This is the first investigation of the relationship between long-latency afferent inhibition (LAI) and the sensory afferent volley. Differences exist between median and digital nerve LAI. For the median nerve, LAI increases until all sensory fibers are presumably recruited. In contrast, digital nerve LAI does not increase with the recruitment of additional sensory fibers but rather is present when a given volume of sensory afferent fibers is recruited (~50% of maximum sensory nerve action potential). This novel data provide practical guidelines and contribute to our understanding of the mechanisms underlying LAI.
Keywords: TMS, LAI, sensory nerve action potential, afferent volley
Abstract
Long-latency afferent inhibition (LAI) is the inhibition of the transcranial magnetic stimulation (TMS) motor-evoked potentials (MEP) by the sensory afferent volley following electrical stimulation of a peripheral nerve. It is unknown how the activation of sensory afferent fibers relates to the magnitude of LAI. This study investigated the relationship between LAI and the sensory nerve action potentials (SNAP) from the median nerve (MN) and the digital nerves (DN) of the second digit. LAI was obtained by delivering nerve stimulation 200 ms before a TMS pulse delivered over the motor cortex. Experiment 1 assessed the magnitude of LAI following stimulation of the contralateral MN or DN using nerve stimulus intensities relative to the maximum SNAP (SNAPmax) of that nerve and two TMS intensities (0.5- and 1-mV MEP). Results indicate that MN LAI is maximal at ~50% SNAPmax, when presumably all sensory afferents are recruited for TMS of 0.5-mV MEP. For DN, LAI appears at ~50% SNAPmax and does not increase with further recruitment of sensory afferents. Experiment 2 investigated the magnitude of LAI following ipsilateral nerve stimulation at intensities relative to SNAPmax. Results show minimal LAI evoked by ipsilateral MN and no LAI following ipsilateral DN stimulation. Implications for future studies investigating LAI include adjusting nerve stimulation to 50% SNAPmax to obtain maximal LAI. Additionally, MN LAI can be used as a marker for neurological disease or injury by using a nerve stimulation intensity that can evoke a depth of LAI capable of increasing or decreasing.
NEW & NOTEWORTHY This is the first investigation of the relationship between long-latency afferent inhibition (LAI) and the sensory afferent volley. Differences exist between median and digital nerve LAI. For the median nerve, LAI increases until all sensory fibers are presumably recruited. In contrast, digital nerve LAI does not increase with the recruitment of additional sensory fibers but rather is present when a given volume of sensory afferent fibers is recruited (~50% of maximum sensory nerve action potential). This novel data provide practical guidelines and contribute to our understanding of the mechanisms underlying LAI.
sensorimotor integration may be investigated by quantifying the influence of peripheral somatosensory inputs on corticospinal excitability using transcranial magnetic stimulation (TMS) paired with preceding nerve stimulation. A single pulse of TMS delivered to the primary motor cortex (M1) results in a motor-evoked potential (MEP) in the muscle of interest, which reflects the integrity of the corticospinal tract (Bestmann and Krakauer 2015). Peripheral nerve stimulation applied at specific interstimulus intervals (ISIs) before the TMS pulse results in MEP inhibition or facilitation. Short-latency afferent inhibition (SAI), a circuit mediated by GABAA and acetylcholine receptors (Di Lazzaro et al. 2000, 2007), occurs when the TMS pulse follows median (MN) or digital nerve (DN) stimulation by ~19–21 ms (Tokimura et al. 2000). MN afferent facilitation is observed at ISIs ranging from 45 to 70 ms (Devanne et al. 2009). However, at longer ISIs ranging from ~200 to 1,000 ms, both MN and DN stimulation inhibit MEPs, a circuit called long-latency afferent inhibition (LAI) (Chen et al. 1999).
LAI occurs through the conditioning of corticospinal output by stimulation of the cutaneous DN or the mixed MN (Chen et al. 1999; Sailer et al. 2003). Peripheral nerve stimulation activates the primary somatosensory (S1), bilateral secondary somatosensory (S2), and contralateral posterior parietal (PPC) cortices (Boakye et al. 2000; Korvenoja et al. 1999). Therefore, the sensory afferent volley is thought to indirectly inhibit M1 through mechanisms involving the widespread activation of sensory areas (Chen 2004; Sailer et al. 2002, 2003). LAI is altered in clinical populations with sensory deficiencies including Parkinson’s disease (Sailer et al. 2003, 2007) and focal hand dystonia (Abbruzzese et al. 2001; Pirio Richardson et al. 2009) further implicating the involvement of sensory areas in the genesis of LAI. LAI is also reduced following the resolution of pain (Burns et al. 2016) and in those with complex regional pain syndrome (Morgante et al. 2017). In healthy individuals, LAI has been shown to enhance surround inhibition, a process that may contribute to movement accuracy through the suppression of nonmoving muscles (Voller et al. 2005). Further research is required to explore the functional significance of the LAI circuit. It is speculated that LAI is of cortical origin since spinal F waves are unchanged during the acquisition of MN LAI (Chen et al. 1999). However, the precise neural mechanism and pharmacological basis of the LAI circuit remains unknown.
Little is known about the relationship between the magnitude of the sensory afferent volley and the depth of LAI. For DN, LAI is greatest at a stimulation intensity of three times the sensory threshold (ST) for detection of the electrical stimulus and plateaus at higher nerve stimulation intensities (Chen et al. 1999). However, it remains unclear how the depth of LAI relates to the sensory afferent volley, a measure that is achievable by recording the peripheral sensory nerve action potential (SNAP) that follows nerve stimulation. To date, no studies have examined the relationship between the SNAP amplitude and the magnitude of LAI for either the DN or MN. In contrast, the SAI circuit evoked by DN and MN is reported to increase until full recruitment of sensory afferent fibers (Bailey et al. 2016).
The magnitude of LAI may also be influenced by the intensity of TMS delivered to M1. LAI decreases when the TMS intensity is increased from 1 to 4 mV (Kukaswadia et al. 2005; Sailer et al. 2002). However, the magnitude of LAI does not change with TMS intensities ranging from 0.2 to 1 mV (Kukaswadia et al. 2005; Sailer et al. 2002). Two TMS intensities were used in this study, 0.5 and 1 mV. Therefore, while the magnitude of LAI should not differ between these two TMS intensities, the influence of the sensory afferent volley on MEP amplitude may be dependent on the TMS intensity and therefore impact the relationship between SNAP and LAI magnitude.
The present study explores the relationship between the sensory afferent volley and LAI for the cutaneous DN and the mixed MN at two TMS intensities. One study has demonstrated ipsilateral MN-evoked LAI (Chen et al. 1999), yet it remains unclear whether ipsilateral DN-evoked LAI exists and whether the magnitude of LAI reflects the sensory afferent volley. Therefore, we also investigated the relationship between DN and MN LAI and SNAPs when nerve stimulation was delivered to the ipsilateral limb (i.e., ipsilateral to the delivery of the TMS pulse). Our results indicate that the magnitude of contralateral MN LAI increases until all sensory fibers are presumably recruited as assessed with the SNAP (i.e., ~50% SNAPmax) (Bailey et al. 2016) and follows a U-shaped function, a relationship that is contingent on the TMS intensity. In contrast, contralateral DN LAI first appears, is maximum, and plateaus by ~50% SNAPmax, and this relationship is unaltered by the TMS intensities tested. LAI was evoked following ipsilateral MN but not DN stimulation. This study provides insight about the neural mechanisms mediating LAI and offers practical implications for studies assessing sensorimotor integration using this circuit.
METHODS
Participants
Young, right-handed individuals participated in one (experiment 1: n = 20; 15 females; 23.4 ± 5.2 yr) or two (experiment 2: n = 18; 14 females; 23.2 ± 5.5 yr) experiments. All individuals were screened for contraindications to TMS, completed a modified handedness questionnaire (Oldfield 1971), and provided written consent before participation. The research was approved by the McMaster Research Ethics Board and conformed to the Declaration of Helsinki.
Electromyography
In experiment 1, surface electrodes (9-mm Ag-AgCl electrodes) were used to record electromyography (EMG) from the right limb including the first dorsal interosseous (FDI) and abductor pollicis brevis (APB) muscles and SNAP from the MN at the elbow proximal to the medial epicondyle. In experiment 2, EMG was recorded from the right FDI muscle and SNAP from the MN on the left arm. A wet ground was secured around the forearm, distal to the elbow. EMG recordings were amplified ×1,000 (Intronix Technologies Corporation Model 2024F, Bolton, Canada) and band-pass filtered between 20 and 2.5 kHz. With the use of an analog-to-digital interface (Power1401; Cambridge Electronics Design, Cambridge, UK), data were digitized at 5 kHz and analyzed using commercial software (Signal v6.02; Cambridge Electronics Design).
TMS Parameters
TMS was performed using a customized figure-of-eight “branding iron” coil (50-mm diameter), connected to a Magstim 2002 stimulator (Magstim) and delivered to the left hemisphere. Specifically, the coil was positioned over the motor hotspot of the right FDI muscle. The motor hotspot was identified as the location that elicited the largest MEP in the relaxed FDI with the coil orientated at a 45° angle from the sagittal plane to induce a posterior-anterior current. The location of the motor hotspot was registered digitally using the Brainsight Neuronavigation system (Rogue Research). The ISI between the nerve stimulus (NS) and TMS pulse was set to 200 ms (Chen et al. 1999).
Sensory Nerve Action Potentials
SNAPs were recorded from the MN at the elbow (described above) from the right (experiment 1) or left (experiment 2) limb. For MN stimulation, SNAPs were elicited using a bar electrode placed over the MN at the wrist with cathode proximal using a constant current stimulator (Digitimer DS7AH; square wave pulses of 0.5 ms, 2 Hz). To determine SNAPmax, the starting nerve stimulation intensity was set to the motor threshold (MT) for the APB muscle. MT was defined as the minimum intensity (in mA) required to evoke a visible twitch in the APB muscle belly. Fifty stimuli were delivered, and the peak-to-peak amplitude of the averaged SNAP was quantified. This procedure continued in stepwise increments of 2 mA until SNAPmax was achieved. The SNAPmax was defined as the intensity (in mA) at which the SNAP ceased to increase by 10% in 3 consecutive blocks of 50 trials. Following the acquisition of SNAPmax, the current was lowered until the following amplitudes of SNAP were found: 25, 50, and 75% SNAPmax (i.e., until the averaged SNAP amplitude after 50 trials matched the amplitude corresponding to ~25, 50, or 75% SNAPmax). Following this procedure, participants rated the sensation of the nerve stimulus on a scale of mild, moderate, strong, or painful for each of the four intensities (25, 50, 75, or 100% SNAPmax). If the sensation was deemed painful, they were asked to rate the pain on a scale of 0 to 10 according to the Numeric Rating Scale (NRS) (Hawker et al. 2011). If participants rated pain greater than 7 on the scale, then the stimulation intensity was not increased further. This ensured that participants never received painful stimulation since LAI is reduced 15 min following the resolution of a painful stimulus (Burns et al. 2016).
For DN stimulation, SNAPs were elicited using ring electrodes placed around the proximal and middle segments of digit 2 (index finger) with the cathode proximal (Digitimer DS7AH; square wave pulses of 0.5 ms, 2 Hz). To determine SNAPmax, the starting nerve stimulus intensity was set to the ST defined as the minimum current (in mA) required for the participant to detect the presence of the electrical stimulus. The peak-to-peak SNAP was quantified as the average of the 100 stimuli, and this procedure was repeated at stepwise increments of 1 mA until SNAPmax was achieved (as defined above). Again, following the acquisition of SNAPmax, the current was lowered until the following amplitudes of SNAP were found: 25, 50, and 75% SNAPmax. The aforementioned pain rating was performed as described above.
Experiment 1: Contralateral LAI
LAI was tested at two TMS intensities whereby the TMS intensity was set to elicit an averaged MEP with peak-to-peak amplitude of ~1 or 0.5 mV in the right FDI muscle. EMG was recorded from the FDI and APB muscles. For each TMS intensity, 20 unconditioned MEPs (i.e., TMS only) and 20 conditioned MEPs were acquired (i.e., nerve stimulation-TMS) for each SNAP percentage (25, 50, 75, or 100% SNAPmax). The order of SNAP percentage delivery was randomized using a William’s square design for each nerve. The orders of TMS intensity (1 and 0.5 mV) and nerve stimulated (MN and DN) were pseudorandomized across participants.
Experiment 2: Ipsilateral LAI
TMS intensity was maintained to evoke an unconditioned MEP of 1 mV. The TMS coil was positioned over the FDI motor hotspot in the left hemisphere for acquiring MEPs from the FDI muscle of the right hand. The ipsilateral (left) arm received DN or MN stimulation in an order pseudorandomized across participants. EMG recordings were recorded from the FDI muscle. SNAPs were acquired as outlined above to obtain 25, 50, 75, and 100% SNAPmax. Twenty unconditioned MEPs were acquired (i.e., TMS alone), and for each SNAP amplitude, 20 conditioned MEPs were acquired.
Statistical Analysis
All MEP data were visually inspected, and any trials that were contaminated with EMG activity before the TMS artifact were discarded. Specifically, a trial was discarded if the peak-to-peak EMG signal within a 160-ms window between the nerve and TMS stimuli was greater than twice the peak-to-peak EMG signal in a 20-ms window before the nerve stimulus. Data for a given participant at a particular SNAP percentage were only included if more than 75% of trials remained following visual inspection. Table 1 shows the number of participants whose data sets were included in the analyses for each SNAP percentage. Group-level analysis included outlier analysis and normality testing via Shapiro-Wilk test. For all normally distributed data, an ANOVA was performed and post hoc tests used Tukey’s tests. For nonnormally distributed data, the data were ranked data and a Conover’s ANOVA was performed whereby post hoc testing used Wilcoxon-signed ranks tests (WSRT) (Conover and Iman 1982). The following statistical analyses were based on the results of the above normality tests. For the analysis of SNAPs, first, the actual SNAP amplitudes recorded corresponding to the intended 25, 50, 75, and 100% SNAPmax bins were computed. The group-averaged SNAP amplitude at each percentage of SNAPmax was compared against neighboring SNAP percentages via WSRT. This analysis was used to determine whether SNAP amplitudes within each percentage bin were statistically different from their neighboring bins. Next, the absolute amplitude of SNAPmax (in µV) was compared between the MN and DN using a two-tailed paired t-test to confirm the larger SNAPs associated with MN as shown previously (Bailey et al. 2016). Finally, the percentage growth of SNAPs was calculated for each bin (25–50, 50–75, and 75–100% SNAPmax) and subject to a two-way Conover’s ANOVA using within-subjects factor INTERVAL (3 levels: 25–50, 50–75, and 75–100%) and NERVE (2 levels; DN, MN).
Table 1.
Data sets included within each condition
| FDI | APB | |||||||||
| Condition | TMS alone | 25% | 50% | 75% | 100% | TMS alone | 25% | 50% | 75% | 100% |
| Contralateral | ||||||||||
| 1-mV MN | 20 | 20 | 20 | 19 | 20 | 19 | 18 | 17 | 18 | 18 |
| 1-mV DN | 19 | 19 | 19 | 19 | 17 | 18 | 18 | 18 | 18 | 18 |
| 0.5-mV MN | 20 | 20 | 20 | 20 | 20 | 19 | 19 | 18 | 19 | 19 |
| 0.5-mV DN | 19 | 19 | 19 | 19 | 19 | 19 | 18 | 19 | 18 | 19 |
| Ipsilateral | ||||||||||
| MN | 16 | 16 | 16 | 16 | 16 | |||||
| DN | 15 | 15 | 15 | 15 | 15 | |||||
FDI, first dorsal interosseous; APB, abductor pollicis brevis; TMS, transcranial magnetic stimulation; MN, median nerve; DN, digital nerve.
For experiment 1, a three-way repeated-measures Conover’s ANOVA was performed using within-subjects factors NERVE (2 levels: DN and MN), MUSCLE (2 levels: FDI and APB), and SNAP (5 levels: TMS alone, 25, 50, 75, or 100% SNAPmax) for each TMS intensity of 1 and 0.5 mV. We note that the unconditioned MEP (i.e., TMS alone) was included in the statistical model above to test for the presence vs. absence of LAI at each SNAP intensity. Identifying the SNAP intensities that yielded statistically significant LAI was an essential component of the research question. All data plotted in the figures are reflective of the unnormalized data used in the statistical analyses. Separate ANOVAs were performed for each TMS intensity (0.5 and 1 mV) to assess the LAI and SNAP relationship within a specific TMS intensity. No statistical comparisons were made between TMS intensities. For experiment 2, a two-way repeated measure Conover’s ANOVA was performed using within-subjects factors NERVE (2 levels: DN and MN) and SNAP (5 levels: TMS alone, 25, 50, 75, or 100% SNAPmax).
Finally, the unconditioned and conditioned MEP amplitudes from the contralateral 0.5 mV TMS condition were plotted and a second-order polynomial trend line was fitted to the data. For all analyses, significance was set to α <0.05. Table 2 provides the results of all statistical analyses.
Table 2.
Conover's ANOVA statistics
| Condition/Factor | Degrees of Freedom | F-Statistic | P Value | Effect Size (partial η2) |
| Contralateral TMS of 1 mV* | ||||
| NERVE | 1, 13† | 5.81† | 0.031† | 0.31† |
| MUSCLE | 1, 13† | 5.04† | 0.043† | 0.28† |
| SNAP | 4, 52† | 21.12† | <0.001† | 0.62† |
| NERVE × MUSCLE | 1, 13 | 0.28 | 0.604 | 0.02 |
| NERVE × SNAP | 4, 52† | 8.74† | <0.001† | 0.40† |
| MUSCLE × SNAP | 4, 52 | 1.07 | 0.361 | 0.07 |
| NERVE × MUSCLE × SNAP | 4, 52 | 1.12 | 0.354 | 0.08 |
| Contralateral TMS of 0.5 mV* | ||||
| NERVE | 1, 15 | 0.78 | 0.390 | 0.07 |
| MUSCLE | 1, 15† | 5.64† | 0.031† | 0.27† |
| SNAP | 4, 60† | 11.16† | >0.001† | 0.43† |
| NERVE × MUSCLE | 1, 15 | 2.12 | 0.16 | 0.12 |
| NERVE × SNAP | 4, 60† | 3.45† | 0.013† | 0.19† |
| MUSCLE × SNAP | 4, 60 | 0.86 | 0.439 | 0.05 |
| NERVE × MUSCLE × SNAP | 4, 60 | 0.41 | 0.718 | 0.03 |
| Ipsilateral** | ||||
| NERVE | 1, 13† | 4.72† | 0.049† | 0.267† |
| SNAP | 4, 52† | 2.86† | 0.032† | 0.181† |
| NERVE × SNAP | 4, 52 | 0.89 | 0.476 | 0.064 |
| DN ipsilateral*** | ||||
| SNAP | 4, 56 | 1.51 | 0.228 | 0.098 |
| MN ipsilateral*** | ||||
| SNAP | 4, 60† | 2.61† | 0.044† | 0.149† |
SNAP, sensory nerve action potential.
Three-way Conover's ANOVA (ranked data).
Two-way Conover's ANOVA (ranked data).
One-way Conover's ANOVA (ranked data).
Significance as shown.
RESULTS
Experiment 1: Contralateral LAI
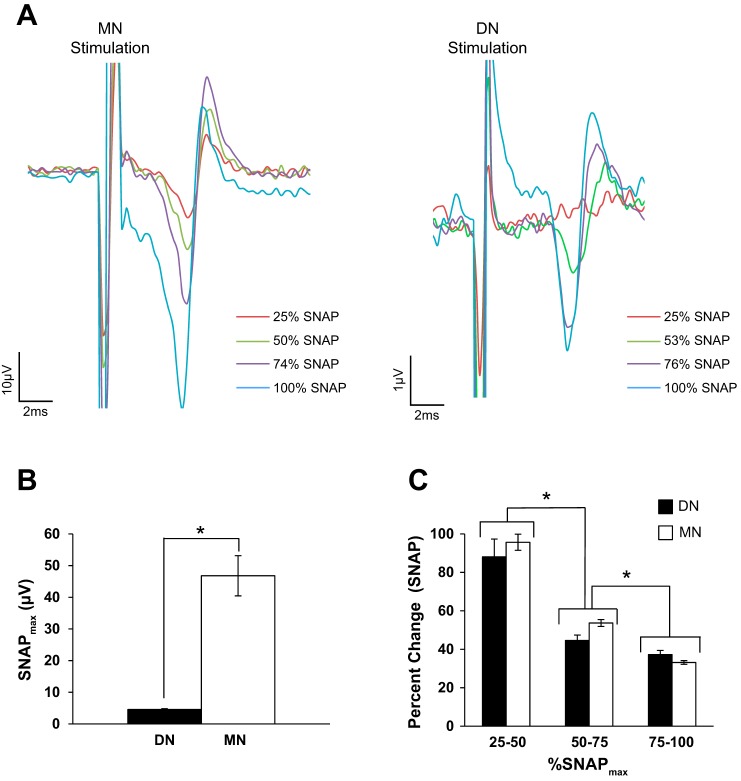
In total, data from 20 and 19 participants were collected for the MN and DN, respectively. One individual opted to not partake in the DN component of this experiment. The precise number of data sets included in the analyses (following trials discarded due to excessive EMG) is listed in Table 1. The actual group-averaged SNAP percentages (±SD) for MN were 25% (±2.2%), 49% (±1.9%), 75% (±2.4%), and 100% SNAPmax and for DN were 29% (±6.9%), 51% (±4.3%), 73% (±4.5%), and 100% SNAPmax, and each SNAP amplitude was significantly different from neighboring percentages (all P < 0.001, WRST). For simplicity, we have used the labels of 25, 50, 75, and 100% on the plots and in our discussion of the results. Three participants for both MN and DN rated the 100% SNAPmax as mild according to the NRS pain scale (NRS average 3.3). No participant rated nerve stimulation as severe (i.e., greater than 7 on the NRS scale). Sample SNAPs from one individual are shown in Fig. 1A following MN (Fig. 1A, left) and DN (Fig. 1A, right) stimulation. The MN SNAPmax was significantly greater than DN SNAPmax as shown in Fig. 1B (P < 0.001, two-tailed paired t-test). Figure 1C displays the percent change in SNAP amplitude with nerve stimulation intensities. A two-way Conover’s ANOVA revealed a main effect of INTERVAL [F(2,30) 72.33, P < 0.001] and NERVE [F(1,15) = 5.20, P = 0.038]. However, post hoc tests indicate no significant difference between nerves (P = 0.195, WSRT). For the main effect of INTERVAL, the largest growth in the SNAP occurred between ~25–50% of SNAPmax (P < 0.001, WSRT). The change in SNAP amplitude, while still significant, was much less between 50 and 75% SNAPmax (P < 0.001, WSRT). Similarly, the change in SNAP amplitude between 75 and 100% SNAPmax was significantly less than the change between 50 and 75% SNAPmax (P < 0.001, WSRT). Therefore, despite the difference in the absolute amplitude of the DN- vs. MN-evoked SNAP, both nerve types display similar growth patterns with the largest changes occurring between 25 and 50% SNAPmax.
Fig. 1.
Contralateral sensory nerve action potential (SNAP). A: average SNAP recorded from 1 participant at each percentage of SNAPmax following median nerve (MN; left) or digital nerve (DN; right) stimulation. The percentages of SNAPmax correspond to that individual’s data and are not the group-averaged percentages of SNAPmax. B: the absolute values (in µV) of group-averaged 100% SNAPmax recorded from the MN and DN. *Significant difference between nerves (Cohen’s d = 2.08). C: the group-averaged percent change of the SNAP for each interval of % SNAPmax. *Significant difference between intervals of % SNAPmax. A significantly greater change in SNAP was seen between 25 and 50% SNAPmax compared with 50 and 75% SNAPmax (Cohen’s d = 1.89). Additionally, the change in SNAP amplitude between 50 and 75% SNAPmax was significantly greater than the change between 75 and 100% SNAPmax (Cohen’s d = 1.54). Furthermore, the SNAP grows similarly for each increment of change between both nerves.
TMS intensity of 1 mV.
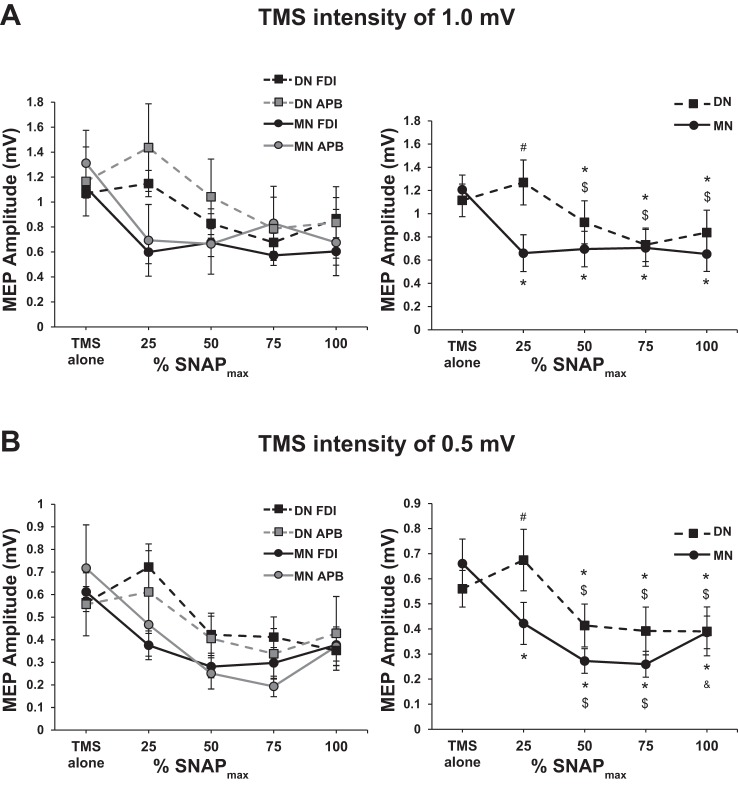
Figure 2A, left, shows the group-averaged MEPs (with SE) for each nerve and muscle. Conover’s ANOVA revealed a main effect of NERVE [F(1,13) = 5.81, P = 0.031], MUSCLE [F(1,13) = 5.04, P = 0.043], SNAP [F(4,52) = 21.12, P < 0.001], and a NERVE × SNAP interaction [F(4,52) = 8.74, P < 0.001] as plotted in Fig. 2A, right. The main effect of muscle revealed that the MEP amplitude from the APB muscle was greater than that of the FDI muscle (P < 0.001, WSRT). The interaction revealed differences between the two nerves at 25% SNAPmax (#P < 0.001, WSRT). Other than at 25% SNAPmax, there was no difference in the magnitude of LAI obtained between nerves. Furthermore, for the MN, LAI existed at all percentages of SNAPmax (*P < 0.001, WSRT) and the maximum depth of LAI was observed by 25% SNAPmax. For the DN, LAI was present at 50, 75, and 100% SNAPmax (*P < 0.01, WRST) and was not increased beyond 50% SNAPmax. Therefore, for both nerve types, once LAI was present it did not increase significantly with additional gains in the SNAP amplitude. Collectively, the data obtained at a TMS intensity of 1 mV indicate that MN and DN LAI do not continue to increase despite the increase in the amplitude of the SNAP.
Fig. 2.
Contralateral long-latency afferent inhibition (LAI). A: transcranial magnetic stimulation (TMS) 1-mV condition: three-way Conover’s ANOVA plot (left) and the NERVE × SNAP interaction (right). Both graphs show the group-averaged motor-evoked potential (MEP) amplitude (±SE) in each condition. #Conditioned MEP amplitude is greater for DN than MN (Cohen’s d = 0.78). *Presence of LAI (i.e., significance difference from TMS alone). $Inhibition greater than that seen at 25% SNAPmax. For the MN, LAI was present at all percentages of SNAPmax (Cohen’s d = 0.85, 0.82, 0.79, and 0.89, respectively). For the DN, LAI was present at 50, 75, and 100% SNAPmax (Cohen’s d = 0.26, 0.62, and 0.38, respectively). For the DN, LAI observed at 25% SNAPmax is significantly smaller than at 50, 75, and 100% SNAPmax (Cohen’s d = 0.41, 0.71, and 0.51, respectively). For the first dorsal interosseous (FDI) muscle, MN stimulation, n = 20, 20, 20, 19, and 20 for each %SNAPmax, respectively. For the FDI muscle, DN stimulation, n = 19, 19, 19, 19, and 17 for each %SNAPmax, respectively. For the abductor pollicis brevis (APB) muscle, MN stimulation, n = 19, 18, 17, 18, and 18, for each %SNAPmax, respectively. For the APB muscle, DN stimulation, n = 18 for all %SNAPmax intensities. B: TMS 0.5-mV condition: three-way ANOVA plot (left) and the NERVE × SNAP interaction (right). Both graphs show the group-averaged MEP amplitude (±SE) in each condition. #Conditioned MEP amplitude is greater for DN than MN (Cohen’s d = 0.55). *Presence of LAI (i.e., significant difference from TMS alone). $Inhibition greater than that seen at 25% SNAPmax. &For the MN, the conditioned MEP amplitude at 100% SNAPmax is greater than at 75% SNAPmax (Cohen’s d = 0.49). For the MN, LAI was present at all percentages of SNAPmax (Cohen’s d = 0.59, 1.12, 1.15, and 0.74, respectively). For the DN, LAI was present at 50, 75, and 100% SNAPmax (Cohen’s d = 0.42, 0.43, and 0.46, respectively). For the MN, LAI observed at 25% SNAPmax is significantly smaller than at 50 and 75% SNAPmax (Cohen’s d = 0.49, 0.52, respectively). For the DN, LAI observed at 25% SNAPmax is significantly smaller than at 50, 75, and 100% SNAPmax (Cohen’s d = 0.57, 0.59, and 0.59, respectively). For the FDI muscle, MN stimulation, n = 20 for all %SNAPmax intensities. For the FDI muscle, DN stimulation, n = 19 for all %SNAPmax intensities. For the APB muscle, MN stimulation, n = 19, 19, 18, 19, and 19, for each %SNAPmax, respectively. For the APB muscle, DN stimulation, n = 19, 18, 19, 18, and 19, for each %SNAPmax, respectively.
TMS intensity of 0.5 mV.
Figure 2B, left, plots the group-averaged MEPs (with SE) for each nerve and muscle. Conover’s ANOVA revealed main effects of MUSCLE [F(1,15) = 5.64, P = 0.031], SNAP [F(3,60) = 11.16, P < 0.001], and a NERVE × SNAP interaction [F(4,60) = 3.45, P = 0.013] shown in Fig. 2B, right. The main effect of muscle revealed that the MEP amplitude from the FDI muscle was greater than that of the APB muscle (WSRT, P < 0.001). The interaction revealed differences between the two nerves at 25% SNAPmax (#P < 0.01, WRST). Other than at 25% SNAPmax, there were no differences in between the nerves. For MN, the interaction revealed that LAI existed at all percentages of SNAPmax (*P < 0.001, WRST) similar to the TMS 1-mV data described above. Additionally, the maximum depth of LAI was observed at 50% SNAPmax and LAI did not grow beyond this intensity. However, in contrast to the TMS 1-mV data above, the depth of MN LAI increased from 25 to 50% SNAPmax (different from 25%; $P < 0.01, WSRT) indicating that the additional contribution of sensory fibers leads to further increases in the magnitude of LAI in this range. Last, for MN stimulation, we note that LAI is decreased from 75 to 100% SNAPmax (&P < 0.01, WSRT). For DN LAI, the results were similar to the TMS 1-mV data. Specifically, LAI was present at 50, 75, and 100% SNAPmax (*P < 0.01, WRST) with no further increases in LAI beyond 50% SNAPmax.
To examine the trend in LAI as a function of current intensity and percentage of SNAPmax, second-order polynomial trend lines were plotted (Fig. 3) for each nerve. Polynomial trends lines were made for the 0.5-mV TMS intensity only since MN LAI in this condition showed a dose-dependent relationship. MT and multiples of ST commonly used in the literature are plotted for comparison. For the MN, MT corresponds with ~50% SNAPmax, while for DN, ST corresponds to ~25% SNAPmax. Two and three times ST correspond to ~50 and 75% SNAPmax, respectively.
Fig. 3.
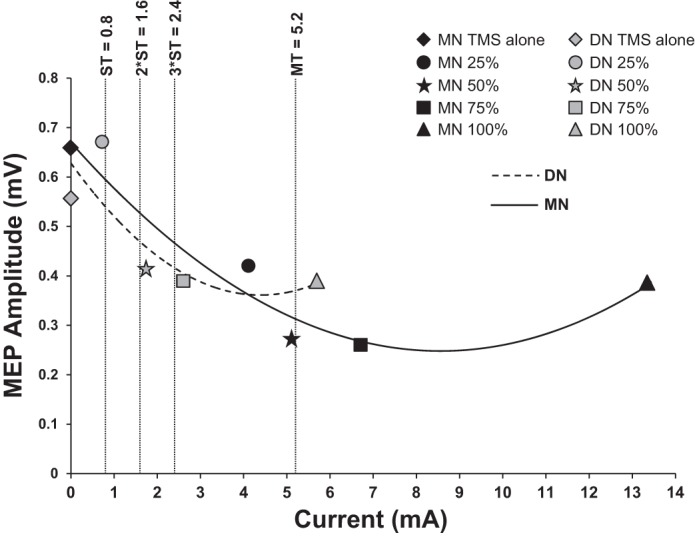
Contralateral polynomial trends (TMS intensity 0.5 mV). Data were averaged across muscles for LAI following contralateral nerve stimulation. Second-order polynomial trend lines show the conditioned MEP amplitude changes following increases in SNAP for each nerve. The conditioned MEP amplitude decreases (i.e., more inhibition) and trends towards a maximum at 50% SNAPmax for both MN and DN. Shown on this graph is the average motor threshold (MT), sensory threshold (ST), 2 × ST, and 3 × ST.
Experiment 2: Ipsilateral LAI
Data from 17 participants was collected in experiment 2. One participant opted not to partake in the MN component, and another did not partake in the DN component of this experiment. Therefore, in total, 16 data sets were collected for each nerve. However, Table 1 displays the precise number of data sets included in each level of the analyses following exclusion of trials based on the inclusion criteria. The actual group-averaged SNAP percentages for MN were 25% (±1.8%), 51% (±2.0%), 75% (±2.5%), and 100% SNAPmax and for DN were 28% (±5.7%), 49% (±4.7%), 75% (±4.5%), and 100% SNAPmax. Each SNAP amplitude was significantly different from neighboring percentages (all P < 0.001, WSRT). Two and three participants rated MN and DN 100% SNAPmax as moderate on the NRS pain scale, respectively (NRS average 5.9). No participants rated nerve stimulation as severe (i.e., >7 on the NRS scale).
Figure 4 plots the group-averaged MEP amplitudes (with SE) at each percentage of SNAPmax. Conover’s ANOVA revealed a main effect of NERVE [F(1,13) = 4.72, P = 0.049] such that the MEP amplitude averaged across stimulus intensities for DN was greater than that observed for MN (WSRT, P = 0.041). Therefore, one-way Conover’s ANOVAs were performed for each nerve separately. For the MN (Fig. 4), there was a main effect of SNAP [F(4,60) = 2.61, P = 0.044] such that LAI occurs at 50, 75, and 100% SNAPmax (all P < 0.05, WSRT). However, the magnitude of inhibition is smaller following ipsilateral stimulation compared with contralateral stimulation (~15% ipsilateral vs. ~40% contralateral). For the DN, there was no effect of SNAP indicating that LAI does not occur following ipsilateral DN stimulation (Fig. 4).
Fig. 4.
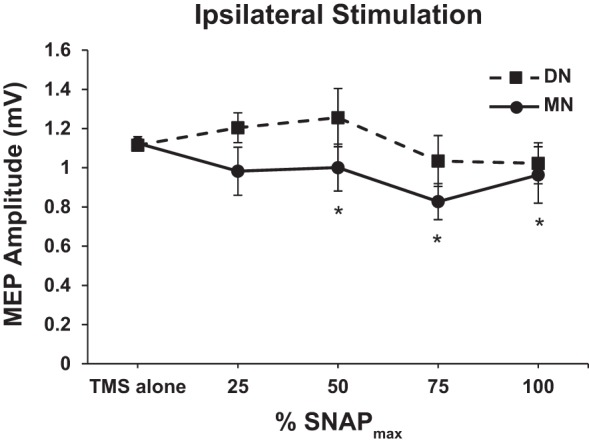
Ipsilateral stimulation (FDI only). Graph of both one-way Conover’s ANOVAs, 1 for each nerve. Group-averaged MEP amplitude (±SE) for each nerve at each percentage of SNAPmax. *Presence of LAI (i.e., significance difference from TMS alone) for ipsilateral MN stimulation at 50, 75, and 100% SNAPmax (Cohen’s d = 0.35, 1.07, 0.39, respectively). LAI was not present at any SNAP amplitude following ipsilateral DN stimulation. For MN stimulation, n = 16 for all %SNAPmax intensities. For DN stimulation, n = 15 for all %SNAPmax intensities.
DISCUSSION
This study examined the relationship between the sensory afferent volley and the depth of LAI. Novel findings include the observation that LAI reflects increases in the volume of the afferent volley up until ~50% SNAPmax for the mixed MN, indicating that LAI increases until presumably all sensory fibers are recruited (see below). Notably, this was only observed for the TMS intensity of 0.5 mV. For the DN, LAI appeared at ~50% SNAPmax and further increases in SNAP did not yield changes in LAI. We also observed that ipsilateral MN but not DN stimulation evokes LAI, although the magnitude of inhibition is quite small compared with the contralateral stimulation (MN: ~15% ipsilateral vs. ~40% contralateral). We discuss these findings and their practical implications to basic and clinical research below.
MN LAI
The MN is a mixed nerve bundle containing both motor efferent and sensory afferent nerve fibers. A previous study examining SNAPs and somatosensory-evoked potentials showed that somatosensory-evoked potentials plateaued at 50% of the MN SNAPmax but continued to increase until 100% of the DN SNAPmax (Bailey et al. 2016). It was speculated that for MN, all sensory afferent fibers were recruited by 50% SNAPmax and subsequent increases in SNAP were contributed by motor efferents (Bailey et al. 2016). Therefore, in this study we speculate that sensory afferents contribute to the MN SNAP until ~50% of the SNAPmax, after which further increases in SNAP may result from the addition of antidromic efferent fibers. Following MN stimulation, we observed LAI at all percentages of SNAPmax, irrespective of TMS intensity. For the TMS intensity of 0.5 mV, the depth of LAI grew from 25 to 50% SNAPmax. It is notable that it is within this range of 25 to 50% SNAPmax when the SNAP itself demonstrated the largest increase (Fig. 1C). That is, where the contribution of sensory afferents was greatest, an increase in LAI was observed. Therefore, we conclude that the magnitude of LAI increases with the added recruitment of sensory afferent fibers. Of note, we did not observe this effect using the stronger 1-mV TMS protocol, which may have masked the subtle effects of the sensory afferent volley that are observable at lower TMS intensities. One explanation is that a TMS intensity that evoked an ~1-mV MEP would activate a larger number of corticospinal neurons compared with an intensity that evoked MEP of 0.5 mV. The afferent volley at a set nerve intensity will activate a given population of neurons within M1. Therefore, for 25% SNAPmax, TMS set to 1 mV may result in the activation of a larger number of neurons that are influenced by the peripheral afferent volley compared with 0.5 mV, thereby leading to maximum LAI for the 1-mV intensity. Increasing TMS intensity from 0.5 to 1 mV would lead to the recruitment of late I waves but also increase the amplitude of early I waves (Di Lazzaro et al. 2012). While SAI predominately inhibits late I waves (Di Lazzaro et al. 2012; Ni et al. 2011), it is not known whether LAI predominately inhibits early or late I waves. How the effects of LAI on different I waves influence the effects of TMS intensity requires further study. Collectively, these data suggest that maximal MN LAI occurs 1) via a TMS intensity to recruit a large population of neurons that are influenced by the sensory afferent volley (i.e., ~1-mV MEP), and 2) by increasing the volume of sensory afferents to achieve the maximum influence on a set population of motor neurons, which may explain the increase in LAI from 25 to 50% for TMS at 0.5 mV.
We observed that the conditioned MEP increased from 75 to 100% SNAPmax (i.e., less inhibition). One explanation for this change may relate to the ascending reafferent signal that occurs following the muscle twitch in APB. Such input may have interfered with the opportunity to observe inhibition. In support of this idea, LAI in the APB muscle is reduced immediately following 15 min of muscle vibration (Lapole and Tindel 2015). It is notable that this occurred for the MN but not the DN stimulation that did not produce muscle twitch. Therefore, the relationship between MN LAI and the sensory afferent volley appears to be very similar to that reported for the SAI circuit whereby SAI increases to ~50% SNAPmax and demonstrates a U-shaped function with modest decreases in SAI beyond 75% SNAPmax (Bailey et al. 2016). Furthermore, we observed that MN stimulation leads to an ~40% inhibition of the MEP for LAI circuit and this is similar to the maximum magnitude of MN SAI (Bailey et al. 2016).
Finally, we observed that LAI occurs following stimulation of the ipsilateral MN at 50% SNAPmax and greater. This is consistent with previous research that showed ipsilateral MN stimulation set to MT is able to elicit LAI (Chen et al. 1999), and in our study 50% SNAPmax aligned with MT (Fig. 3). Chen et al. (1999) showed an ~20% inhibition in the FDI muscle at this ipsilateral nerve stimulation intensity, and our data demonstrates ~10% inhibition at 50% SNAPmax. Slight differences in the amount of inhibition may be attributed to the muscle hotspot targeted [FDI here vs. APB in Chen et al. (1999)].
DN LAI
DN is composed of purely sensory fibers that determine the SNAP amplitude until presumably SNAPmax is achieved (Bailey et al. 2016). Following DN stimulation, LAI was present at ~50% SNAPmax and beyond for both TMS intensities tested. It is notable that ~50% of SNAPmax equated with 2 × ST (Fig. 3), and one study demonstrated that 2 × ST was the minimum intensity to observe DN LAI (Chen et al. 1999). Activation of nerve fibers is dependent on stimulation intensity, such that larger diameter fibers have a lower activation threshold (Hennings et al. 2005). Lower stimulation intensities activate large diameter fibers such as the Aα- and Aβ-afferent fibers, and smaller diameter fibers, such as the sensory Aδ and C fibers, are recruited with increasing stimulus intensities (Boyd and Kalu 1979). It is likely that DN stimulation causes activation of lower threshold (i.e., larger diameter) sensory fibers, leading to DN LAI mediated by these fibers.
Despite the growth in SNAP that was contributed by the recruitment of sensory afferent fibers up to 100% SNAPmax, the depth of LAI did not increase beyond its first appearance at ~50% SNAPmax, a finding that occurred irrespective of TMS intensity. These data indicate that the sensory afferent volley dictates the appearance of LAI such that a certain volume of sensory afferent fibers is required to evoke the circuit (i.e., ~50% SNAPmax and 2 × ST). Therefore, the addition of sensory fibers does not yield concomitant changes in the magnitude of LAI beyond its first appearance. However, we note that DN LAI is reported to be maximum at 3 × ST (Chen et al. 1999), which corresponds to ~75% SNAPmax in our study. Therefore, we conclude that DN LAI is maximum in the range of 2–3 × ST and is not impacted by further recruitment of sensory fibers that act to increase the amplitude of the SNAP. We also note that DN LAI has a different relationship with the sensory afferent volley compared with the DN SAI circuit. DN SAI increases until 100% SNAPmax is achieved (i.e., all cutaneous afferent fibers are recruited) (Bailey et al. 2016). Our data indicate that DN LAI is less reliant on the sensory afferent volley once a minimum afferent volley to activate the circuit is achieved. We did not observe LAI following ipsilateral DN stimulation at any SNAP percentage.
Comparison of LAI Between Nerves
For contralateral nerve stimulation, we did not observe a difference in the magnitude of LAI between the nerve types. That is, when LAI existed for DN, its depth was not different for the MN. This was somewhat surprising since the stimulated MN includes proprioceptive afferents and is responsible for the cutaneous innervation of digits 1, 2, and 3, while DN stimulation was selective for the cutaneous innervation of digit 2 only (Tubbs et al. 2011). However, there were differences between nerves that may indeed be attributed to the difference in sensory afferent volume. First, MN LAI was observed at 25% SNAPmax while a greater afferent volley was required of the DN to reach the threshold for LAI appearance (~50% SNAPmax). Differences in the induction of LAI between the nerves may also be attributed to differences in the composition of the nerve fibers, such as the contributions of muscle spindle afferent fibers in the MN but not in the DN. Muscle spindle afferents provide proprioceptive input that projects directly to M1 from the thalamus or is relayed through area 3a (Huffman and Krubitzer 2001). It is likely that this proprioceptive information will reach M1 quicker than the sensory information obtained by the DN that first project to S1. The differences in timing or path traversed between the types nerves may account for the observation that DN does not demonstrate LAI at 25% SNAPmax. We studied four increments of SNAPmax, and it is possible that smaller increments may have exposed the stimulus-response relationship more precisely for the DN LAI between 25 and 50% SNAPmax (i.e., 35 and 45% SNAPmax). Second, once present, DN LAI does not demonstrate evidence of a response contingent on the sensory afferent volley while MN LAI is increased from 25 to 50% SNAPmax (as described above). Last, we observed ipsilateral MN LAI but not DN LAI, a finding that may be explained by the wider spread cortical termination of the MN vs. DN afferent volley whereby the MN activates somatosensory loci responsive to inputs derived from cutaneous and proprioceptive sources and their combination.
Practical Implications of the Findings
This study provides practical methodological implications for future studies aiming to evoke LAI. The first implication of this research stems from the stimulus-response relationship between the depth of LAI and the SNAP amplitude. For MN stimulation, LAI grew up until 50% SNAPmax, which equated to the stimulus intensity required to obtain MT in the APB muscle (Fig. 3). Therefore, stimulation at MT would be expected to evoke the maximum LAI. Furthermore, studies using DN stimulation to evoke LAI would benefit from nerve stimulation intensities at 50% SNAPmax or greater, as no LAI was observed at 25% SNAPmax in this study. Therefore, based on our results, stimulation at the ST in the index finger would not be expected to evoke LAI. Rather, stimulation at 2–3 × ST would be expected to evoke maximum LAI. A second implication is the use of LAI as marker for recovery following neurological disease or injury. To reveal changes in the magnitude of MN LAI, MN stimulation should be set between 25 and 50% SNAPmax, with a TMS intensity adjusted to a 0.5-mV MEP. For DN LAI, one might consider delivering smaller incremental changes in nerve intensity to achieve SNAPs between 25 and 50% or, alternatively, test both 25% SNAPmax and 50% SNAPmax. In a healthy young population, this would demonstrate the emergence of LAI.
Limitations
We studied LAI using an ISI of 200 ms since this was previously shown to evoke the maximum amount and most consistent magnitude of LAI in the FDI muscle (Chen et al. 1999; Sailer et al. 2002). However, LAI can be evoked from ISIs ranging from 200 to 1,000 ms (Chen et al. 1999). Therefore, these findings may or may not extend to other ISIs. Next, our population tested consisted of healthy young adults, and it is unclear whether the same results would exist in older or special populations. Finally, the majority of our participants were females. While the effects of biological sex on LAI are unknown, it is possible that our observations are driven largely by the female population.
GRANTS
This study is supported by Natural Sciences and Engineering Research Council operating grant (to A. J. Nelson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.V.T., J.E.-S., R.C., and A.J.N. conceived and designed research; C.V.T., J.E.-S., H.J.F., and A.J.N. performed experiments; C.V.T., J.E.-S., and A.J.N. analyzed data; C.V.T., J.E.-S., H.J.F., R.C., and A.J.N. interpreted results of experiments; C.V.T. and J.E.-S. prepared figures; C.V.T., J.E.-S., H.J.F., and A.J.N. drafted manuscript; C.V.T., J.E.-S., H.J.F., R.C., and A.J.N. edited and revised manuscript; C.V.T., J.E.-S., H.J.F., R.C., and A.J.N. approved final version of manuscript.
REFERENCES
- Abbruzzese G, Marchese R, Buccolieri A, Gasparetto B, Trompetto C. Abnormalities of sensorimotor integration in focal dystonia: a transcranial magnetic stimulation study. Brain 124: 537–545, 2001. doi: 10.1093/brain/124.3.537. [DOI] [PubMed] [Google Scholar]
- Bailey AZ, Asmussen MJ, Nelson AJ. Short-latency afferent inhibition determined by the sensory afferent volley. J Neurophysiol 116: 637–644, 2016. doi: 10.1152/jn.00276.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S, Krakauer JW. The uses and interpretations of the motor-evoked potential for understanding behaviour. Exp Brain Res 233: 679–689, 2015. doi: 10.1007/s00221-014-4183-7. [DOI] [PubMed] [Google Scholar]
- Boakye M, Huckins SC, Szeverenyi NM, Taskey BI, Hodge CJ Jr. Functional magnetic resonance imaging of somatosensory cortex activity produced by electrical stimulation of the median nerve or tactile stimulation of the index finger. J Neurosurg 93: 774–783, 2000. doi: 10.3171/jns.2000.93.5.0774. [DOI] [PubMed] [Google Scholar]
- Boyd IA, Kalu KU. Scaling factor relating conduction velocity and diameter for myelinated afferent nerve fibres in the cat hind limb. J Physiol 289: 277–297, 1979. doi: 10.1113/jphysiol.1979.sp012737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns E, Chipchase LS, Schabrun SM. Reduced short- and long-latency afferent inhibition following acute muscle pain: a potential role in the recovery of motor output. Pain Med pii: pnv104, 2016. doi: 10.1093/pm/pnv104. [DOI] [PubMed] [Google Scholar]
- Chen R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp Brain Res 154: 1–10, 2004. doi: 10.1007/s00221-003-1684-1. [DOI] [PubMed] [Google Scholar]
- Chen R, Corwell B, Hallett M. Modulation of motor cortex excitability by median nerve and digit stimulation. Exp Brain Res 129: 77–86, 1999. doi: 10.1007/s002210050938. [DOI] [PubMed] [Google Scholar]
- Conover WJ, Iman RL. Analysis of covariance using the rank transformation. Biometrics 38: 715–724, 1982. doi: 10.2307/2530051. [DOI] [PubMed] [Google Scholar]
- Devanne H, Degardin A, Tyvaert L, Bocquillon P, Houdayer E, Manceaux A, Derambure P, Cassim F. Afferent-induced facilitation of primary motor cortex excitability in the region controlling hand muscles in humans. Eur J Neurosci 30: 439–448, 2009. doi: 10.1111/j.1460-9568.2009.06815.x. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Pennisi MA, Di Giovanni S, Zito G, Tonali P, Rothwell JC. Muscarinic receptor blockade has differential effects on the excitability of intracortical circuits in the human motor cortex. Exp Brain Res 135: 455–461, 2000. doi: 10.1007/s002210000543. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Profice P, Ranieri F, Ricci V, Bria P, Tonali PA, Ziemann U. Segregating two inhibitory circuits in human motor cortex at the level of GABAA receptor subtypes: a TMS study. Clin Neurophysiol 118: 2207–2214, 2007. doi: 10.1016/j.clinph.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Profice P, Ranieri F, Capone F, Dileone M, Oliviero A, Pilato F. I-wave origin and modulation. Brain Stimulat 5: 512–525, 2012. doi: 10.1016/j.brs.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken) 63, Suppl 11: S240–S252, 2011. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- Hennings K, Arendt-Nielsen L, Christensen SS, Andersen OK. Selective activation of small-diameter motor fibres using exponentially rising waveforms: a theoretical study. Med Biol Eng Comput 43: 493–500, 2005. doi: 10.1007/BF02344731. [DOI] [PubMed] [Google Scholar]
- Huffman KJ, Krubitzer L. Thalamo-cortical connections of areas 3a and M1 in marmoset monkeys. J Comp Neurol 435: 291–310, 2001. doi: 10.1002/cne.1031. [DOI] [PubMed] [Google Scholar]
- Korvenoja A, Huttunen J, Salli E, Pohjonen H, Martinkauppi S, Palva JM, Lauronen L, Virtanen J, Ilmoniemi RJ, Aronen HJ. Activation of multiple cortical areas in response to somatosensory stimulation: combined magnetoencephalographic and functional magnetic resonance imaging. Hum Brain Mapp 8: 13–27, 1999. doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukaswadia S, Wagle-Shukla A, Morgante F, Gunraj C, Chen R. Interactions between long latency afferent inhibition and interhemispheric inhibitions in the human motor cortex. J Physiol 563: 915–924, 2005. doi: 10.1113/jphysiol.2004.080010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapole T, Tindel J. Acute effects of muscle vibration on sensorimotor integration. Neurosci Lett 587: 46–50, 2015. doi: 10.1016/j.neulet.2014.12.025. [DOI] [PubMed] [Google Scholar]
- Morgante F, Naro A, Terranova C, Russo M, Rizzo V, Risitano G, Girlanda P, Quartarone A. Normal sensorimotor plasticity in complex regional pain syndrome with fixed posture of the hand. Mov Disord 32: 149–157, 2017. doi: 10.1002/mds.26836. [DOI] [PubMed] [Google Scholar]
- Ni Z, Charab S, Gunraj C, Nelson AJ, Udupa K, Yeh IJ, Chen R. Transcranial magnetic stimulation in different current directions activates separate cortical circuits. J Neurophysiol 105: 749–756, 2011. doi: 10.1152/jn.00640.2010. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pirio Richardson S, Bliem B, Voller B, Dang N, Hallett M. Long-latency afferent inhibition during phasic finger movement in focal hand dystonia. Exp Brain Res 193: 173–179, 2009. doi: 10.1007/s00221-008-1605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer A, Cunic DI, Paradiso GO, Gunraj CA, Wagle-Shukla A, Moro E, Lozano AM, Lang AE, Chen R. Subthalamic nucleus stimulation modulates afferent inhibition in Parkinson disease. Neurology 68: 356–363, 2007. doi: 10.1212/01.wnl.0000252812.95774.aa. [DOI] [PubMed] [Google Scholar]
- Sailer A, Molnar GF, Cunic DI, Chen R. Effects of peripheral sensory input on cortical inhibition in humans. J Physiol 544: 617–629, 2002. doi: 10.1113/jphysiol.2002.028670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer A, Molnar GF, Paradiso G, Gunraj CA, Lang AE, Chen R. Short and long latency afferent inhibition in Parkinson’s disease. Brain 126: 1883–1894, 2003. doi: 10.1093/brain/awg183. [DOI] [PubMed] [Google Scholar]
- Tokimura H, Di Lazzaro V, Tokimura Y, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol 523: 503–513, 2000. doi: 10.1111/j.1469-7793.2000.t01-1-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubbs RS, Rogers JM, Loukas M, Cömert A, Shoja MM, Cohen-Gadol AA. Anatomy of the palmar branch of the ulnar nerve: application to ulnar and median nerve decompressive surgery. J Neurosurg 114: 263–267, 2011. doi: 10.3171/2010.3.JNS091249. [DOI] [PubMed] [Google Scholar]
- Voller B, St Clair Gibson A, Lomarev M, Kanchana S, Dambrosia J, Dang N, Hallett M. Long-latency afferent inhibition during selective finger movement. J Neurophysiol 94: 1115–1119, 2005. doi: 10.1152/jn.00333.2005. [DOI] [PubMed] [Google Scholar]