Neuropeptide-Y (NPY) has been shown to act on mesolimbic dopamine circuits to increase motivated behaviors toward food, but it is unclear exactly how NPY causes these responses. Here, we demonstrate that NPY directly inhibited a subset of ventral tegmental area (VTA) dopamine neurons through the activation of G protein-coupled inwardly rectifying potassium currents, and it inhibited both excitatory postsynaptic currents and inhibitory postsynaptic currents onto subsets of dopamine neurons through a presynaptic mechanism. Thus NPY uses multiple mechanisms to dynamically control VTA dopamine neuron activity.
Keywords: NPY, dopamine, VTA, GIRK
Abstract
The mesocorticolimbic dopamine system, the brain’s reward system, regulates many different behaviors including food intake, food reward, and feeding-related behaviors, and there is increasing evidence that hypothalamic feeding-related neuropeptides alter dopamine neuron activity to affect feeding. For example, neuropeptide-Y (NPY), a strong orexigenic hypothalamic neuropeptide, increases motivation for food when injected into the ventral tegmental area (VTA). How NPY affects the activity of VTA dopamine neurons to regulate feeding behavior is unknown, however. In these studies we have used whole cell patch-clamp electrophysiology in acute brain slices from mice to examine how NPY affects VTA dopamine neuron activity. NPY activated an outward current that exhibited characteristics of a G protein-coupled inwardly rectifying potassium channel current in ~60% of dopamine neurons tested. In addition to its direct effects on VTA dopamine neurons, NPY also decreased the amplitude and increased paired-pulse ratios of evoked excitatory postsynaptic currents in a subset of dopamine neurons, suggesting that NPY decreases glutamatergic transmission through a presynaptic mechanism. Interestingly, NPY also strongly inhibited evoked inhibitory postsynaptic currents onto dopamine neurons by a presynaptic mechanism. Overall these studies demonstrate that NPY utilizes multiple mechanisms to affect VTA dopamine neuron activity, and they provide an important advancement in our understanding of how NPY acts in the VTA to control feeding behavior.
NEW & NOTEWORTHY Neuropeptide-Y (NPY) has been shown to act on mesolimbic dopamine circuits to increase motivated behaviors toward food, but it is unclear exactly how NPY causes these responses. Here, we demonstrate that NPY directly inhibited a subset of ventral tegmental area (VTA) dopamine neurons through the activation of G protein-coupled inwardly rectifying potassium currents, and it inhibited both excitatory postsynaptic currents and inhibitory postsynaptic currents onto subsets of dopamine neurons through a presynaptic mechanism. Thus NPY uses multiple mechanisms to dynamically control VTA dopamine neuron activity.
over one-third of the U.S. adult population is obese (Flegal et al. 2012; Ogden et al. 2014), putting these individuals at increased risk for numerous other deleterious conditions, including diabetes, cardiovascular disease, stroke, high blood pressure, and some forms of cancer (Kopelman 2007). As there are currently few effective treatments available to combat obesity (Kaplan 2010), it is essential to understand how the brain controls feeding and weight gain to identify new targets that can be used to develop effective treatments for obesity and weight gain.
The mesocorticolimbic dopamine system is the primary neural circuit regulating reward-related and motivational behaviors, and this system plays an important role in controlling feeding and body weight, including the appetitive and consummatory aspects of feeding (Kenny 2011; Lutter and Nestler 2009; Palmiter 2007; Rui 2013; Volkow et al. 2011; Wise 2004). For example, dopamine-deficient mice are aphagic and will starve to death by 4 wk of age if they are not treated with l-DOPA, a dopamine precursor (Zhou and Palmiter 1995). Food intake, food reward, and stimuli associated with food also cause phasic increases in dopamine release (Bassareo and Di Chiara 1999; Hernandez and Hoebel 1988a, 1988b), and blocking dopamine receptors systemically or in the nucleus accumbens decreases operant responding for food in rats (Beninger et al. 1987; Cousins et al. 1994; Koch et al. 2000). Impairments in the mesocorticolimbic dopamine system have also been associated with obesity and dysregulated feeding in humans. For example, dopamine agonists cause increased compulsive eating and weight gain in Parkinson’s patients (Nirenberg and Waters 2006), and obese individuals show increased activity in mesocorticolimbic areas in response to pictures of palatable food but decreased responses to food consumption compared with lean individuals (Dimitropoulos et al. 2012; Gautier et al. 2000; Rothemund et al. 2007; Stice et al. 2008; Stoeckel et al. 2008). However, overall, we have an incomplete understanding of how the mesocorticolimbic dopamine system regulates feeding. This includes an incomplete understanding of how other brain systems and circuits interact with dopamine circuits to regulate feeding and body weight.
Neuropeptide-Y (NPY) is a strong orexigenic neuropeptide and an important regulator of energy homeostasis (Chambers and Woods 2012; Loh et al. 2015). For example, central administration of NPY robustly increases food intake (Clark et al. 1984; Vettor et al. 1994), activation of NPY-expressing neurons in the arcuate nucleus of the hypothalamus increases feeding (Aponte et al. 2011), and ablation of NPY neurons reduces food intake and body weight (Gropp et al. 2005; Luquet et al. 2005). There is also evidence that NPY interacts with the mesocorticolimbic dopamine system to regulate feeding. NPY neurons project to the VTA (Dietrich et al. 2012), NPY receptors are expressed in the VTA (Kishi et al. 2005; Korotkova et al. 2006; Wolak et al. 2003), and intra-VTA and intranucleus accumbens injection of NPY increases operant responding for food in rats (Pandit et al. 2014). However, there are conflicting data on exactly how NPY acts in the VTA to affect feeding. Intracerebroventricular NPY has been shown to increase dopamine efflux in the nucleus accumbens suggesting that NPY may activate dopamine neurons (Heilig et al. 1990; Kerkerian-Le Goff et al. 1992; Quarta et al. 2011), but a separate study has shown that NPY decreases the firing rate of VTA dopamine neurons in ex vivo brain slice preparations (Korotkova et al. 2006). Thus, overall, it is unknown how NPY affects VTA dopamine neurons to regulate feeding. Therefore, in these studies, we have used patch-clamp electrophysiology in acute brain slice preparations to test whether NPY inhibits VTA dopamine neurons through direct action on dopamine neurons or through the presynaptic regulation of their synaptic inputs.
MATERIALS AND METHODS
Animals.
Male and female mice (5–14 wk old) on a C57Bl/6J or a mixed C57/129 background were used in all experiments. All protocols and procedures were approved by the Institutional Animal Care and Use Committee at Georgia State University and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Slice preparation and electrophysiology.
Acute brain slices were prepared as previously described (Roseberry et al. 2007; Stuhrman and Roseberry 2015). Briefly, adult mice were anesthetized with isofluorane and decapitated. The brain was then removed and placed in carbogen (95% O2-5% CO2)-saturated ice-cold artificial cerebral spinal fluid (aCSF), containing the following (in mM): 126 NaCl, 2.5 KCl, 2.4 CaCl2, 1.2 NaH2PO4, 1.2 MgCl2, 11.1 glucose, and 21.4 NaHCO3. A brain block containing the VTA was made, and pseudohorizontal sections (220 μm) were cut with a vibrating blade microtome. Slices were then incubated in aCSF (~35°C) containing 10 μM MK-801 {(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate} for 30–60 min before recording. Slices were placed in a recording chamber and perfused with carbogen-saturated aCSF at a flow rate of ~1–2 ml/min. Whole cell recordings were made using an Axon multiclamp 700B microelectrode amplifier and Axograph software. Putative dopamine neurons were identified by their location relative to the medial terminal nucleus of the accessory optic tract, the presence of hyperpolarization-activated cation currents (H current), and the presence of spontaneous pacemaker firing (Johnson and North 1992).
Electrodes (2.0–3.0 MΩ) were filled with a potassium gluconate (KGluconate)-based internal solution containing the following (in mM): 128 KGluconate, 10 NaCl, 1 MgCl2, 10 HEPES, 2 ATP, 0.3 GTP, 10 creatine phosphate, and 10 BAPTA or 0.1 EGTA. The internal solution contained EGTA for the experiments examining the direct effect of NPY on dopamine neuron activity under reduced calcium-buffering conditions and for the experiments examining the effect of NPY on excitatory postsynaptic currents (EPSCs). The internal solution contained BAPTA for all other experiments, with the exception of the measurement of inhibitory postsynaptic currents (IPSCs), where a potassium methylsulfate-based internal solution containing a high concentration of Cl− was used as follows (in mM): 57 KCl, 70 KMeSO4, 20 NaCl, 1.5 MgCl2, 5 HEPES, 0.1 EGTA, 2 ATP, 0.3 GTP, and 10 creatine phosphate. Series resistance values were ~3–15 MΩ. If the series resistance increased by more than 20%, the experiment was terminated or excluded from analysis. In addition, if the holding current changed by more than 10 pA during baseline recording or during the first minute of NPY application, the experiment was terminated or excluded from analysis. Neurons were voltage clamped at −60 mV for most experiments. Corrections were not made for the liquid junction potential, which was calculated to be the following for each internal: KGluconate 10 mM BAPTA, 13.9 (normal aCSF), 13.6 (high K+ external solution); KGluconate 0.1 mM EGTA, 14.8; and K methylsufate/KCl, 6. EPSCs/IPSCs were evoked using a bipolar stimulating electrode placed 100–300 μm from the recorded cell. The electrode was placed anterior to the recorded cell to evoke EPSCs and posterior to the recorded cell to evoke IPSCs. Pairs of PSCs were evoked with a 50-ms interpulse interval every 20 s. EPSCs were isolated by including picrotoxin (100 μM) in the perfusion solution, and IPSCs were isolated by including DNQX (10 μM) in the perfusion solution. For all experiments, cells were held for at least 10 min before drug application to allow for diffusion of the internal solution into the cell. To determine the current-voltage relationship and the reversal potential of the NPY current, cells were perfused with a high K+ external solution containing tetrodotoxin (TTX) as follows (in mM): 118.5 NaCl, 10 KCl, 2.4 CaCl2, 1.2 NaH2PO4, 1.2 MgCl2, 11.1 glucose, 21.4 NaHCO3, and 0.001 TTX. The cells were then held at −40 mV, and slow voltage ramps were applied from −100 to 0 mV at 100 mV/s every 30 s.
Drugs.
Neuropeptide-Y and BIBP3226 were purchased from Bachem (Torrance, CA). TTX was purchased from Tocris Biosciences (Minneapolis, MN). All other reagents were from common commercial sources.
Data analysis and statistics.
Data are represented as the means ± SE unless otherwise noted. For all PSC measurements, the effect of NPY on EPSCs/IPSCs was determined by comparing the average value of the PSCs measured 5 min before the onset of NPY to the average values 5 min after the onset of NPY treatment. The pair-pulse ratio (PPR) was calculated by dividing the amplitude of the second PSC by the amplitude of the first PSC. The coefficient of variation was calculated by dividing the SD by the mean of the PSC amplitude. Data were analyzed using Axograph X (v1.3.5), LabChart (v7.3.6; ADInstruments), and Excel (v14.0; Microsoft) software. Statistics were calculated using Sigmastat (v11.0; Systat Software). Data were initially tested for normality using the Shapiro-Wilk test and were then analyzed with Student’s t-tests, Wilcoxon signed-rank tests, or a two-way ANOVA with Holm-Sidek post hoc tests as appropriate with a significance level of P < 0.05 set a priori. For the experiments comparing the responses to NPY and baclofen with the BAPTA and EGTA internal solutions (see Fig. 4), the data was log transformed to achieve normality before a two-way ANOVA was run.
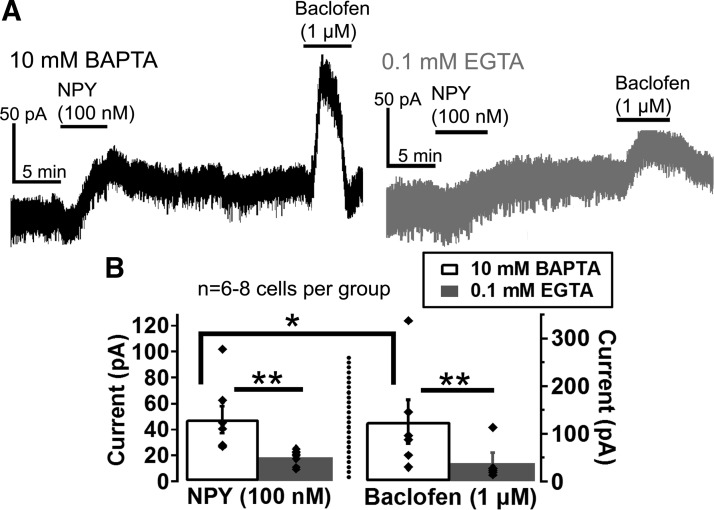
Fig. 4.
NPY and baclofen currents are affected by intracellular Ca2+ levels in VTA dopamine neurons. A and B: sample traces (A) and mean peak amplitudes (B) of the NPY (100 nM)- and baclofen (1 μM)-induced currents using internal solutions containing 10 mM BAPTA (black trace) or 0.1 mM EGTA (gray trace). Bars in A indicate time of NPY and baclofen application. Scale bars = 50 pA/5 min; n = 6–8 cells from 5 to 8 mice for each group. *P ≤ 0.05; **P ≤ 0.01.
RESULTS
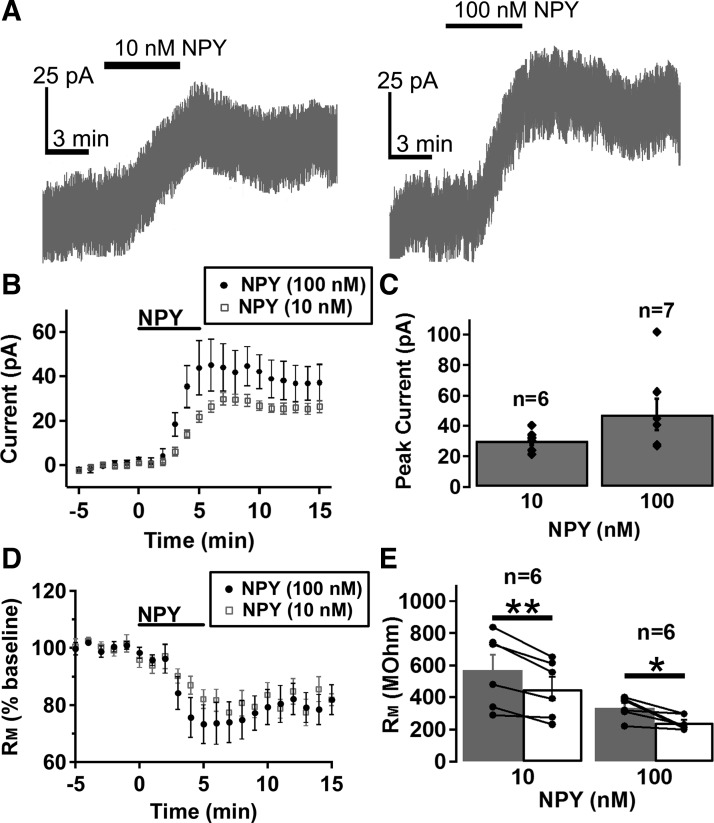
There are conflicting data on whether NPY increases or decreases VTA dopamine neuron activity (Heilig et al. 1990; Kerkerian-Le Goff et al. 1992; Korotkova et al. 2006; Quarta et al. 2011). Thus we used patch-clamp electrophysiology in acute brain slice preparations to test whether NPY directly regulates VTA dopamine neuron activity. NPY activated an outward current in ~58% of VTA dopamine neurons tested (Fig. 1, A–C; 37 out of 64 neurons total; 10 nM = 6 of 12; 100 nM = 27 of 42; and 300 nM 4 of 10). The NPY-activated current was concentration-dependent (Fig. 1, A–C) and was accompanied by a significant decrease in membrane resistance (Fig. 1, D and E), suggesting that NPY directly activates an ionic conductance in VTA dopamine neurons. Thus it appears that NPY directly inhibits VTA dopamine neurons. The 100- and 300-nM concentrations of NPY were used in all subsequent experiments, as both appeared to be saturating concentrations.
Fig. 1.
Neuropeptide Y (NPY) concentration dependently activated an outward current and reduced membrane resistance (RM) in a subset of ventral tegmental area (VTA) dopamine neurons. A–C: sample traces (A), mean effect (B), and mean peak amplitude (C) of the NPY activated current at different concentrations. D and E: mean effect of NPY on RM (D) and mean RM before and after NPY application (E) at different concentrations. Bars in A, B, and D indicate time of NPY application. n = 6–7 cells from 5 to 6 mice for each group. Scale bars = 25 pA/3 min. *P < 0.05; **P < 0.01.
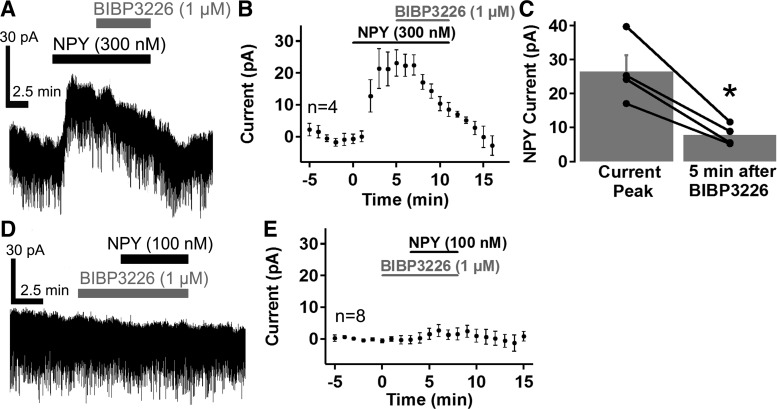
We next sought to identify the NPY receptor mediating the NPY-induced current in VTA dopamine neurons. Previous studies have reported that the postsynaptic effects of NPY are mediated by NPY acting on Y1 and Y2 receptors (Acuna-Goycolea et al. 2005; Fu et al. 2004; Melnick 2012; Roseberry et al. 2004; Sosulina et al. 2008; Sun et al. 2001). We initially tested whether Y1 receptors (Y1R) mediated this effect using the Y1R antagonist BIBP3226 (Acuna-Goycolea et al. 2005; Fu et al. 2004; Melnick 2012; Roseberry et al. 2004; Sosulina et al. 2008; Sun et al. 2001). BIBP3226 (1 µM) reversed the NPY-induced current when it was applied at the peak of the NPY current (Fig. 2, A–C, n = 4, note the rate of reversal of the NPY-induced current with BIBP3226 compared with NPY alone in Fig. 1, A and B). In addition, pretreatment with BIBP3226 (1 µM) completely prevented the NPY-induced current in all cells tested (Fig. 2, D and E, n = 8). Thus NPY appears to directly inhibit VTA dopamine neurons by activating Y1Rs.
Fig. 2.
The NPY-induced current was mediated by NPY Y1Rs in VTA dopamine neurons. A–C: the Y1R antagonist BIBP3226 (1 µM) reversed the NPY (300 nM)-induced current. Sample trace (A) and mean response (B; n = 4) of the NPY current before and during BIBP3326 application, and mean NPY current amplitude before and after BIBP3226 application (C; n = 4). D and E: sample trace (D) and mean response (E; n = 8) of VTA dopamine neurons pretreated with BIBP3226 (1 µM) to NPY (100 nM). Bars in A, B, D, and E indicate time of NPY and BIBP3226 application. Scale bars = 30 pA/2.5 min. *P < 0.05.
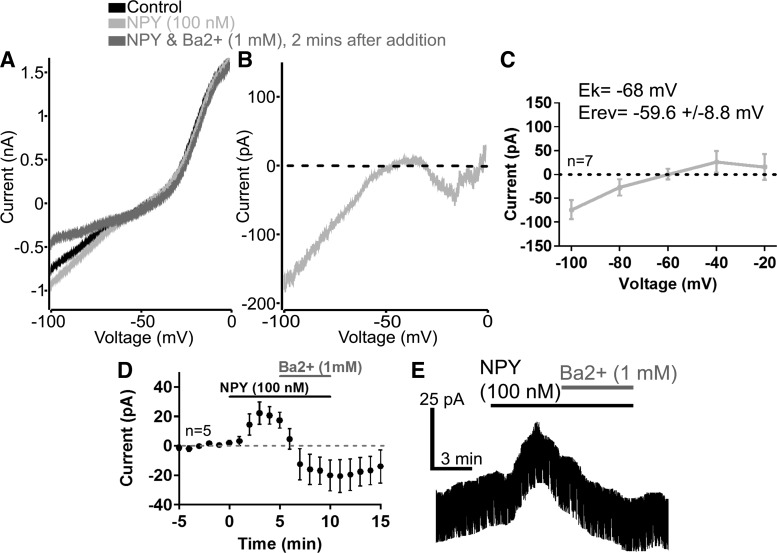
We next sought to determine the identity of the channel mediating the NPY-activated current in VTA dopamine neurons. We tested the current-voltage relationship of the NPY current by applying slow voltage ramps (−100 to 0 mV 100 mV/s) in a high K+ (10 mM) external solution containing TTX (1 μM). The current obtained from these slow voltage ramps exhibited inward rectification and had a reversal potential near that of the reversal potential for potassium ions under these conditions (Fig. 3, A–C; EK = −68 mV; NPY Erev = −59.6 ± 8.8 mV). These results indicated that NPY induced a potassium current in VTA dopamine neurons that is likely mediated by activation of GIRK channels. We then tested whether extracellular barium (1 mM) could inhibit the NPY-induced current (Fig. 3, A, D, and E). Barium is a known blocker of inwardly rectifying potassium channels, including GIRK channels (Lesage et al. 1995; Yamada et al. 1998), and it has been shown to block NPY-induced GIRK currents in many different central nervous system neurons (Acuna-Goycolea et al. 2005; Fu et al. 2004; Melnick 2012; Roseberry et al. 2004; Sosulina et al. 2008; Sun et al. 2001). Extracellular barium reversed and blocked the NPY current along with a basal leak current in VTA dopamine neurons (Fig. 3, A, D, and E). Thus it appears that NPY activated GIRK channels in VTA dopamine neurons.
Fig. 3.
The NPY-induced current in VTA dopamine neurons exhibited characteristics of a G protein-coupled inwardly rectifying K+ (GIRK) channel. A: sample current traces resulting from slow voltage ramps (−100 to 0 mV at 100 mV/s) before (black trace) and after NPY (100 nM; light gray trace) application and 2 min after the addition of Ba2+ (1 mM; NPY + Ba2+, dark gray trace) using a high K+ (10 mM) external solution containing tetrodotoxin (TTX; 1 μM). B: sample trace of the net NPY (100 nM)-induced current. C: mean current-voltage relationship of the NPY (100 nM)-induced current. D and E: mean effect (D) and sample trace (E) of the NPY (100 nM)-induced current at a holding potential of −40 mV before and during Ba2+ (1 mM) application using a high K+ (10 mM) external solution containing TTX (1 μM). Bars in D and E indicate time of NPY and Ba2+ application; n = 5–7 cells from 5 to 7 mice for each group. Scale bars = 25 pA/3 min.
We next tested whether the NPY-activated current in VTA dopamine neurons was sensitive to intracellular calcium levels, because previous studies have shown that GIRK currents are smaller when intracellular calcium buffering is reduced in VTA dopamine neurons (Beckstead and Williams 2007; Perra et al. 2011). As a positive control, we also tested whether GIRK currents activated by the GABAB receptor agonist baclofen (1 μM) were dependent on the strength of intracellular calcium buffering. The NPY-induced currents were significantly smaller than the baclofen-induced currents (Fig. 4), and, as expected, both NPY (100 nM) and baclofen (1 μM) currents were significantly smaller with reduced intracellular calcium buffering (0.1 mM EGTA) compared with strong calcium buffering (10 mM BAPTA) {Fig. 4; significant main effects of drug [F(1,23) = 7.807, P = 0.010] and calcium buffering [F(1, 23) = 19.165, P < 0.001)}, demonstrating that intracellular calcium regulates GABAB- and NPY-induced currents in a similar manner. Thus these results further suggest that NPY activates a GIRK channel current in VTA dopamine neurons and demonstrates that this current is sensitive to intracellular calcium levels.
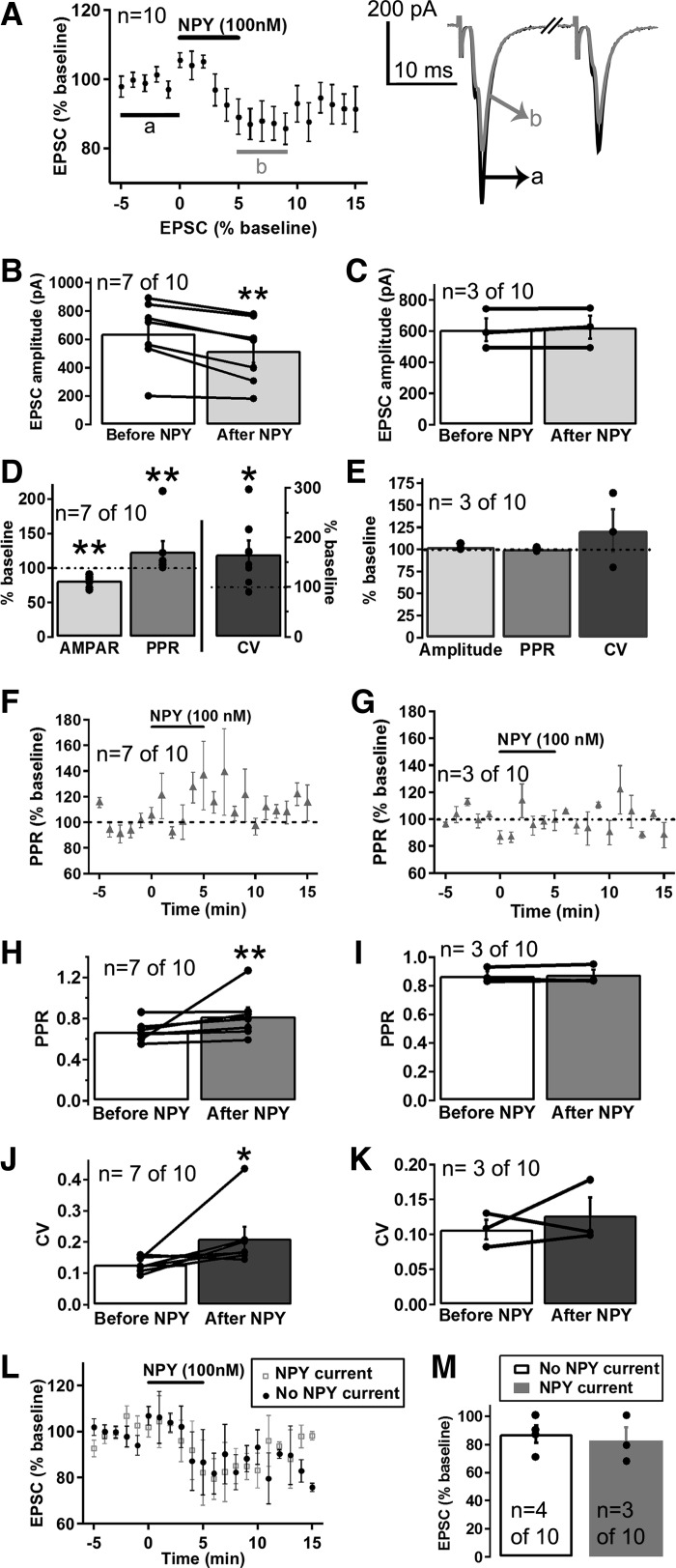
In addition to directly inhibiting VTA dopamine neurons, it is possible that NPY could regulate the activity of dopamine neurons indirectly through modulation of their afferent inputs. Glutamatergic and GABAergic afferent inputs are important regulators of dopamine neuron activity (Grace et al. 2007; Morikawa and Paladini 2011; Paladini and Roeper 2014), and NPY has been shown to affect glutamatergic and GABAergic transmission in other areas of the CNS (Acuna-Goycolea et al. 2005; Fu et al. 2004; Melnick 2012; Molosh et al. 2013). Thus we next examined whether NPY altered glutamatergic inputs to dopamine neurons. NPY decreased the amplitude of evoked EPSCs in 7 of the 10 VTA dopamine neurons tested (Fig. 5, A–E, range of effect = 68-91% of baseline). To examine the mechanism by which NPY decreased EPSCs, we assessed whether there were changes to the PPR and coefficient of variation (CV) of the EPSCs after treatment with NPY. PPR and CV are measures used to determine whether a change in synaptic strength is due to a presynaptic or postsynaptic modification, and PPR and CV values have been shown to significantly increase when the probability of presynaptic neurotransmitter release is decreased but do not change when the amplitude of PSCs is affected by a postsynaptic modification (Choi and Lovinger 1997; Michaeli and Yaka 2010). NPY (100 nM) significantly increased both the PPR and CV of the EPSCs inhibited by NPY (Fig. 5, D, F, H, and J; n = 7 of 10) without affecting the PPR or CV of the EPSCs whose amplitude was not affected by NPY (Fig. 5, E, G, I, and K; n = 3 of 10). Thus it appears that NPY decreased glutamatergic transmission onto a subset of VTA dopamine neurons through an inhibition of presynaptic release. We next examined whether the NPY-induced current and the inhibition of EPSCs were related effects by assessing whether NPY activated GIRK currents and inhibited EPSCs in the same neurons or in distinct populations of VTA dopamine neurons. NPY inhibited EPSCs in both dopamine neurons that showed an NPY-induced outward current (n = 3 of 10) and in neurons that did not directly respond to NPY (n = 4 of 10), and the magnitude of the inhibition of the EPSCs was similar for both sets of neurons (Fig. 5, L–M). These results suggest that NPY inhibited EPSCs independent of the NPY-induced GIRK current and that NPY inhibits EPSCs and activates inhibitory GIRK currents in both distinct and overlapping sets of VTA dopamine neurons.
Fig. 5.
NPY decreased excitatory postsynaptic currents (EPSCs) in a subset of VTA dopamine neurons through a presynaptic decrease in glutamate release. A: mean effect of NPY (100 nM) on EPSCs (n = 10 cells from 10 mice) and sample trace of an EPSC before (a; black trace) and after (b; gray trace) NPY. B and C: mean EPSC amplitude before and after NPY (100 nM) application for the EPSCs inhibited by NPY (B; n = 7 of 10) and for the EPSCs not affected by NPY (C; n = 3 of 10). D and E: mean effect of NPY (100 nM) on the EPSC amplitude, paired-pulse ratio (PPR), and coefficient of variation (CV) for the EPSCs inhibited by NPY (D; n = 7 of 10) and for the EPSCs not affected by NPY (E; n = 3 of 10). F and G: time course of the effect of NPY (100 nM) on EPSC PPRs for the EPSCs inhibited by NPY (F; n = 7 of 10) and for the EPSCs not affected by NPY (G; n = 3 of 10). H–K: mean PPR (H and I) and mean CV (J and K) before and after NPY (100 nM) application for the EPSCs inhibited by NPY (H and J; n = 7 of 10) and for the EPSCs not affected by NPY (I and K; n = 3 of 7). L and M: mean EPSC response to NPY (100 nM) (L) and mean EPSC amplitude after NPY (100 nM) application (M) in neurons in which NPY caused an outward current (n = 3 of 10) compared with neurons that did not show an NPY-induced current (n = 4 of 10). Bars in A, F, G, and L indicate time of NPY application. Scale bar = 200 pA/10 ms. *P ≤ 0.05; **P ≤ 0.01.
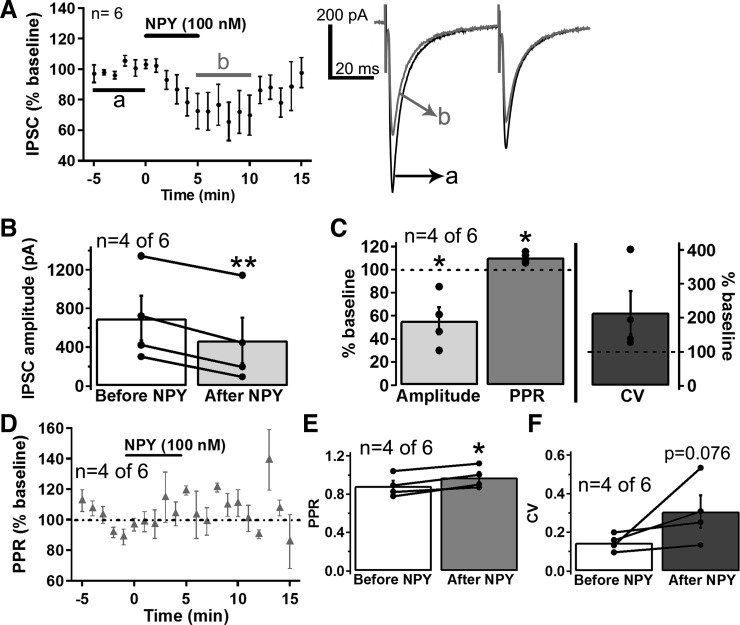
NPY has also been reported to inhibit VTA GABA neurons (Korotkova et al. 2006), which provide important inhibitory input to VTA dopamine neurons (Grace et al. 2007; Morikawa and Paladini 2011; Omelchenko and Sesack 2009; Paladini and Roeper 2014). Therefore, we next tested whether NPY also altered GABAergic inputs to VTA dopamine neurons. NPY strongly inhibited evoked IPSCs in four out of six dopamine neurons tested (Fig. 6, A–C; range of effect = 30-85% of baseline) and increased the PPR and CV of the affected IPSCs (Fig. 6, C–F; n = 4 of 6), although the CV did not reach statistical significance. NPY did not decrease evoked IPSCs in two out of six dopamine neurons tested but did activate GIRK currents in both of these neurons, suggesting that, as with NPY’s effect on EPSCs, the inhibition of the IPSCs is not related to the NPY-induced GIRK current. Thus NPY appears to also decrease GABAergic transmission onto a subset of VTA dopamine neurons through an inhibition of presynaptic release.
Fig. 6.
NPY inhibited inhibitory postsynaptic currents (IPSCs) in a subset of VTA dopamine neurons through a presynaptic decrease in GABA release. A: mean effect of NPY (100 nM) on IPSCs (n = 6 cells from 5 mice) and sample trace of an IPSC before (a; black trace) and after (b; gray trace) NPY. B: mean IPSC amplitude before and after NPY (100 nM) application for the IPSCs inhibited by NPY (n = 4 of 6). C: mean effect of NPY (100 nM) on the IPSC amplitude, PPR, and CV for the IPSCs inhibited by NPY (n = 4 of 6). D: time course of the effect of NPY (100 nM) on IPSC PPRs for the IPSCs inhibited by NPY (n = 4 of 6). E and F: mean PPR (E) and mean CV (F) before and after NPY (100 nM) application for the IPSCs inhibited by NPY (n = 4 of 6). Bars in A and D indicate time of NPY application. Scale bar = 200 pA/20 ms. *P ≤ 0.05; **P ≤ 0.01.
DISCUSSION
In these studies we have used patch-clamp electrophysiology in acute brain slice preparations to determine how NPY alters VTA dopamine neuron activity to affect feeding. NPY inhibited a subset of dopamine neurons through two mechanisms; NPY directly inhibited dopamine neurons through Y1R-mediated activation of GIRK channels, and NPY indirectly inhibited dopamine neurons by decreasing glutamatergic transmission onto dopamine neurons. Interestingly, NPY also decreased GABAergic transmission onto a subset of dopamine neurons, indicating that NPY could cause excitation of some VTA dopamine neurons.
A previous study found that NPY decreases the firing rate of a subset of VTA dopamine neurons in ex vivo brain slices from rats (Korotkova et al. 2006), but the mechanism of this NPY caused inhibition of dopamine neurons was unknown. NPY mediates its effects through five known receptors, Y1, Y2, Y4, Y5, and Y6 (Blomqvist and Herzog 1997; Ingenhoven and Beck-Sickinger 1999). All of the NPY receptors are G protein-coupled receptors that signal through Gi/o G-proteins (Blomqvist and Herzog 1997; Ingenhoven and Beck-Sickinger 1999), and NPY causes a GIRK channel current in neurons located in different areas of the CNS (Acuna-Goycolea et al. 2005; Fu et al. 2004; Melnick 2012; Roseberry et al. 2004; Sosulina et al. 2008; Sun et al. 2001). Thus we hypothesized that NPY inhibited dopamine neurons through a similar mechanism. Indeed, the results presented here support the hypothesis that NPY activates GIRK channels in VTA dopamine neurons as NPY caused a concentration-dependent outward current that was accompanied by a decrease in membrane resistance, reversed at the reversal potential for K+ ions, exhibited inward rectification, and was sensitive to extracellular barium, which is similar to what has been reported in numerous other brain regions (Acuna-Goycolea et al. 2005; Fu et al. 2004; Melnick 2012; Roseberry et al. 2004; Sosulina et al. 2008; Sun et al. 2001). Thus we can conclude that NPY activates Y1Rs that in turn release activated Gi/o-proteins to open GIRK channels.
The NPY-induced current was also sensitive to intracellular calcium levels, which is an interesting characteristic of GIRK currents in VTA dopamine neurons (Beckstead and Williams 2007; Perra et al. 2011). For example, GIRK currents activated by GABAB and dopamine D2 receptor agonists are reported to be smaller when intracellular calcium buffering is reduced and calcium levels are high in VTA dopamine neurons (Beckstead and Williams 2007; Perra et al. 2011). We found that, like the baclofen-induced currents, the NPY-induced currents were significantly smaller under reduced calcium-buffering conditions. One potential caveat in these experiments is that the NPY currents could have affected the amplitude of the subsequent baclofen currents through heterologous desensitization, although this would not affect the interpretation of these results, as we would expect this to be true for both low and high calcium buffering. Thus, taken together, our findings indicate that NPY directly inhibits VTA dopamine neurons by activating a GIRK current that is sensitive to intracellular calcium levels.
In addition to the direct effects of NPY on VTA dopamine neurons, we also examined whether NPY indirectly affected dopamine neuron activity through modulation of their glutamatergic and GABAergic afferent inputs, which play an important role in controlling dopamine neuron activity (Grace et al. 2007; Morikawa and Paladini 2011; Paladini and Roeper 2014). Glutamatergic afferents primarily control dopamine neuron burst firing, and GABAergic afferents strongly inhibit dopamine neurons, demonstrating that these afferent inputs are important regulators of dopamine neuron activity (Grace et al. 2007; Morikawa and Paladini 2011; Paladini and Roeper 2014). Surprisingly, NPY decreased both excitatory glutamatergic and inhibitory GABAergic transmission onto VTA dopamine neurons, although not to the same extent (Figs. 5 and 6). NPY decreased both glutamatergic and GABAergic transmission through a decrease in presynaptic release, which is similar to what has been reported in other areas of the CNS (Acuna-Goycolea et al. 2005; Fu et al. 2004; Melnick 2012). Thus NPY modulates VTA dopamine neuron activity through two different presynaptic mechanisms.
The net effect of NPY on the overall activity of VTA dopamine neurons is unclear, because the responses observed here would result in both activation and inhibition of dopamine neurons. The inhibitory effects of NPY on VTA dopamine neurons were relatively small [a small (~50 pA) direct inhibition and a modest ~18% decrease in EPSCs], whereas the excitatory effect of NPY was more robust (~44% decrease in IPSCs), suggesting that NPY could have a net excitatory effect on VTA dopamine neuron activity. This possibility is supported by previous studies suggesting that NPY excites VTA dopamine neurons (Heilig et al. 1990; Kerkerian-Le Goff et al. 1992; Quarta et al. 2011). For example, centrally delivered NPY increases dopamine release at VTA dopamine efferent sites (Heilig et al. 1990; Kerkerian-Le Goff et al. 1992; Quarta et al. 2011) and increases dopamine-associated behaviors (Jewett et al. 1992, 1995; Maric et al. 2008, 2009; Pandit et al. 2014) suggesting that NPY increases the activity of dopamine neurons to stimulate dopamine release. In contrast, Korotkova et al. (2006) have shown that NPY inhibits firing of VTA dopamine neurons in ex vivo slice preparations, indicating that NPY inhibits dopamine neurons, which is supported by our studies showing that NPY activates an outward GIRK current and inhibits EPSCs in dopamine neurons. We attempted to examine the net effect of NPY on dopamine neuron activity by testing the effect of NPY on the firing rate of VTA dopamine neurons in the cell-attached configuration in the presence and absence of inhibitors of synaptic transmission (DNQX and picrotoxin). However, due to the small effects of NPY on dopamine neuron firing rate in these experiments, we could not conclusively determine whether NPY had an excitatory, inhibitory, or no effect on the activity of all the dopamine neurons tested. Thus it is still unclear whether the net effect of NPY on dopamine neuron activity in vivo would be excitatory or inhibitory.
One possible explanation for NPY causing both excitatory and inhibitory effects on dopamine neuron activity is that NPY could differentially modulate separate subpopulations of VTA dopamine neurons through distinct mechanisms. NPY only affected a subset of VTA dopamine neurons for each of the responses measured (direct current, EPSCs, and IPSCs). Thus NPY could excite one subpopulation of dopamine neurons and inhibit another distinct subpopulation of dopamine neurons. Historically, dopamine neurons have been thought of as a uniform population of neurons, but recent research has demonstrated that there are subpopulations of VTA dopamine neurons that project to different efferent target regions and show distinct electrophysiological and molecular properties (Lammel et al. 2014; Roeper 2013; Volman et al. 2013; Wenzel et al. 2015). In addition, aversive stimuli and rewards have also been shown to excite distinct subpopulations of dopamine neurons (Lammel et al. 2014; Roeper 2013; Volman et al. 2013; Wenzel et al. 2015). Thus NPY could excite a specific subpopulation of dopamine neurons while inhibiting a distinct subset of neurons to differentially regulate distinct aspects of behavior (e.g., reward vs aversion). For example, dopamine neurons encoding reward and reinforcement project to the nucleus accumbens while dopamine neurons encoding aversion project to the prefrontal cortex (Lammel et al. 2012). Thus it is possible that NPY could excite dopamine neurons projecting to the nucleus accumbens to promote food reward while inhibiting dopamine neurons projecting to the prefrontal cortex to decrease aversion. This possibility is supported by the overall effects of NPY on food-motivated behavior, as injection of NPY either intracerebroventricularly or into the VTA increases operant responding for sucrose and food pellets in rats (Jewett et al. 1992; 1995; Pandit et al. 2014), and this response is associated with increased dopamine release in the nucleus accumbens (Adamantidis et al. 2011; Koch et al. 2000). Further experiments will be required to identify the net effect of NPY on overall dopamine neuron activity and dopamine output and to determine whether NPY is activating and inhibiting distinct subpopulations of VTA dopamine neurons to promote food-seeking behaviors.
In summary, we have demonstrated that NPY modulates subsets of VTA dopamine neurons through three independent mechanisms, including both presynaptic and postsynaptic mechanisms. NPY directly inhibited VTA dopamine neurons through activation of a postsynaptic GIRK channel current and indirectly inhibited VTA dopamine neurons through a presynaptic reduction in glutamate release. NPY also decreased GABAergic transmission onto dopamine neurons through a presynaptic reduction in GABA release. These results advance our understanding of how VTA dopamine neuron activity is regulated and provide further understanding of how NPY interacts with the mesocorticolimbic dopamine system to regulate feeding behavior.
GRANTS
Funding for these studies was provided by the Department of Biology, the Brains and Behavior Program, and the Center for Obesity Reversal at Georgia State University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.S.W. and A.G.R. conceived and designed research; K.S.W. performed experiments; K.S.W. and A.G.R. analyzed data; K.S.W. and A.G.R. interpreted results of experiments; K.S.W. and A.G.R. prepared figures; K.S.W. drafted manuscript; K.S.W. and A.G.R. edited and revised manuscript; K.S.W. and A.G.R. approved final version of manuscript.
REFERENCES
- Acuna-Goycolea C, Tamamaki N, Yanagawa Y, Obata K, van den Pol AN. Mechanisms of neuropeptide Y, peptide YY, and pancreatic polypeptide inhibition of identified green fluorescent protein-expressing GABA neurons in the hypothalamic neuroendocrine arcuate nucleus. J Neurosci 25: 7406–7419, 2005. doi: 10.1523/JNEUROSCI.1008-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamantidis AR, Tsai HC, Boutrel B, Zhang F, Stuber GD, Budygin EA, Touriño C, Bonci A, Deisseroth K, de Lecea L. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J Neurosci 31: 10829–10835, 2011. doi: 10.1523/JNEUROSCI.2246-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci 14: 351–355, 2011. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G. Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience 89: 637–641, 1999. doi: 10.1016/S0306-4522(98)00583-1. [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Williams JT. Long-term depression of a dopamine IPSC. J Neurosci 27: 2074–2080, 2007. doi: 10.1523/JNEUROSCI.3251-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beninger RJ, Cheng M, Hahn BL, Hoffman DC, Mazurski EJ, Morency MA, Ramm P, Stewart RJ. Effects of extinction, pimozide, SCH 23390, and metoclopramide on food-rewarded operant responding of rats. Psychopharmacology (Berl) 92: 343–349, 1987. doi: 10.1007/BF00210842. [DOI] [PubMed] [Google Scholar]
- Blomqvist AG, Herzog H. Y-receptor subtypes–how many more? Trends Neurosci 20: 294–298, 1997. doi: 10.1016/S0166-2236(96)01057-0. [DOI] [PubMed] [Google Scholar]
- Chambers AP, Woods SC. The role of neuropeptide Y in energy homeostasis. Handb Exp Pharmacol 209: 23–45, 2012. doi: 10.1007/978-3-642-24716-3_2. [DOI] [PubMed] [Google Scholar]
- Choi S, Lovinger DM. Decreased probability of neurotransmitter release underlies striatal long-term depression and postnatal development of corticostriatal synapses. Proc Natl Acad Sci USA 94: 2665–2670, 1997. doi: 10.1073/pnas.94.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology 115: 427–429, 1984. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Wei W, Salamone JD. Pharmacological characterization of performance on a concurrent lever pressing/feeding choice procedure: effects of dopamine antagonist, cholinomimetic, sedative and stimulant drugs. Psychopharmacology (Berl) 116: 529–537, 1994. doi: 10.1007/BF02247489. [DOI] [PubMed] [Google Scholar]
- Dietrich MO, Bober J, Ferreira JG, Tellez LA, Mineur YS, Souza DO, Gao XB, Picciotto MR, Araújo I, Liu ZW, Horvath TL. AgRP neurons regulate development of dopamine neuronal plasticity and nonfood-associated behaviors. Nat Neurosci 15: 1108–1110, 2012. doi: 10.1038/nn.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitropoulos A, Tkach J, Ho A, Kennedy J. Greater corticolimbic activation to high-calorie food cues after eating in obese vs. normal-weight adults. Appetite 58: 303–312, 2012. doi: 10.1016/j.appet.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 307: 491–497, 2012. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- Fu LY, Acuna-Goycolea C, van den Pol AN. Neuropeptide Y inhibits hypocretin/orexin neurons by multiple presynaptic and postsynaptic mechanisms: tonic depression of the hypothalamic arousal system. J Neurosci 24: 8741–8751, 2004. doi: 10.1523/JNEUROSCI.2268-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier JF, Chen K, Salbe AD, Bandy D, Pratley RE, Heiman M, Ravussin E, Reiman EM, Tataranni PA. Differential brain responses to satiation in obese and lean men. Diabetes 49: 838–846, 2000. doi: 10.2337/diabetes.49.5.838. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci 30: 220–227, 2007. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, Barsh GS, Horvath TL, Brüning JC. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci 8: 1289–1291, 2005. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- Heilig M, Vècséi L, Wahlestedt C, Alling C, Widerlöv E. Effects of centrally administered neuropeptide Y (NPY) and NPY13-36 on the brain monoaminergic systems of the rat. J Neural Transm (Vienna) 79: 193–208, 1990. doi: 10.1007/BF01245130. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG. Feeding and hypothalamic stimulation increase dopamine turnover in the accumbens. Physiol Behav 44: 599–606, 1988a. doi: 10.1016/0031-9384(88)90324-1. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG. Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci 42: 1705–1712, 1988b. doi: 10.1016/0024-3205(88)90036-7. [DOI] [PubMed] [Google Scholar]
- Ingenhoven N, Beck-Sickinger AG. Molecular characterization of the ligand-receptor interaction of neuropeptide Y. Curr Med Chem 6: 1055–1066, 1999. [PubMed] [Google Scholar]
- Jewett DC, Cleary J, Levine AS, Schaal DW, Thompson T. Effects of neuropeptide Y on food-reinforced behavior in satiated rats. Pharmacol Biochem Behav 42: 207–212, 1992. doi: 10.1016/0091-3057(92)90517-J. [DOI] [PubMed] [Google Scholar]
- Jewett DC, Cleary J, Levine AS, Schaal DW, Thompson T. Effects of neuropeptide Y, insulin, 2-deoxyglucose, and food deprivation on food-motivated behavior. Psychopharmacology (Berl) 120: 267–271, 1995. doi: 10.1007/BF02311173. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol 450: 455–468, 1992. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan LM. Pharmacologic therapies for obesity. Gastroenterol Clin North Am 39: 69–79, 2010. doi: 10.1016/j.gtc.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron 69: 664–679, 2011. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkerian-Le Goff L, Forni C, Samuel D, Bloc A, Dusticier N, Nieoullon A. Intracerebroventricular administration of neuropeptide Y affects parameters of dopamine, glutamate and GABA activities in the rat striatum. Brain Res Bull 28: 187–193, 1992. doi: 10.1016/0361-9230(92)90178-Z. [DOI] [PubMed] [Google Scholar]
- Kishi T, Aschkenasi CJ, Choi BJ, Lopez ME, Lee CE, Liu H, Hollenberg AN, Friedman JM, Elmquist JK. Neuropeptide Y Y1 receptor mRNA in rodent brain: distribution and colocalization with melanocortin-4 receptor. J Comp Neurol 482: 217–243, 2005. doi: 10.1002/cne.20432. [DOI] [PubMed] [Google Scholar]
- Koch M, Schmid A, Schnitzler HU. Role of muscles accumbens dopamine D1 and D2 receptors in instrumental and Pavlovian paradigms of conditioned reward. Psychopharmacology (Berl) 152: 67–73, 2000. doi: 10.1007/s002130000505. [DOI] [PubMed] [Google Scholar]
- Kopelman P. Health risks associated with overweight and obesity. Obes Rev 8, Suppl 1: 13–17, 2007. doi: 10.1111/j.1467-789X.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Brown RE, Sergeeva OA, Ponomarenko AA, Haas HL. Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. Eur J Neurosci 23: 2677–2685, 2006. doi: 10.1111/j.1460-9568.2006.04792.x. [DOI] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Malenka RC. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology 76: 351–359, 2014. doi: 10.1016/j.neuropharm.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature 491: 212–217, 2012. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage F, Guillemare E, Fink M, Duprat F, Heurteaux C, Fosset M, Romey G, Barhanin J, Lazdunski M. Molecular properties of neuronal G-protein-activated inwardly rectifying K+ channels. J Biol Chem 270: 28660–28667, 1995. doi: 10.1074/jbc.270.48.28660. [DOI] [PubMed] [Google Scholar]
- Loh K, Herzog H, Shi YC. Regulation of energy homeostasis by the NPY system. Trends Endocrinol Metab 26: 125–135, 2015. doi: 10.1016/j.tem.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310: 683–685, 2005. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- Lutter M, Nestler EJ. Homeostatic and hedonic signals interact in the regulation of food intake. J Nutr 139: 629–632, 2009. doi: 10.3945/jn.108.097618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maric T, Cantor A, Cuccioletta H, Tobin S, Shalev U. Neuropeptide Y augments cocaine self-administration and cocaine-induced hyperlocomotion in rats. Peptides 30: 721–726, 2009. doi: 10.1016/j.peptides.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Maric T, Tobin S, Quinn T, Shalev U. Food deprivation-like effects of neuropeptide Y on heroin self-administration and reinstatement of heroin seeking in rats. Behav Brain Res 194: 39–43, 2008. doi: 10.1016/j.bbr.2008.06.023. [DOI] [PubMed] [Google Scholar]
- Melnick IV. Cell type-specific postsynaptic effects of neuropeptide Y in substantia gelatinosa neurons of the rat spinal cord. Synapse 66: 640–649, 2012. doi: 10.1002/syn.21550. [DOI] [PubMed] [Google Scholar]
- Michaeli A, Yaka R. Dopamine inhibits GABA(A) currents in ventral tegmental area dopamine neurons via activation of presynaptic G-protein coupled inwardly-rectifying potassium channels. Neuroscience 165: 1159–1169, 2010. doi: 10.1016/j.neuroscience.2009.11.045. [DOI] [PubMed] [Google Scholar]
- Molosh AI, Sajdyk TJ, Truitt WA, Zhu W, Oxford GS, Shekhar A. NPY Y1 receptors differentially modulate GABAA and NMDA receptors via divergent signal-transduction pathways to reduce excitability of amygdala neurons. Neuropsychopharmacology 38: 1352–1364, 2013. doi: 10.1038/npp.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa H, Paladini CA. Dynamic regulation of midbrain dopamine neuron activity: intrinsic, synaptic, and plasticity mechanisms. Neuroscience 198: 95–111, 2011. doi: 10.1016/j.neuroscience.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenberg MJ, Waters C. Compulsive eating and weight gain related to dopamine agonist use. Mov Disord 21: 524–529, 2006. doi: 10.1002/mds.20757. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA 311: 806–814, 2014. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Ultrastructural analysis of local collaterals of rat ventral tegmental area neurons: GABA phenotype and synapses onto dopamine and GABA cells. Synapse 63: 895–906, 2009. doi: 10.1002/syn.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladini CA, Roeper J. Generating bursts (and pauses) in the dopamine midbrain neurons. Neuroscience 282: 109–121, 2014. doi: 10.1016/j.neuroscience.2014.07.032. [DOI] [PubMed] [Google Scholar]
- Palmiter RD. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci 30: 375–381, 2007. doi: 10.1016/j.tins.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Pandit R, Luijendijk MC, Vanderschuren LJ, la Fleur SE, Adan RA. Limbic substrates of the effects of neuropeptide Y on intake of and motivation for palatable food. Obesity (Silver Spring) 22: 1216–1219, 2014. doi: 10.1002/oby.20718. [DOI] [PubMed] [Google Scholar]
- Perra S, Clements MA, Bernier BE, Morikawa H. In vivo ethanol experience increases D(2) autoinhibition in the ventral tegmental area. Neuropsychopharmacology 36: 993–1002, 2011. doi: 10.1038/npp.2010.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarta D, Leslie CP, Carletti R, Valerio E, Caberlotto L. Central administration of NPY or an NPY-Y5 selective agonist increase in vivo extracellular monoamine levels in mesocorticolimbic projecting areas. Neuropharmacology 60: 328–335, 2011. doi: 10.1016/j.neuropharm.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Roeper J. Dissecting the diversity of midbrain dopamine neurons. Trends Neurosci 36: 336–342, 2013. doi: 10.1016/j.tins.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Roseberry AG, Liu H, Jackson AC, Cai X, Friedman JM. Neuropeptide Y-mediated inhibition of proopiomelanocortin neurons in the arcuate nucleus shows enhanced desensitization in ob/ob mice. Neuron 41: 711–722, 2004. doi: 10.1016/S0896-6273(04)00074-1. [DOI] [PubMed] [Google Scholar]
- Roseberry AG, Painter T, Mark GP, Williams JT. Decreased vesicular somatodendritic dopamine stores in leptin-deficient mice. J Neurosci 27: 7021–7027, 2007. doi: 10.1523/JNEUROSCI.1235-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, Klapp BF. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage 37: 410–421, 2007. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Rui L. Brain regulation of energy balance and body weight. Rev Endocr Metab Disord 14: 387–407, 2013. doi: 10.1007/s11154-013-9261-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosulina L, Schwesig G, Seifert G, Pape HC. Neuropeptide Y activates a G-protein-coupled inwardly rectifying potassium current and dampens excitability in the lateral amygdala. Mol Cell Neurosci 39: 491–498, 2008. doi: 10.1016/j.mcn.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol 117: 924–935, 2008. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW III, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage 41: 636–647, 2008. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Stuhrman K, Roseberry AG. Neurotensin inhibits both dopamine- and GABA-mediated inhibition of ventral tegmental area dopamine neurons. J Neurophysiol 114: 1734–1745, 2015. doi: 10.1152/jn.00279.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun QQ, Huguenard JR, Prince DA. Neuropeptide Y receptors differentially modulate G-protein-activated inwardly rectifying K+ channels and high-voltage-activated Ca2+ channels in rat thalamic neurons. J Physiol 531: 67–79, 2001. doi: 10.1111/j.1469-7793.2001.0067j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vettor R, Zarjevski N, Cusin I, Rohner-Jeanrenaud F, Jeanrenaud B. Induction and reversibility of an obesity syndrome by intracerebroventricular neuropeptide Y administration to normal rats. Diabetologia 37: 1202–1208, 1994. doi: 10.1007/BF00399793. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci 15: 37–46, 2011. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volman SF, Lammel S, Margolis EB, Kim Y, Richard JM, Roitman MF, Lobo MK. New insights into the specificity and plasticity of reward and aversion encoding in the mesolimbic system. J Neurosci 33: 17569–17576, 2013. doi: 10.1523/JNEUROSCI.3250-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel JM, Rauscher NA, Cheer JF, Oleson EB. A role for phasic dopamine release within the nucleus accumbens in encoding aversion: a review of the neurochemical literature. ACS Chem Neurosci 6: 16–26, 2015. doi: 10.1021/cn500255p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci 5: 483–494, 2004. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wolak ML, DeJoseph MR, Cator AD, Mokashi AS, Brownfield MS, Urban JH. Comparative distribution of neuropeptide Y Y1 and Y5 receptors in the rat brain by using immunohistochemistry. J Comp Neurol 464: 285–311, 2003. doi: 10.1002/cne.10823. [DOI] [PubMed] [Google Scholar]
- Yamada M, Inanobe A, Kurachi Y. G protein regulation of potassium ion channels. Pharmacol Rev 50: 723–760, 1998. [PubMed] [Google Scholar]
- Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell 83: 1197–1209, 1995. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]