at higher field strengths. A commercially available R2-based method (FerriScan®) offers HIC assessment up to about 43 mg/g dry wt at 1.5 T (57), but might also have limited precision for massive HIC levels because the associated T2 times will be too short to be reliably detected with conventional spin-echo sequences. A T2-based calibration for 3 T does not exist. The overall lack of a precise MR-based method for HIC estimation in massive iron overload or at 3 T might support the need for a new biopsy calibration study in such patients.
The FB-imUTE sequence presented here is currently being tested in a larger, on-going clinical trial to systematically compare and evaluate GRE and UTE sequences for R2*-based assessment of massive HIC at 1.5 T and 3 T (www.clinicaltrials.gov #NCT01572922). In the study on the general feasibility of 2D UTE imaging in the liver by Chappell et al. (31), the authors also applied fat suppression but did not use additional echo interleaves. The authors did not comment on either implemented mechanisms to counteract slice selection distortions due to eddy currents or other confounding factors in UTE-based T2* measurements of the liver. As mentioned in the Introduction, Chappell et al. reported a mean hepatic T2* value of 7 ms for their hemochromatosis patients which is substantially longer than the T2* times of < 1ms seen in our massive HIC cases. Therefore, some of the pitfalls of UTE-based T2* quantitation were probably not as apparent in their analysis as in our study which aims at providing a robust 2D UTE technique to quantify T2*/R2* in massive iron overload at both clinically relevant field strengths. We present the necessary technical developments which were required to enable quantitative 2D UTE imaging that is not confounded by artifacts from out-of-slice signal contributions or streaking. Proof-of-concept of the clinical applicability of the FB-imUTE sequence was demonstrated in five subjects with massively high HIC levels.
The current acquisition time of the FB-imUTE sequence of about 1:40 min is longer compared to BH techniques. However, the sequence was well tolerated during in vivo testing as it acquires data in regular free breathing and does not require any breath holding or motion correction concepts (58). The radial sampling pattern makes the FB-imUTE images very robust against respiratory motion. This can be appreciated from Fig. 5 which compares images acquired at the first echo time of TE1 = 0.1 ms during breath holding with the BH-mGRE sequence (f–j) and free breathing with the FB-imUTE sequence (k–o). Barely any additional artifacts are introduced due to breathing motion. This is in concordance with the pilot work by Chappell et al. (31), in which the authors also report robustness of 2D UTE imaging against abdominal motion even over the specified acquisition time of about 8.5 min. The motion-insensitivity of radial sampling has been exploited lately in motion-robust stack-of-stars implementations (59–61) as a powerful alternative to BH acquisitions in patients with impaired BH capabilities. The FB-imUTE capitalizes on the motion-insensitivity of radial sampling to collect interleaved echo trains and densely sample the fast signal decay, and thus, to improve T2* quantitation without suffering from degraded image quality due to motion artifacts. Dense temporal sampling could also be achieved by multiple BH-mUTE acquisitions with ΔTE-shifted echo trains in sequential BH maneuvers. However, T2*/R2* quantification may be hindered in such an approach by inconsistent BH locations. Continuous free breathing data acquisition cannot be applied to conventional Cartesian mGRE imaging without motion compensation strategies (58), e.g. navigator-based concepts, as it would inevitably lead to motion artifacts (62,63) so that hepatic R2* mapping would be severely hampered.
In the current implementation, image reconstruction was done with Kaiser-Bessel re-gridding techniques. Improved reconstruction strategies such as iterative approaches (64) or compressed sensing (65) might be exploited to achieve a reduction of the total acquisition time via radial data undersampling. As shown in the Supporting Information, temporal delays between readout gradients and ADC, due to hardware imperfections, have to be accounted for to ensure optimal image quality in radial acquisitions. In our case, a simple, manual approach based on phantom pre-scans was sufficient to minimize associated image artifacts (please refer to Supporting Figures S1 and S2 for details). The pre-scan was only required once per magnet and the resulting correction times could be maintained during all imaging studies (phantom and in vivo). Although this method necessitates another pre-scan for delay adjustment as soon as the sequence is used on different systems, it avoids potentially more complex strategies for delay correction via reconstruction or pre-distortion of gradient waveforms (44,66–68).
Currently, the FB-imUTE sequence collects all echoes as half echoes which ensures identical readout conditions for all echoes. Theoretically, fully sampled echoes could be collected for the 2nd and higher echoes of each echo train which would yield higher SNR. We used half spoke sampling in this initial implementation as half spoke sampling requires only half the time of fully sampled spokes which allows for shorter echo spacing. Furthermore, full echo sampling might also require modifications in the T2* fitting procedure to account for differences in the noise level of half and full echoes. A reduction of the echo spacing within one interleave (currently 1.8 ms) could also be achieved by using a smaller readout matrix size (e.g. 64). This would, however, lead to a lower spatial resolution. Although larger voxel sizes would also yield higher SNR, the spatial resolution still has to be high enough to clearly distinguish e.g. blood vessels from liver parenchyma which is important to avoid contamination of the hepatic T2* assessment due to partial volume effects. Nevertheless, both concepts – a full echo acquisition for 2nd and higher echoes and a reduced readout matrix size – could be investigated to optimize SNR and scan time in the future.
A potential limitation of the FB-imUTE sequence is that the half pulse excitation together with the application of sSAT bands limits its multi-slice capability. The sequence only provides data from a single plane and does not cover the whole liver. Multiple slices could be measured via sequential acquisitions or in an interleaved fashion if the slices are separated by the width of the sSAT bands. Both strategies may not be advantageous, though, from a total scan time perspective. However, existing R2*-HIC calibrations have been established for single slice mGRE acquisitions through the center of the liver (12–15) so that a single plane seems to be sufficient for HIC assessment.
In summary, the lack of precision of R2*-based HIC assessment with mGRE methods poses shortcomings in the clinical management of patients with massive iron overload. In previous studies, about 40% of patients with iron overload showed HIC levels of 15 mg Fe/g dry wt or more (13,14). Current mGRE techniques are intrinsically limited when detecting signals from tissues with short T2* times on the scale of 1 ms or less which corresponds to HIC levels above approximately 25 mg Fe/g dry wt at 1.5 T and 12.5 mg Fe/g dry wt at 3 T. We conclude that the proposed UTE sequence offers the opportunity to provide a means of precisely measuring T2*/R2* in iron overloaded patients over the entire clinically relevant HIC range at 1.5 T and 3 T (including massive iron overload settings). This is achieved by incorporating sequence modules which minimize out-of-slice signal contribution and streaking artifacts and which can be easily implemented on any scanner. Before clinical adoption of these methods, it will be necessary to perform a thorough biopsy-referenced trial and such a systematic clinical study is currently underway.
Supplementary Material
Supporting Figure S1 Manual correction of delay parameters between readout gradient and ADC due to system imperfections. (a) UTE image after delay adjustment. (b) Delay parameter too short – artifacts emerge as dark rim (red arrow). (c) Delay parameter too long – artifacts emerge as bright rim (green arrow). For illustration purposes, the delays in (b) and (c) were deliberately adjusted to yield a symmetric artifact shape. (d) Intensity profiles along the horizontal axis (x-axis) through the center of phantom (indicated by blue (a), red (b), and green (c) lines). After correction for temporal delays, a uniform intensity profile is seen (blue).
Supporting Figure S2 Iterative, manual adjustment of delay parameters in a series of axial phantom pre-scans. The delay parameters can be adjusted independently for each physical gradient direction (here x- and y-gradient direction). (a) Adjustment delay parameter for x-gradient direction. (b) Adjustment of y-gradient. The UTE image in the middle of each row represents the image with correctly adjusted delay parameters.
Supporting Table S1 Summary of mean hepatic R2* values measured at 1.5 T and 3 T with the BH-mGRE, BH-mUTE, and FB-imUTE sequences for the five subjects with massive hepatic iron content together with the respective R2* ratios. Mean R2* values are given with the physical unit [1/s] together with their standard deviation (mean ± standard deviation).
Acknowledgments
Funding: This study was supported by NIH grant 5 R01 DK088988 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and ALSAC – the fund-raising organization of St. Jude Children’s Research Hospital.
The authors would like to acknowledge Gail Fortner, RN, Karen Wodowski, PNP, and Darla Pickett, PA-C, MHS, for patient recruitment and enrollment, Kamisha Flowers, BS, for managing regulatory aspects of the study, and Robert Gold, MD, and Kimberly Proctor, PNP, for performing liver biopsies (all from St. Jude Children’s Research Hospital, Memphis, TN). The authors are indebted to Raymond Osarogiagbon, MD, and Laura M. McHugh, RN (both from Baptist Memorial Hospital, Memphis, TN), and Patricia Adams-Graves, MD, Vanessa Steele, MSW, and Angela Hudson, FNP (all three from Regional One Health, Memphis, TN), for support with patient recruitment. The authors would also like to thank Dr. Mark Bydder (University of California, San Diego, CA) for support with implementation of image reconstruction.
Footnotes
Parts of this study were presented as an oral contribution (abstract number: 87) at the 23rd Annual Meeting of the ISMRM in Toronto, Ontario, Canada, 2015.
References
- 1.Taher AT, Musallam KM, Inati A. Iron overload: consequences, assessment, and monitoring. Hemoglobin. 2009;33(Suppl 1):S46–57. doi: 10.3109/03630260903346676. [DOI] [PubMed] [Google Scholar]
- 2.Kohgo Y, Ikuta K, Ohtake T, Torimoto Y, Kato J. Body iron metabolism and pathophysiology of iron overload. Int J Hematol. 2008;88:7–15. doi: 10.1007/s12185-008-0120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olynyk JK, St Pierre TG, Britton RS, Brunt EM, Bacon BR. Duration of hepatic iron exposure increases the risk of significant fibrosis in hereditary hemochromatosis: a new role for magnetic resonance imaging. Am J Gastroenterol. 2005;100:837–41. doi: 10.1111/j.1572-0241.2005.41287.x. [DOI] [PubMed] [Google Scholar]
- 4.Harmatz P, Butensky E, Quirolo K, Williams R, Ferrell L, Moyer T, Golden D, Neumayr L, Vichinsky E. Severity of iron overload in patients with sickle cell disease receiving chronic red blood cell transfusion therapy. Blood. 2000;96:76–9. [PubMed] [Google Scholar]
- 5.Angelucci E, Brittenham GM, McLaren CE, Ripalti M, Baronciani D, Giardini C, Galimberti M, Polchi P, Lucarelli G. Hepatic iron concentration and total body iron stores in thalassemia major. N Engl J Med. 2000;343:327–31. doi: 10.1056/NEJM200008033430503. [DOI] [PubMed] [Google Scholar]
- 6.Olivieri NF. Progression of iron overload in sickle cell disease. Semin Hematol. 2001;38:57–62. doi: 10.1016/s0037-1963(01)90060-5. [DOI] [PubMed] [Google Scholar]
- 7.Prati D, Maggioni M, Milani S, et al. Clinical and histological characterization of liver disease in patients with transfusion-dependent beta-thalassemia. A multicenter study of 117 cases. Haematologica. 2004;89:1179–86. [PubMed] [Google Scholar]
- 8.Brittenham GM, Griffith PM, Nienhuis AW, McLaren CE, Young NS, Tucker EE, Allen CJ, Farrell DE, Harris JW. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. N Engl J Med. 1994;331:567–73. doi: 10.1056/NEJM199409013310902. [DOI] [PubMed] [Google Scholar]
- 9.Ladis V, Chouliaras G, Berdousi H, Kanavakis E, Kattamis C. Longitudinal study of survival and causes of death in patients with thalassemia major in Greece. Ann N Acad Sci. 2005;1054:445–50. doi: 10.1196/annals.1345.067. [DOI] [PubMed] [Google Scholar]
- 10.Sirlin CB, Reeder SB. Magnetic resonance imaging quantification of liver iron. Magn Reson Imaging Clin N Am. 2010;18:359–81. ix. doi: 10.1016/j.mric.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angelucci E, Baronciani D, Lucarelli G, et al. Needle liver biopsy in thalassaemia: analyses of diagnostic accuracy and safety in 1184 consecutive biopsies. Br J Haematol. 1995;89:757–61. doi: 10.1111/j.1365-2141.1995.tb08412.x. [DOI] [PubMed] [Google Scholar]
- 12.Anderson LJ, Holden S, Davis B, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171–9. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- 13.Wood JC, Enriquez C, Ghugre N, Tyzka JM, Carson S, Nelson MD, Coates TD. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood. 2005;106:1460–5. doi: 10.1182/blood-2004-10-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hankins JS, McCarville MB, Loeffler RB, Smeltzer MP, Onciu M, Hoffer FA, Li CS, Wang WC, Ware RE, Hillenbrand CM. R2* magnetic resonance imaging of the liver in patients with iron overload. Blood. 2009;113:4853–5. doi: 10.1182/blood-2008-12-191643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henninger B, Zoller H, Rauch S, Finkenstedt A, Schocke M, Jaschke W, Kremser C. R2* Relaxometry for the Quantification of Hepatic Iron Overload: Biopsy-Based Calibration and Comparison with the Literature. Rofo. 2015;187:472–479. doi: 10.1055/s-0034-1399318. [DOI] [PubMed] [Google Scholar]
- 16.Storey P, Thompson AA, Carqueville CL, Wood JC, de Freitas RA, Rigsby CK. R2* imaging of transfusional iron burden at 3T and comparison with 1.5T. J Magn Reson Imaging. 2007;25:540–7. doi: 10.1002/jmri.20816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hillenbrand CM, Loeffler RB, McCarville MB, et al. Evaluation of Hepatic Iron Concentrations by R2* Magnetic Resonance Imaging in 35 Patients with Iron Overload: Comparison of R2* Measurements at 1.5T and 3T and Validation with Liver Biopsies. Proc. 16th Annual Meeting ISMRM; Toronto, Ontario, Canada. 2008; Abstract 90. [Google Scholar]
- 18.Ghugre NR, Doyle EK, Storey P, Wood JC. Relaxivity-iron calibration in hepatic iron overload: Predictions of a Monte Carlo model. Magn Reson Med. 2015;74:879–83. doi: 10.1002/mrm.25459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robson MD, Gatehouse PD, Bydder M, Bydder GM. Magnetic resonance: an introduction to ultrashort TE (UTE) imaging. J Comput Assist Tomogr. 2003;27:825–46. doi: 10.1097/00004728-200311000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Bergin CJ, Pauly JM, Macovski A. Lung parenchyma: projection reconstruction MR imaging. Radiology. 1991;179:777–81. doi: 10.1148/radiology.179.3.2027991. [DOI] [PubMed] [Google Scholar]
- 21.Ohno Y, Koyama H, Yoshikawa T, Matsumoto K, Takahashi M, Van Cauteren M, Sugimura K. T2* Measurements of 3-T MRI With Ultrashort TEs: Capabilities of Pulmonary Function Assessment and Clinical Stage Classification in Smokers. Am J Roentgenol. 2011;197:W279–W285. doi: 10.2214/AJR.10.5350. [DOI] [PubMed] [Google Scholar]
- 22.Robson MD, Bydder GM. Clinical ultrashort echo time imaging of bone and other connective tissues. NMR Biomed. 2006;19:765–80. doi: 10.1002/nbm.1100. [DOI] [PubMed] [Google Scholar]
- 23.Serai SD, Laor T, Dwek JR, Zbojniewicz AM, Carl M. Feasibility of ultrashort TE (UTE) imaging of children at 1.5 T. Pediatr Radiol. 2014;44:103–8. doi: 10.1007/s00247-013-2758-2. [DOI] [PubMed] [Google Scholar]
- 24.Du J, Carl M, Bydder M, Takahashi A, Chung CB, Bydder GM. Qualitative and quantitative ultrashort echo time (UTE) imaging of cortical bone. J Magn Reson. 2010;207:304–11. doi: 10.1016/j.jmr.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 25.Biswas R, Bae W, Diaz E, Masuda K, Chung CB, Bydder GM, Du J. Ultrashort echo time (UTE) imaging with bi-component analysis: bound and free water evaluation of bovine cortical bone subject to sequential drying. Bone. 2012;50:749–55. doi: 10.1016/j.bone.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du J, Bydder GM. Qualitative and quantitative ultrashort-TE MRI of cortical bone. NMR Biomed. 2013;26:489–506. doi: 10.1002/nbm.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du J, Sheth V, He Q, Carl M, Chen J, Corey-Bloom J, Bydder GM. Measurement of T1 of the Ultrashort T2* Components in White Matter of the Brain at 3T. PLOS ONE. 2014;9:e103296. doi: 10.1371/journal.pone.0103296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du J, Ma G, Li S, Carl M, Szeverenyi NM, VandenBerg S, Corey-Bloom J, Bydder GM. Ultrashort Echo Time (UTE) Magnetic Resonance Imaging of the Short T2 Components in White Matter of the Brain Using a Clinical 3T Scanner. Neuro Image. 2014;87:32–41. doi: 10.1016/j.neuroimage.2013.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wansapura JP, Daniel BL, Pauly J, Butts K. Temperature mapping of frozen tissue using eddy current compensated half excitation RF pulses. Magn Reson Med. 2001;46:985–992. doi: 10.1002/mrm.1285. [DOI] [PubMed] [Google Scholar]
- 30.Lu A, Daniel BL, Pauly JM, Butts Pauly K. Improved slice selection for R2* mapping during cryoablation with eddy current compensation. J Magn Reson Imaging. 2008;28:190–198. doi: 10.1002/jmri.21396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chappell KE, Patel N, Gatehouse PD, Main J, Puri BK, Taylor-Robinson SD, Bydder GM. Magnetic resonance imaging of the liver with ultrashort TE (UTE) pulse sequences. J Magn Reson Imaging. 2003;18:709–13. doi: 10.1002/jmri.10423. [DOI] [PubMed] [Google Scholar]
- 32.Pauly JM, Conolly S, Nishimura D, Macovski A. Slice-selective excitation for very short T2 species. Proc. 8th Annu. Meet. SMRM Amst. Neth; 1989; p. 28. [Google Scholar]
- 33.Pauly JM. eMagRes. John Wiley & Sons, Ltd; 2012. Selective Excitation for Ultrashort Echo Time Imaging. [DOI] [Google Scholar]
- 34.Tyler DJ, Robson MD, Henkelman RM, Young IR, Bydder GM. Magnetic resonance imaging with ultrashort TE (UTE) PULSE sequences: technical considerations. J Magn Reson Imaging. 2007;25:279–89. doi: 10.1002/jmri.20851. [DOI] [PubMed] [Google Scholar]
- 35.Josan S, Pauly JM, Daniel BL, Pauly KB. Double half RF pulses for reduced sensitivity to eddy currents in UTE imaging. Magn Reson Med. 2009;61:1083–9. doi: 10.1002/mrm.21879. [DOI] [PubMed] [Google Scholar]
- 36.Josan S, Kaye E, Pauly JM, Daniel BL, Pauly KB. Improved half RF slice selectivity in the presence of eddy currents with out-of-slice saturation. Magn Reson Med. 2009;61:1090–5. doi: 10.1002/mrm.21914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harkins KD, Horch RA, Does MD. Simple and robust saturation-based slice selection for ultrashort echo time MRI. Magn Reson Med. 2015;73:2204–11. doi: 10.1002/mrm.25361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hargreaves BA, Cunningham CH, Nishimura DG, Conolly SM. Variable-rate selective excitation for rapid MRI sequences. Magn Reson Med. 2004;52:590–7. doi: 10.1002/mrm.20168. [DOI] [PubMed] [Google Scholar]
- 39.Block KT, Chandarana H, Milla S, et al. Towards Routine Clinical Use of Radial Stack-of-Stars 3D Gradient-Echo Sequences for Reducing Motion Sensitivity. J Korean Soc Magn Reson Med. 6(18):87–106. [Google Scholar]
- 40.Haase A, Frahm J, Hanicke W, Matthaei D. 1H NMR chemical shift selective (CHESS) imaging. Phys Med Biol. 1985;30:341–4. doi: 10.1088/0031-9155/30/4/008. [DOI] [PubMed] [Google Scholar]
- 41.Glover GH, Pauly JM. Projection reconstruction techniques for reduction of motion effects in MRI. Magn Reson Med. 1992;28:275–89. doi: 10.1002/mrm.1910280209. [DOI] [PubMed] [Google Scholar]
- 42.Jackson JI, Meyer CH, Nishimura DG, Macovski A. Selection of a convolution function for Fourier inversion using gridding [computerised tomography application] IEEE Trans Med Imaging. 1991;10:473–8. doi: 10.1109/42.97598. [DOI] [PubMed] [Google Scholar]
- 43.Pipe JG, Menon P. Sampling density compensation in MRI: rationale and an iterative numerical solution. Magn Reson Med. 1999;41:179–86. doi: 10.1002/(sici)1522-2594(199901)41:1<179::aid-mrm25>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 44.Peters DC, Derbyshire JA, McVeigh ER. Centering the projection reconstruction trajectory: reducing gradient delay errors. Magn Reson Med. 2003;50:1–6. doi: 10.1002/mrm.10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng Y, He T, Gatehouse PD, Li X, Harith Alam M, Pennell DJ, Chen W, Firmin DN. Improved MRI R2 * relaxometry of iron-loaded liver with noise correction. Magn Reson Med. 2013;70:1765–74. doi: 10.1002/mrm.24607. [DOI] [PubMed] [Google Scholar]
- 46.McCarville MB, Hillenbrand CM, Loeffler RB, Smeltzer MP, Song R, Li CS, Hankins JS. Comparison of whole liver and small region-of-interest measurements of MRI liver R2* in children with iron overload. Pediatr Radiol. 2010;40:1360–7. doi: 10.1007/s00247-010-1596-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scheffler K, Hennig J. Reduced circular field-of-view imaging. Magn Reson Med. 1998;40:474–80. doi: 10.1002/mrm.1910400319. [DOI] [PubMed] [Google Scholar]
- 48.Block KT, Fenchel M. Simple Method for Attenuation of Streaking Artifacts from Peripheral Intensity Accumulation. Proc. 20th Annual Meeting ISMRM; Melbourne, Australia. 2012; Abstract 2444. [Google Scholar]
- 49.Xue Y, Yu J, Kang HS, Englander S, Rosen MA, Song HK. Automatic coil selection for streak artifact reduction in radial MRI. Magn Reson Med. 2012;67:470–6. doi: 10.1002/mrm.23023. [DOI] [PubMed] [Google Scholar]
- 50.Kholmovski EG, Parker DL, Di Bella EV. Streak Artifact Suppression in Multi-coil MRI with Radial Sampling. Proc. 15th Annuaul Meeting ISMRM; Berlin, Germany. 2007; Abstract 1902. [Google Scholar]
- 51.Bley TA, Wieben O, Francois CJ, Brittain JH, Reeder SB. Fat and water magnetic resonance imaging. J Magn Reson Imaging. 2010;31:4–18. doi: 10.1002/jmri.21895. [DOI] [PubMed] [Google Scholar]
- 52.Hu HH, Bornert P, Hernando D, Kellman P, Ma J, Reeder S, Sirlin C. ISMRM workshop on fat-water separation: insights, applications and progress in MRI. Magn Reson Med. 2012;68:378–88. doi: 10.1002/mrm.24369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meloni A, Tyszka JM, Pepe A, Wood JC. Effect of inversion recovery fat suppression on hepatic R2* quantitation in transfusional siderosis. AJR Am J Roentgenol. 2015;204:625–9. doi: 10.2214/AJR.14.12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krafft AJ, Loeffler RB, Song R, Bian X, McCarville MB, Hankins JS, Hillenbrand CM. Does fat suppression via chemically selective saturation affect R2*-MRI for transfusional iron overload assessment? A clinical evaluation at 1.5T and 3T. Magn Reson Med. 2016;76:591–601. doi: 10.1002/mrm.25868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Henkelman RM, Stanisz GJ, Graham SJ. Magnetization transfer in MRI: a review. NMR Biomed. 2001;14:57–64. doi: 10.1002/nbm.683. [DOI] [PubMed] [Google Scholar]
- 56.Gandon Y, Olivie D, Guyader D, Aube C, Oberti F, Sebille V, Deugnier Y. Non-invasive assessment of hepatic iron stores by MRI. Lancet. 2004;363:357–62. doi: 10.1016/S0140-6736(04)15436-6. [DOI] [PubMed] [Google Scholar]
- 57.St Pierre TG, Clark PR, Chua-anusorn W, Fleming AJ, Jeffrey GP, Olynyk JK, Pootrakul P, Robins E, Lindeman R. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood. 2005;105:855–61. doi: 10.1182/blood-2004-01-0177. [DOI] [PubMed] [Google Scholar]
- 58.Zaitsev M, Maclaren J, Herbst M. Motion artifacts in MRI: A complex problem with many partial solutions. J Magn Reson Imaging. 2015;42:887–901. doi: 10.1002/jmri.24850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Azevedo RM, de Campos RO, Ramalho M, Heredia V, Dale BM, Semelka RC. Free-breathing 3D T1-weighted gradient-echo sequence with radial data sampling in abdominal MRI: preliminary observations. AJR Am J Roentgenol. 2011;197:650–7. doi: 10.2214/AJR.10.5881. [DOI] [PubMed] [Google Scholar]
- 60.Chandarana H, Block TK, Rosenkrantz AB, Lim RP, Kim D, Mossa DJ, Babb JS, Kiefer B, Lee VS. Free-breathing radial 3D fat-suppressed T1-weighted gradient echo sequence: a viable alternative for contrast-enhanced liver imaging in patients unable to suspend respiration. Invest Radiol. 2011;46:648–53. doi: 10.1097/RLI.0b013e31821eea45. [DOI] [PubMed] [Google Scholar]
- 61.Chandarana H, Block KT, Winfeld MJ, Lala SV, Mazori D, Giuffrida E, Babb JS, Milla SS. Free-breathing contrast-enhanced T1-weighted gradient-echo imaging with radial k-space sampling for paediatric abdominopelvic MRI. Eur Radiol. 2014;24:320–6. doi: 10.1007/s00330-013-3026-4. [DOI] [PubMed] [Google Scholar]
- 62.Axel L, Summers RM, Kressel HY, Charles C. Respiratory effects in two-dimensional Fourier transform MR imaging. Radiology. 1986;160:795–801. doi: 10.1148/radiology.160.3.3737920. [DOI] [PubMed] [Google Scholar]
- 63.Wood ML, Henkelman RM. MR image artifacts from periodic motion. Med Phys. 1985;12:143–51. doi: 10.1118/1.595782. [DOI] [PubMed] [Google Scholar]
- 64.Block KT, Uecker M, Frahm J. Undersampled radial MRI with multiple coils. Iterative image reconstruction using a total variation constraint. Magn Reson Med. 2007;57:1086–98. doi: 10.1002/mrm.21236. [DOI] [PubMed] [Google Scholar]
- 65.Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007;58:1182–95. doi: 10.1002/mrm.21391. [DOI] [PubMed] [Google Scholar]
- 66.Moussavi A, Untenberger M, Uecker M, Frahm J. Correction of gradient-induced phase errors in radial MRI. Magn Reson Med. 2014;71:308–12. doi: 10.1002/mrm.24643. [DOI] [PubMed] [Google Scholar]
- 67.Kramer M, Biermann J, Reichenbach JR. Intrinsic correction of system delays for radial magnetic resonance imaging. Magn Reson Imaging. 2015;33:491–6. doi: 10.1016/j.mri.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 68.Harkins KD, Does MD, Grissom WA. Iterative method for predistortion of MRI gradient waveforms. IEEE Trans Med Imaging. 2014;33:1641–7. doi: 10.1109/TMI.2014.2320987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Figure S1 Manual correction of delay parameters between readout gradient and ADC due to system imperfections. (a) UTE image after delay adjustment. (b) Delay parameter too short – artifacts emerge as dark rim (red arrow). (c) Delay parameter too long – artifacts emerge as bright rim (green arrow). For illustration purposes, the delays in (b) and (c) were deliberately adjusted to yield a symmetric artifact shape. (d) Intensity profiles along the horizontal axis (x-axis) through the center of phantom (indicated by blue (a), red (b), and green (c) lines). After correction for temporal delays, a uniform intensity profile is seen (blue).
Supporting Figure S2 Iterative, manual adjustment of delay parameters in a series of axial phantom pre-scans. The delay parameters can be adjusted independently for each physical gradient direction (here x- and y-gradient direction). (a) Adjustment delay parameter for x-gradient direction. (b) Adjustment of y-gradient. The UTE image in the middle of each row represents the image with correctly adjusted delay parameters.
Supporting Table S1 Summary of mean hepatic R2* values measured at 1.5 T and 3 T with the BH-mGRE, BH-mUTE, and FB-imUTE sequences for the five subjects with massive hepatic iron content together with the respective R2* ratios. Mean R2* values are given with the physical unit [1/s] together with their standard deviation (mean ± standard deviation).