Abstract
Purpose
To develop a novel surgical approach to provide consistent delivery of cell suspension into the subretinal space without cell leakage into the vitreous.
Methods
Cell viability was assessed following mock injections to determine the optimal size cannula for delivery of the cells. A pars plana without vitrectomy approach was used to create a subretinal bleb with balanced salt solution using a 41ga cannula. GFP-labeled RPE cells were injected through transretinal (n=8) and transscleral (n=16) injection approaches. OCT, fundus photography and autofluorescence, and histological analysis were used to evaluate surgical success.
Results
The 30ga cannula yielded the highest recovery of cells with highest viability. The transretinal approach consistently resulted in transplanted cells in the vitreous, with some cells coming to rest on the inner limiting membrane. Conversely, the transscleral approach resulted in transplantation of cells into the subretinal space in 100% of cases. Histological analysis confirmed these results.
Conclusions
We have developed a novel surgical approach that resulted in encapsulation of transplanted cells into the subretinal space with a 100% success rate. This approach will provide a useful tool for further cell transplantation study and may provide an approach for clinical application of delivering cells to the subretinal space.
Keywords: Cell transplantation, Detachment, Subretinal injection, Surgical technique
Introduction
Cell based therapies are of great interest for the treatment of macular degeneration and other retinal diseases that result in loss of the RPE or retinal cell layers. There are currently several human clinical trials underway to evaluate the safety and efficacy of cell based therapy1,2. Many of these trials have as a common element the delivery of suspensions of cells to the subretinal space through a cannula. The surgical approach to delivering these cells could be a substantial factor in the success of the procedure and the type of complications that might occur as a result of surgery. For instance, there is likely to be an optimal concentration or absolute number of cells to deliver to the subretinal space. But passage of cells through cannulas may lead to cell death, so that the actual number of cells delivered to the subretinal space is less than expected. Additionally, if cells are injected transretinally via a vitreous approach, there is likely to be some loss of cells through the injection site3. This loss of cells into the vitreous (leakage) reduces the number of cells in the subretinal space, and could potentially lead to membrane formation on the surface of the retina. Such membrane formation might increase the possibility of retinal detachment and proliferative vitreoretinopathy.
In this study, we have evaluated the effect of different cannula sizes on the recovery and viability of cells. In addition, we have documented the leakage of cells during trans-retinal injection of the cells from a vitreous approach. Based on these evaluations we have developed a novel method for transplanting cells in the subretinal space that maximizes cell viability, minimizes loss of cells, and minimizes escape of cells into the vitreous cavity.
Methods
Determining appropriate cannula size
GFP labeled Human iPSC-derived RPE cells (courtesy of Kapil Bharti) were trypsinized and resuspended at a concentration of 10,000 cells per μL divided into 3 centrifuge tubes containing 100μL and 1 tube containing 30μL. For each 100μL aliquot, the cell suspension was drawn up into microtubing connected to a 250μL Hamilton syringe through a blunt tipped 18g needle. Once the cell suspension was drawn fully into the tubing, the 18g needle was replaced with either a 30g, 38g, or 41g subretinal injection cannula. The suspension was then pushed to the tip of the cannula. Five 10μL injections into new centrifuge tubes were performed for each cannula gauge. These 10μL injections were diluted in BSS+ and trypan blue. Cells were counted for each injection providing 5 counts per cannula. Control counts were made from the single tube containing 30μL. For these, 10μL aliquots were pipetted into 3 new centrifuge tubes and diluted in BSS+ and Trypan blue.
Surgical approaches
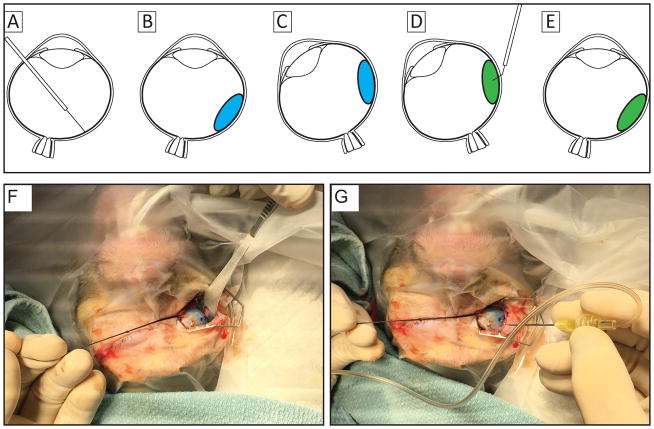
Evaluation of surgical approaches to cell delivery was carried out in Rhesus Macaques. A pars plana without vitrectomy approach was used to create a subretinal bleb with balanced salt solution using a 41 gauge cannula. Based on findings from the in vitro cell recovery and viability experiments, cell injections were performed with 30 gauge cannulas. The cells (10,000 cells/μL; 50 μL total volume) were injected into the subretinal space with two different approaches. In 8 eyes of 4 animals, cells were injected transretinally into the subretinal space (within the previously created saline bleb) using a 30 gauge cannula, while maintaining intraocular pressure with a combined light pipe infusion cannula. In 16 eyes of 8 primates, cells were delivered with a combined technique illustrated in Figure 1. In this combined technique, the location of the saline bleb was marked externally where cells were then injected through the sclera into the preformed bleb using a 30 gauge angulated sharp tip cannula (Hurricane Medical #7509, Bradenton, FL).
Figure 1.
Illustrations of the surgical approach for transscleral delivery of cell suspension resulting in high success rates that places cells within the subretinal space without leakage into the vitreous. (A) a subretinal bleb (panel B;blue; ~300 μL) is created using a 41g cannula. The location of the bleb is marked externally and the eye is rotated to ease access to location for injection (C), cells are injected into the subretinal bleb using a 30 gauge cannula (D; green). The eye is then returned to resting position (E) and examined using an indirect ophthalmoscope to ensure proper location of the delivered cells. Panel F illustrates rotation of the right eye and marking the location at which the injection cannula will be inserted, seen in panel G.
Results
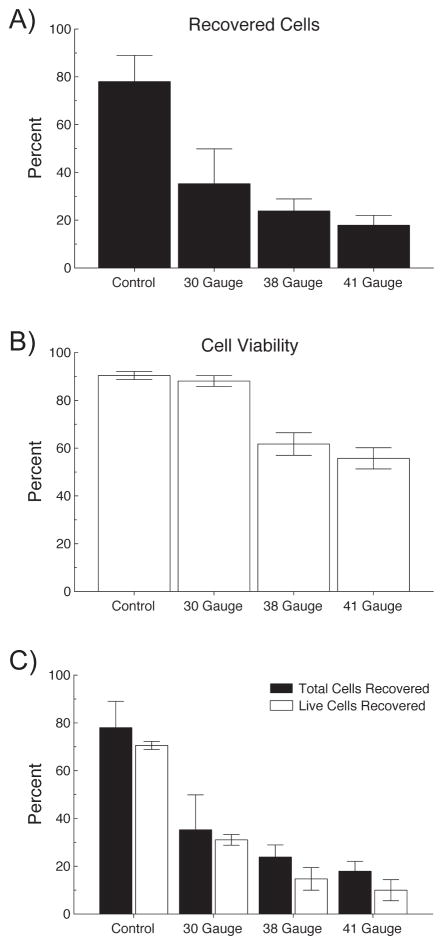
Approximately 20% of cells were lost from standard pipetting and redistributing the cells in a single cell suspension. A significant number of cells were also lost in the process of loading and injecting through the cannulas. Many of these cells were lost because they remain stuck to the inside of the hub of the injection cannula indicated by residual pigmentation within the needle hub. Figure 2a illustrates the percentage of cells recovered following injection through 30, 38 and 41 gauge cannulas. Figure 2b shows the percentage of the recovered cells that were viable for each size cannula. Figure 2c combines Figures 2a and 2b to show the overall percentage of viable cells for each gauge cannula. Using a 30g cannula, approximately 40% of the original number of cells are retained; however, with high viability (~90%). When using 38 or 41g, only 15–20% of cells are recovered and approximately 50% of them are dead or nonviable. Notably, it is clear that smaller cannula calibers result in dramatic reduction in the number of cells recovered. Additionally, of the cells that are recovered after injection through the smaller cannulas, fewer of the remaining cells are viable when compared to the injections through the larger cannula.
Figure 2.
Determination of minimal cannula size for safe passage of cells. Top panel: Percentage of recovered (both live and dead) cells after pipetting only (Control) and injecting RPE cells through 30, 38 and 41 gauge cannulas. Middle panel: Viability of the recovered cells measured as the proportion of live cells to total recovered cells. Viability remained constant between control and the 30g cannula (90%), but was reduced to ~50% in the 38 and 41g cannulas. Panel C combines panels A and B and illustrates the total cells recovered for each cannula and the cell viability within each recovered sample.
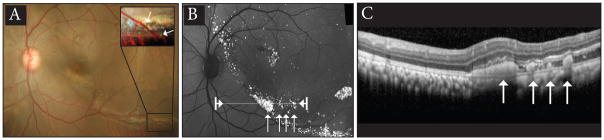
Surgical observations of the trans-retinal injections from a vitreous approach revealed cell leakage around the cannula in 100% of cases. In cases performed with the trans-scleral approach, escape of cells into the vitreous has been nearly completely eliminated. Retinal imaging at one-day post transplantation revealed 1) GFP positive cells under the retina in color fundus photographs (Fig. 3a), 2) bright GFP fluorescence of the transplanted cells under the retina (Fig. 3b), and 3) transplanted cells in the subretinal space visualized with optical coherence tomography (Fig. 3c). The color fundus and autofluorescence images also confirm the absence of transplanted cells in the vitreous.
Figure 3.
One-day post injection color fundus photograph (A), fundus autofluorescence (B), and optical coherence tomography (C) illustrating the cells are located in the subretinal space. Arrows in panel A inset indicate fluorescent cells in the subretinal space. Arrows in B indicate location of cells in panel C, also indicated by the arrows. The line in panel B between arrowheads indicates location of OCT scan in panel C.
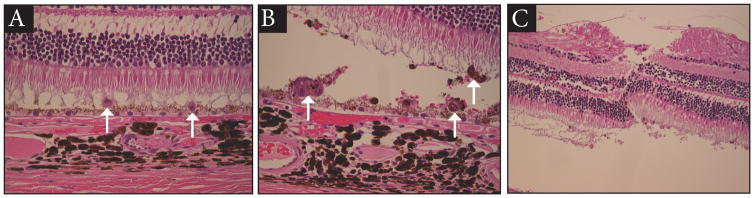
The serous retinal detachment produced with this surgical technique generally resolves within 24–48 hours. The histopathologic appearance of the cells in the subretinal space after 24 hours is shown in Figure 4. Cells are present as individual cells (Fig 4a) and as small clumps of cells (Fig 4b). The retinotomy site is shown in Figure 4c.
Figure 4.
Hematoxylin and Eosin stained retina one-day post subretinal transplantation of RPE cells. Panel A illustrates RPE cells delivered and distributed as single cells. Panel B illustrates examples where RPE cells remained clustered in small groups. Panel C illustrates the retinotomy used to generate the initial subretinal saline bleb.
Discussion
The findings in this study demonstrate that technical factors with the surgical delivery of cells to the subretinal space have major impacts on the number of viable cells that are successfully delivered to the subretinal space. Having a standard, predictable method for delivering cells will be essential to interpret the findings from studies of cell based therapies in animal experiments as well as in human trials.
Also of importance is minimizing the delivery of non-viable cells and cell debris into the subretinal space. Although only indirectly evaluated in this study, our results suggest that with use of smaller cannulas a large amount of cell debris and non-viable cells are delivered into the subretinal space with smaller cannulas. It is likely this material will elicit some form of tissue reaction. Activation of microglial cells and/or greater disruption of the blood retinal barrier are undesirable events and so the introduction of material that will need to be cleared from the subretinal space should be avoided if possible4.
Each cell type used in cell based therapy could have different responses to passage through a cannula. Properties of the cells, properties of the cannulas used for injection, the liquid media used with injection, and the velocity with which the cells are injected could all be variables in survival of cells.
The surgical approach to delivering cells is also an important variable affecting the number of cells successfully delivered to the subretinal space. Our experience is that there is substantial leakage of cells back into the vitreous when injecting through the retina from a vitreous approach. This leakage of cells not only reduces the number of cells in the desired subretinal location, but also increases the risk of membrane formation on the surface of the retina, and perhaps even retinal detachment. Preretinal cellular proliferations have been noted in patients undergoing trials introducing cells into the subretinal space1. We have found that injecting with the technique illustrated in Fig. 1 is very effective in eliminating this back leakage. Although, this approach is slightly more difficult technically, it relies only on skills that are very familiar to most vitreoretinal surgeons. A potential disadvantage of this approach is that there could be a greater exposure of the injected cells to uveal tissue. However, our preliminary studies with this technique have not provided any evidence of intra-uveal injections.
In conclusion, the size of the cannula for injection of cells in the subretinal space needs to be optimized to allow for a standard dose of viable cells to be delivered, and to avoid delivering cell debris or non-viable cells into the subretinal space. We have found that larger cannulas are necessary for this purpose. However, use of large cannulas from a vitreous approach leads to a large amount of cell leakage into the vitreous cavity. A trans-scleral approach permits use of a larger cannula while still delivering the cells into the subretinal space.
Supplementary Material
Supplemental Digital Content 1: Wilson Surgical Technique Video.mp4
Summary Statement.
We developed a novel transscleral surgical approach for delivery of cell suspension into the subretinal space of non-human primates that was successful in all cases. Use of this approach may be appropriate for clinical application of cell-based therapy.
Acknowledgments
Funding: The project was supported by NIH grants R01 EY021214 (MN), Unrestricted departmental funding from Research to Prevent Blindness (New York, NY), departmental core grants P30 EY010572 and P51 OD011092 from the National Institutes of Health (Bethesda, MD), Sybil B. Harrington Special Scholar Award from Research to Prevent Blindness (TJM).
Footnotes
Disclosures: The authors have no financial/conflicting interests to disclose.
References
- 1.Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman JP, Davis JL, Heilwell G, Spirn M, Maguire J, Gay R, Bateman J, Ostrick RM, Morris D, Vincent M, Anglade E, Del Priore LV, Lanza R. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015 Feb 7;385(9967):509–16. doi: 10.1016/S0140-6736(14)61376-3. Epub 2014 Oct 15. [DOI] [PubMed] [Google Scholar]
- 2.StemCells, Inc. Study of Human Central Nervous System Stem Cells (HuCNS-SC) in Age-Related Macular Degeneration (AMD) www.clinicaltrials.gov.
- 3.Kundu J, Michaelson A, Baranov P, Young MJ, Carrier RL. Approaches to cell delivery: substrates and scaffolds for cell therapy. Dev Ophthalmol. 2014;53:143–54. doi: 10.1159/000357369. [DOI] [PubMed] [Google Scholar]
- 4.Xian B1, Huang B2. The immune response of stem cells in subretinal transplantation. Stem Cell Res Ther. 2015 Sep 14;6:161. doi: 10.1186/s13287-015-0167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1: Wilson Surgical Technique Video.mp4