Abstract
Objective:
Allium elburzense is an endemic plant of the family Amaryllidaceae that grows wild in northern Iran with some nutritional and medicinal applications. The present study was aimed to investigate the fibrinolytic and antioxidant effects of A. elburzense bulb extracts.
Materials and Methods:
Hydroalcoholic, aqueous, chloroformic and butanolic extracts were evaluated in this research. In vitro antioxidant assays were performed using total phenolic, DPPH, and FRAP methods. In the in vivo analysis, animals received i.p. injection of A. elburzense hydroalcoholic extract for 21 days and hydroperoxides level, FRAP value, PT and aPTT were determined in serum samples. The fibrinolytic activity of different extracts was quantitatively evaluated by measurement of clot weight.
Results:
In vitro antioxidant assay showed that A. elburzense aqueous extract had the highest DPPH scavenging and the highest total antioxidant capacity. In the in vivo assay, A. elburzense hydroalcoholic extract reduced serum hydroperoxides level and increased serum total antioxidant capacity in rats. In vitro fibrinolytic assay revealed remarkable thrombolytic activity for this plant with the highest effect for the aqueous extract. However, coagulation parameters including PT and aPTT were not affected by administration of A. elburzense hydroalcoholic extract in rats.
Conclusion:
In conclusion, the results of this study revealed the potential antioxidant and fibrinolytic effects of A. elburzense bulb extracts. For developing novel thrombolytic agents, further investigations for isolation of bioactive constituents and finding the underlying mechanisms are suggested.
Key Words: Allium elburzense Wendelbo, Antioxidant, Fibrinolytic agents
Introduction
Venous thromboembolism consisting of serious and life-threating complications such as deep vein thrombosis, post-thrombotic syndrome and pulmonary embolism, is of great clinical concern worldwide. This common preventable disorder contributes to long-term morbidity and high cost of health-care (Kesieme et al., 2011 ▶). Surgery, prolonged bed rest, congestive heart failure, pregnancy, nephrotic syndrome, cancer, obesity, chemotherapy and oral contraceptive therapy are some of important risk factors for venous thromboembolism (Anderson et al., 2003 ▶).
In spite of different pharmacological treatments including antithrombotic and fibrinolytic drugs, venous thromboembolism remains a major cause of mortality and disability. Therefore, investigations for finding efficacious and safe antithrombotic drugs with low risk of bleeding are ongoing (Sikka et al., 2010 ▶). Recent studies have focused on herbal medicines as one of the potential sources of prevention and treatment of venous thromboembolism (Kim et al., 2015 ▶).
Allium is the major and most important genus of Amaryllidaceae family with more than 800 species in the world (Neshati et al., 2009 ▶). Some of important species such as onion, garlic and scallion have been used as foods, spices and medicine for a long time. Allium species have many biological effects and high potential for prevention and treatment of cardiovascular diseases including hypercholesterolemia, hypertension and atherosclerosis (Lister et al., 2007 ▶). Some species of this genus have been evaluated as possible anti-platelet-aggregation and antithrombotic agents (Srivastava et al., 1993 ▶; Hiyasat et al., 2009 ▶).
Allium elburzense Wendelbo is an endemic plant growing in the Alburz Mountains in northern Iran. This plant grows and blooms from April to May and its aerial parts are usually used as food. It has been traditionally used as an antidiabetic, antirheumatic, antihelminthic and aphrodisiac medicine. However, limited information has been reported on the pharmacological properties of A. elburzense (Zolfaghari et al., 2012 ▶).
The present study was aimed to investigate the anticoagulant, fibrinolytic and antioxidant effects of A. elburzense bulb extract.
Materials and Methods
Chemicals
Streptokinase (SK) was purchased from Karma-Kinase Pharmatech GmbH (Germany). The standard assay kits for evaluation of 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging, hydroperoxides concentration and ferric reducing antioxidant power (FRAP) assay were purchased from Hakiman Shargh Research Co. (Isfahan, Iran). Folin-Ciocalteu and all other reagents were purchased from Merck Co. (Mumbai, India).
Plant material and preparation of extracts
The bulbs of A. elburzense were collected from the Mount Damavand in the Alburz Mountains, Tehran province of Iran in March 2015. After identification of the plant by a Botanist (Dr Iraj Mehregan, Department of Biology, Science and Research Branch, Islamic Azad University, Tehran, Iran), a voucher specimen (No. 1145) was deposited at the Herbarium of the School of Pharmacy and Pharmaceutical Sciences, Isfahan, Iran. For preparation of hydroalcoholic extract, the air-dried bulbs of the plant (1200 g) were powdered and extracted with ethanol: water (70:30) using percolation method for 48 hr at room temperature. After filtration of the extract, the solvent was removed using a rotary evaporator and the extract was freeze-dried (Zolfaghari et al., 2015 ▶).
For preparation of other extracts, dried ground bulbs (100 g) were sequentially extracted with hexane, chloroform, chloroform-methanol (9:1), water and butanol. After removal of saccharides, amino acids and solvents through aqueous solvent, the viscous residues of different extracts were obtained. All extracts were stored at -20 ºC (Zolfaghari et al., 2012 ▶).
Animals
Male Wistar albino rats weighing 180 to 220 g were obtained from the animal house of the School of Pharmacy and Pharmaceutical Sciences, Isfahan, Iran. The animals had free access to water and standard rodent diet and were kept under standard laboratory condition with a 12 hr light/12 hr dark cycle. Rats were acclimatized for 1 week before the experiment. The experiment was conducted according to the international guidelines for laboratory animal use and care. For in vivo study, rats were randomly divided into 5 groups of 6 rats. Animals received daily intraperitoneal (i.p.) injections of A. elburzense hydroalcoholic extract (100, 200 and 400 mg/kg) or vitamin C (30 mg/kg, as a positive control) for 21 days (Zolfaghari et al., 2012 ▶; Kini et al., 2011 ▶; Onoja et al., 2014 ▶). Normal saline (i.p.) was used as the negative control. After the 21st day, blood was collected through direct cardiac puncture under mild ether anesthesia and serum samples were used for further experiments.
In vitro antioxidant assay
Total phenolic assay
Total phenolic content of the A. elburzense hydroalcoholic extract was determined using Folin-Ciocalteu reagent as previously described by Everette and coworkers (Everette et al., 2010 ▶). Results were obtained using a standard curve plotted based on different concentrations of gallic acid and expressed as milligram of gallic acid equivalent (GAE) /g of the dried plant extract.
DPPH radical scavenging assay
Free radical scavenging activity of all extracts was analyzed using 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging method. Different concentrations of plant extracts (100 µl of 25-1000 µg/ml) were mixed with 100 µl of methanolic solution of DPPH (100 µM). The absorbance was measured at 517 nm after 30 min incubation in the dark at room temperature. The percentage of free radical inhibition was calculated using the formula:
[(A0-A1)/A0] × 100
where A0 is the absorbance of the control (containing all reagents except the test compound), and A1 is the absorbance of the extract/standard. Ascorbic acid was used as the reference standard (Iwalewa et al., 2008 ▶). The half maximal inhibitory concentration (IC50) was calculated through a series of dose-response data and using an equation which was fitted to the curve.
FRAP assay
The total antioxidant capacity of different plant extracts was determined by ferric reducing antioxidant power (FRAP) method. This assay is based on the reduction of ferric-tripyridyltriazine complex to ferrous form by colorimetric method. Briefly, the FRAP reagent (200 µl) containing tripyridyltriazine/ferric chloride/acetate buffer was freshly prepared according to the manufacturer’s protocol and added to 10 µl of the extracts (25-1000 µg/ml). The mixture was incubated for 40 min at 37 °C and then the absorbance of colored solutions was measured at 570 nm using a spectrophotometer (Bio-Tek, PowerWave XS, USA). The FRAP value of samples was calculated using a standard curve of FeSO4. 7H2O and expressed as µM of ferrous sulfate equivalents per liter (Benzie et al., 1996 ▶).
In vivo antioxidant assay FOX1 assay
The effect of hydroalcoholic extract of A. elburzense on serum hydroperoxides level was determined based on the ferrous ion oxidation by xylenol orange reagent (FOX1) method. Briefly, the FOX-1 reagent (200 µl) containing ammonium ferric sulfate was freshly prepared in aqueous medium with sorbitol according to the manufacturer’s protocol. Then, serum samples (10 µl) were mixed with the reagent and after incubation for 30 min at 37ºC, the absorbance of solutions was measured at 540 nm using a spectrophotometer (Bio-Tek, PowerWave XS, USA). The hydroperoxides concentration in serum samples was calculated using a standard curve of hydrogen peroxide (Wolf et al., 2014 ▶).
FRAP assay
The effect of hydroalcoholic extract of A. elburzense on serum total antioxidant capacity was evaluated by FRAP method as described previously in in vitro assay.
PT and aPTT assay
Prothrombin time (PT) and activated partial thromboplastin time (aPTT) were determined using plasma within two hours of sample collection by standard hematological methods as described by Dacie and Lewis (Dacie et al., 1995 ▶).
In vitro fibrinolytic assay
The fibrinolytic activity was quantitatively evaluated by measurement of clot weight. Whole blood samples were collected from healthy human volunteers (n = 10) (aged 20-23 years) without a history of oral contraceptive or anticoagulant therapy after taking informed written consent. The blood specimen (400 µl) was moved to previously-weighed sterilized micro-centrifuge tubes and incubated at 37˚C for 30 min to form clots. After formation of clot, serum was completely removed without disturbing the clot and each tube was again weighed to determine the weight of the clot. For evaluation of thrombolytic activity, 100 μl of different plant extracts (5mg/ml) was added to micro-centrifuge tubes containing the clots. Streptokinase (SK; 30,000 I.U.; equivalent to its IC50) was used as a positive control and sterilized normal saline was considered as a negative non-thrombolytic control. After incubation of all tubes at 37ºC for 90 minutes, the released fluid was removed from the tubes and they were again weighed and the percentage of clot lysis was calculated (Ali et al., 2014 ▶).
Statistical analysis
Data were represented as the mean±SEM. For statistical significance, a one-way analysis of variance (ANOVA) followed by Tukey post-hoc test was used (SPSS software version 16.0). A P<0.05 was considered significant.
Results
Plant extracts
The yield of the plant extract was 27.5 % (w/w) for hydroalcoholic, 3.15 % (w/w) for aqueous, 2.43 % (w/w) for chloroformic and 2.51 % (w/w) for butanolic extract.
In vitro antioxidant experiments
Total phenolic assay
Evaluation of total phenolic content of the A. elburzense hydroalcoholic extract showed 33.52 ± 1.3 mg GAE/g of dried bulbs of the plant extract.
DPPH radical scavenging assay
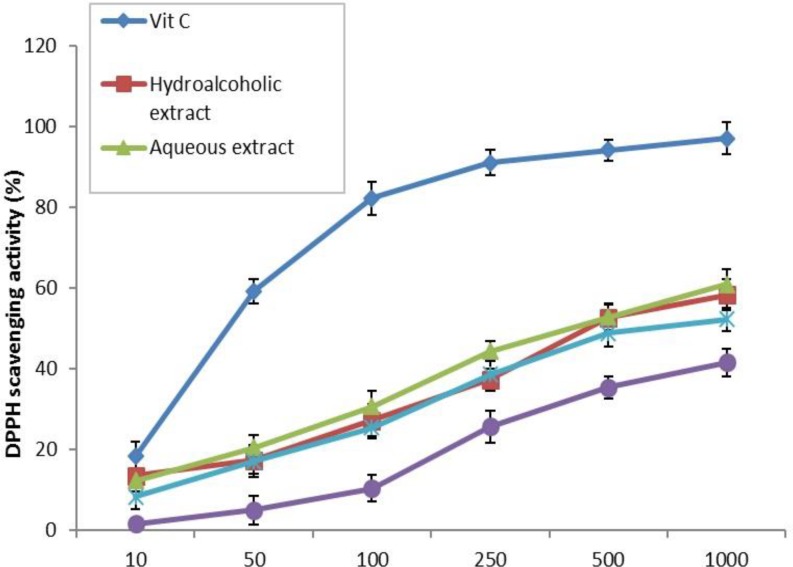
Antiradical activity of A. elburzense extracts was determined by DPPH scavenging test (Figure 1). IC50 of vitamin C as a standard antioxidant, was 0.034 mg/ml. The scavenging activity of different extracts was in the following order: aqueous extract (IC50 = 0.49 mg/ml) > hydroalcoholic extract (IC50 = 0.57 mg/ml) > chloroformic extract (IC50 = 0.94 mg/ml) > butanolic extract (IC50 = 1.68 mg/ml).
Figure 1.
Scavenging activity of hydroalcoholic, aqueous, chloroformic and butanolic extracts of A. elburzense bulb and vitamin C (25-1000 µg/ml) against 1,1-diphenyl-2-picrylhydrazyl (DPPH). Results are means + SEM of three independent experiments
FRAP assay
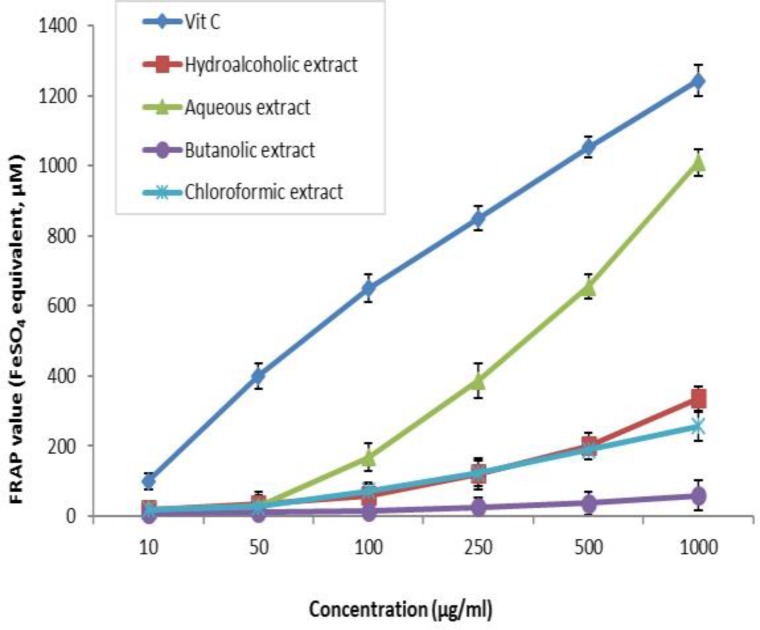
The total antioxidant capacity of different A. elburzense extracts was determined by ferric reducing antioxidant power (FRAP) method and expressed as the equivalents of ferrous sulfate. The extract showed a concentration-dependent increase in total antioxidant capacity (Figure 2). The antioxidant activity of plant extracts was in the following order: aqueous extract> hydroalcoholic extract ≈ chloroformic extract > butanolic extract.
Figure 2.
FRAP values of hydroalcoholic, aqueous, chloroformic and butanolic extracts of A. elburzense and vitamin C (25-1000 µg/ml) determined as ferrous sulfate equivalents. Values are means + SEM of three independent experiments
In vivo antioxidant experiments
FOX1 assay
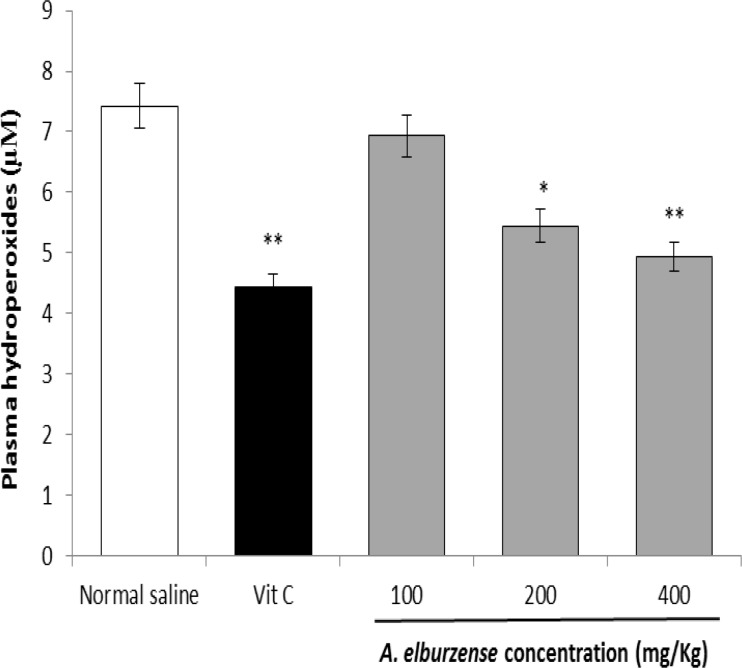
The effect of hydroalcoholic extract of A. elburzense on rats serum hydroperoxides level was determined using FOX1 Method. Administration of vitamin C as a reference standard, reduced serum hydroperoxides compared to the negative control rats (P<0.001). A. elburzense hydroalcoholic extract also significantly decreased serum hydroperoxides level at the doses of 200 and 400 mg/kg (P<0.5 and P<0.01, respectively) (Figure 3).
Figure 3.
Effects of 21-day administration of A. elburzense hydroalcoholic extract (100-400 mg/kg) and vitamin C (30 mg/kg) on plasma hydroperoxides concentrations (as H2O2 equivalents) determined by FOX1 method. Values are means + SEM of six rats (*P<0.05, **P<0.01 and ***P<0.001 versus normal saline-treated control group
FRAP assay
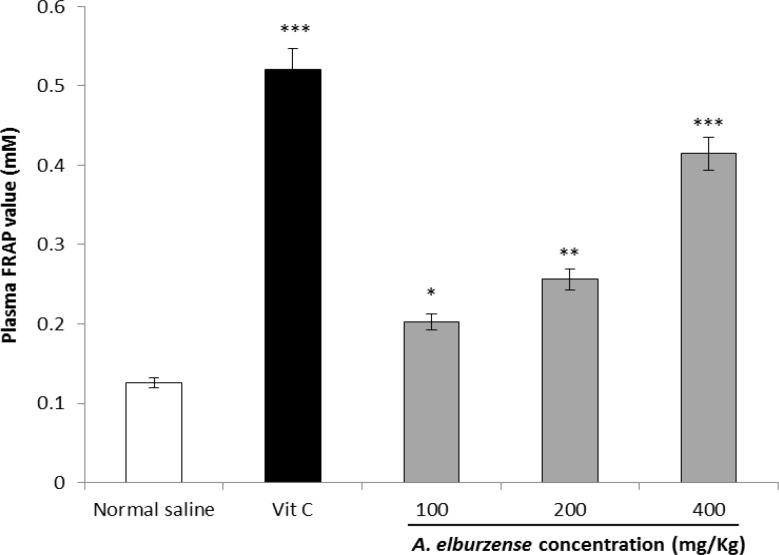
In the in vivo analysis, 21-day administration of vitamin C significantly elevated serum total antioxidant capacity compared to the control group (P<0.001). A. elburzense hydroalcoholic extract also increased the FRAP value dose-dependently (Figure 4).
Figure 4.
Effects of 21-day administration of A. elburzense hydroalcoholic extract (100-400 mg/kg) and vitamin C (30 mg/kg) on plasma FRAP value determined as ferrous sulfate equivalents. Values are means + SEM of six rats (**P<0.01 and ***P<0.001 versus normal saline-treated control group
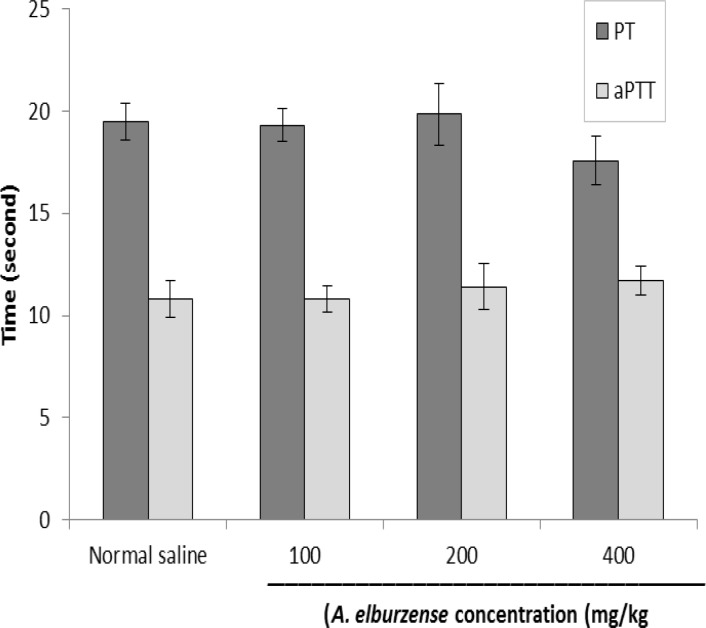
PT and aPTT assay
Administration of different doses of A. elburzense hydroalcoholic extract had no effect on coagulation factors PT and aPTT in the 21-day experiment (Figure 5).
Figure 5.
Effects of 21-day administration of A. elburzense hydroalcoholic extract (100-400 mg/kg) on coagulation parameters (PT and aPTT). Values are means + SEM of six rats
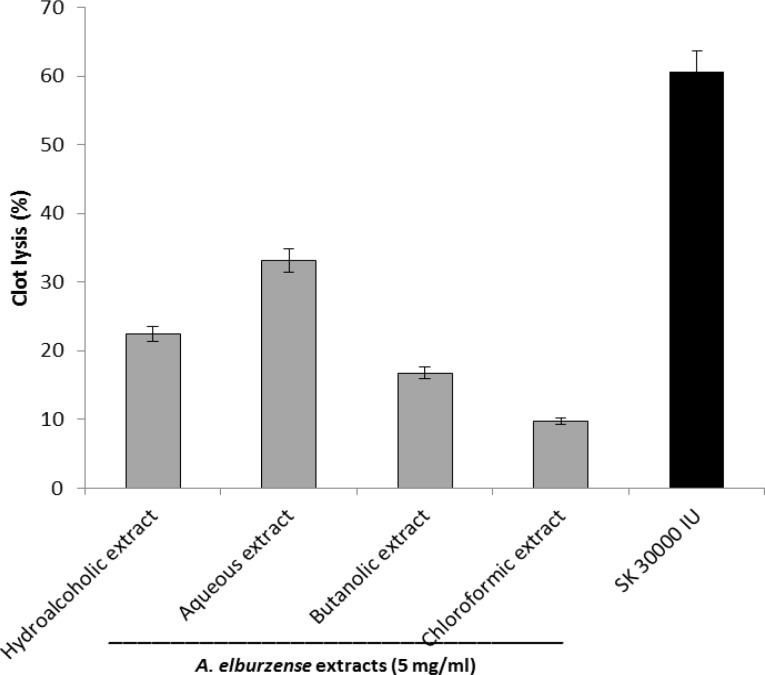
In vitro fibrinolytic assay
Streptokinase as a positive control (30,000 I.U.) caused 60.59% clot lysis after incubation for 90 min at 37°C. The thrombolytic activity of different extracts of the plant was in the following order: aqueous extract (33.11%)> hydroalcoholic extract (22.40%)> butanolic extract (16.75) > chloroformic (9.77%) extract (Figure 6).
Figure 6.
Thrombolytic activity of hydroalcoholic, aqueous, chloroformic and butanolic extracts of A. elburzense and streptokinase (SK; 30,000 I.U.) determined by measurement of clot weight. Values are means + SEM of three independent experiments
Discussion
Herbal medicine as a source of numerous bioactive compounds, is nowadays employed to alleviate various disease conditions (Babaee et al., 2016 ▶; Ghadirkhomi et al., 2016 ▶). A. elburzense is an endemic plant of northern Iran with nutritional and medicinal applications. However, pharmacological activities of this plant have not been completely studied (Zolfaghari et al., 2012 ▶). This study evaluated the anticoagulant, fibrinolytic and antioxidant effects of A. elburzense bulb extracts.
In vitro antioxidant analysis of different extracts of the plant showed that A. elburzense aqueous extract had the highest DPPH scavenging and the highest total antioxidant capacity . In the in vivo analysis, A. elburzense hydroalcoholic extract reduced serum hydroperoxides level and increased serum total antioxidant capacity in rats. In vitro fibrinolytic assay also showed that A. elburzense aqueous extract had the highest thrombolytic activity . However, coagulation parameters including PT and aPTT were not affected by administration of A. elburzense hydroalcoholic extract in rats.
The antioxidant activities of various Allium spp have been reported. Garlic (Allium sativum) as a popular plant of Amaryllidaceae family, is rich in antioxidants. This plant has been widely investigated for prevention and treatment of many diseases associated with oxidative stress (Neil et al., 1994 ▶). Various antioxidant activities including ROS scavenging, increasing the cellular antioxidants (catalase, superoxide dismutase, glutathione peroxidase and glutathione), inhibition of lipid peroxidation and LDL oxidation, and protection of endothelial cells against oxidative damage have been reported for aged garlic extract (Borek et al., 2001 ▶). The antioxidant effects of Allium species may be attributed to different bioactive constituents including sulfur-containing compounds microelements, dietary fibers and polyphenols (Lanzotti., 2006 ▶; Gorinstein et al., 2005 ▶). The presence of flavonoids, phenols, steroids, glycosides and saponins has been reported in total extract of A. elburzense (Zolfaghari et al., 2012 ▶). In our study, the highest antioxidant effect of aqueous A. elburzense extract may be mainly due to the presence of phenolic and water-soluble organosulfur compounds in the aqueous soluble fraction.
Allium spp. especially garlic, also have valuable fibrinolytic and anti-platelet activities (Elsabban., 2009 ▶). Some bioactive components such as adenosine, allicin and thiosulfinates rgar are present in garlic are responsible for fibrinolysis effect, preventing platelet aggregation and inhibiting thromboxane formation (Ackerman et al., 2001 ▶). Steroid saponins from garlic and other Allium species are also involved in their anticoagulant effects (Matsuura et al., 2001 ▶). It has been reported that garlic does not change PT or PTT because it exerts its anticoagulant activity through interfering with platelet aggregation not with coagulation cascade (Fugh-Berman., 2000 ▶). Similarly, the results of our study showed no alteration in PT or PTT suggesting that the extrinsic and intrinsic pathways of coagulation were not affected by A. elburzense extract in rats.
The results of investigation for finding antithrombotic agents have led to the isolation of steroid saponins with fibrinolytic potential including proto-isoeruboside-B and isoeruboside-B from garlic (Matsuura et al., 2001 ▶). Yoshikawa and co-workers isolated two novel fibrinolytic saponins including lucyosides N and P from the seeds of Luffa cylindrica (Cucurbitaceae) (Yoshikawa et al., 1991 ▶). Anti-thrombotic activities have been reported for diosgenyl saponins extracted from the rhizome of Dioscorea zingiberensis through inhibition of factor VIII activities and platelet aggregation (Zhang et al., 2013 ▶). Several sapogenins and new saponins namely elburzensoides have also been isolated from the bulb of A. elburzense (Barlie et al., 2004 ▶; Barlie et al., 2005 ▶).
Moreover, some other recognized compounds of A. elburzense, such as flavonoids and sulfuric compounds may be responsible for its fibrinolytic effect (Zolfaghari et al., 2012 ▶). Dar and his coworkers showed thrombolytic activity of a flavonoid compound through blocking an enzyme protein disulfide isomerase which was participated in blood clotting process (Dar et al., 2012). Some flavonoids isolated from the roots of Scutellaria baicalensis such as baicalein have shown fibrinolytic effect in cellular model (Kimura et al., 1997 ▶). Kaempferol is a flavonol with fibrinolytic potential which has been found in various plants including Allium spp. (Barlie et al., 2005 ▶, Rajput et al., 2011 ▶).
In conclusion, this study revealed the beneficial antioxidant and fibrinolytic effects of A. elburzense bulb extracts. Further investigations are needed for isolation of bioactive constituents responsible for thrombolytic activity of this plant and finding the underlying mechanisms and determining the therapeutic value of this herbal medicine.
Acknowledgments
The content of this paper was extracted from a Pharm.D thesis. Authors would like to thank Vice Chancellor of Research of Isfahan University of Medical Sciences, for the financial support (research project No. 394403).
Conflict of interest
The authors declare no conflicts of interest.
References
- Ackermann RT, Mulrow CD, Ramirez G, Gardner CD, Morbidoni L, Lawrence VA. Garlic shows promise for improving some cardiovascular risk factors. Arch Intern Med. 2001;161:813–824. doi: 10.1001/archinte.161.6.813. [DOI] [PubMed] [Google Scholar]
- Ali R, Hossain M, Runa JF, Hasanuzzaman M, Islam M. Evaluation of thrombolytic potential of three medicinal plants available in Bangladesh, as a potent source of thrombolytic compounds. Avicenna J Phytomed. 2014;4:430–436. [PMC free article] [PubMed] [Google Scholar]
- Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107:9–16. doi: 10.1161/01.CIR.0000078469.07362.E6. [DOI] [PubMed] [Google Scholar]
- Babaee F, Safaeian L, Zolfaghari B, Haghjoo Javanmard S. Cytoprotective effect of hydroalcoholic extract of Pinus eldarica bark against H2O2-induced oxidative stress in human endothelial cells. Iran Biomed J. 2016;20:161–167. doi: 10.7508/ibj.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barile E, Zolfaghari B, Sajjadi SE, Lanzotti V. Saponins of Allium elburzense. J Nat Prod. 2004;67:2037–2042. doi: 10.1021/np0497752. [DOI] [PubMed] [Google Scholar]
- Barile E, Capasso R, Izzo A, Lanzotti V, Sajjadi SE, Zolfaghari B. Structure-activity relationships for saponins from Allium hirtifolium and Alliumelburzense and their antispasmodic activity. Planta Med. 2005;71:1010–1018. doi: 10.1055/s-2005-873134. [DOI] [PubMed] [Google Scholar]
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Borek C. Antioxidant Health Effects of Aged Garlic Extract. J Nutr. 2001;131:1010–1015. doi: 10.1093/jn/131.3.1010S. [DOI] [PubMed] [Google Scholar]
- Dacie JV, Lewis SM. practical haematology. 8th ed. New York: Churchill Livingstone; 1996. Tests for acute phase response; pp. 559–563. [Google Scholar]
- El-Sabban F. Garlic as an antithrombotic and antiplatelet aggregation agent. J Chin Clin Med. 2009;4:288–294. [Google Scholar]
- Everette JD, Bryant QM, Green AM, Abbey YA, Wangila GW, Walker RB. Thorough study of reactivity of various compound classes toward the Folin-Ciocalteu reagent. J Agric Food Chem. 2010;58:8139–8144. doi: 10.1021/jf1005935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugh-Berman A. Herbs and dietary supplements in the prevention and treatment of cardiovascular disease. Prev Cardiol. 2000;3:24–32. doi: 10.1111/j.1520-037x.2000.80355.x. [DOI] [PubMed] [Google Scholar]
- Ghadirkhomi A, Safaeian L, Zolfaghari B, Agha Ghazvini MR, Rezaei P. Evaluation of acute and sub-acute toxicity of Pinus eldarica bark extract in Wistar rats. Avicenna J Phytomed. 2016;6:558–566. [PMC free article] [PubMed] [Google Scholar]
- Gorinstein S, Drzewiecki J, Leontowicz H, Leontowicz M, Najman K, Jastrzebski Z, et al. Comparison of the bioactive compounds and antioxidant potentials of fresh and cooked Polish, Ukrainian and Israeli garlic. J Agric Food Chem. 2005;53:2726–2732. doi: 10.1021/jf0404593. [DOI] [PubMed] [Google Scholar]
- Hiyasat B, Sabha D, Grotzinger K. Antiplatelet activity of Allium ursinum and Allium sativum. Pharmacology. 2009;83:197–204. doi: 10.1159/000196811. [DOI] [PubMed] [Google Scholar]
- Iwalewa EO, Adewale IO, Taiwo BJ. Effects of Harungana madagascariensis stem bark extract on the antioxidant markers in alloxan induced diabetic and carrageenan induced inflammatory disorders in rats. J Complement Integr Med. 2008;5:1–2. [Google Scholar]
- Kesieme E, Kesieme C, Jebbin N, Irekpita E, Dongo A. Deep vein thrombosis: a clinical review. J Blood Med. 2011;2:59–69. doi: 10.2147/JBM.S19009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Lee KM, Hong ND, Jung YS. Anti-platelet and anti-thrombotic effect of a traditional herbal medicine Kyung-Ok-Ko. J Ethnopharmacol. 2015;3:172–179. doi: 10.1016/j.jep.2015.11.040. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Okuda H, Ogita Z. Effects of flavonoids isolated from Scutellariae radix on fibrinolytic system induced by trypsin in human umbilical vein endothelial cells. J Nat Prod. 1997;60:598–601. doi: 10.1021/np970035l. [DOI] [PubMed] [Google Scholar]
- Kini RD, Tripathi Y, Raghuveer CV, Pai SR, Ramaswamy C, Kamath P. Role of vitamin c as an antioxidant in cadmium chloride induced testicular damage. Int J Appl Biol Pharm Technol. 2011;2:484–488. [Google Scholar]
- Lanzotti V. The analysis of onion and garlic. J Chromatogr A. 2006;1112:3–22. doi: 10.1016/j.chroma.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Lister CE, Hedges LJ. The nutritional attributes of Allium species. 2007;1814:1–44. [Google Scholar]
- Matsuura H. Saponins in garlic as modifiers of the risk of cardiovascular disease. J Nutr. 2001;131:1000–1005. doi: 10.1093/jn/131.3.1000S. [DOI] [PubMed] [Google Scholar]
- Neil A, Silagy C. Garlic: Its cardioprotective properties. Curr Opin Lipidol. 1994;5:6–10. doi: 10.1097/00041433-199402000-00002. [DOI] [PubMed] [Google Scholar]
- Neshati F, Fritsch RM. Seed characters and testa sculptures of some Iranian Allium L species (Alliaceae) Feddes Repert . 2009;120:322–332. [Google Scholar]
- Onoja SO, Omeh YN, Ezeja MI, Chukwu MN. Evaluation of the in vitro and in vivo antioxidant potentials of Aframomum melegueta methanolic seed extract. J Trop Med. 2014;2014:159343. doi: 10.1155/2014/159343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajput MS, Mathur V, Agrawal P, Chandrawanshi HK, Pilaniya U. Fibrinolytic activity of kaempferol isolated from the fruits of Lagenaria siceraria (Molina) Standley. Nat Prod Res. 2011;25:1870–1875. doi: 10.1080/14786419.2010.540760. [DOI] [PubMed] [Google Scholar]
- Sikka P, Bindra VK. Newer antithrombotic drugs. Indian J Crit Care Med. 2010;14:188–195. doi: 10.4103/0972-5229.76083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava KC, Tayagi OD. Effects of a garlic derived principle (Ajoene) on aggregation and arachidonic acid metabolism in human blood platelets. Prostaglandins Leukot Essent Fatty Acids. 1993;49:587–595. doi: 10.1016/0952-3278(93)90165-s. [DOI] [PubMed] [Google Scholar]
- Wolf SP. Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol. 1994;233:182–189. [Google Scholar]
- Yoshikawa K, Arihara S, Wang JD, Narui T, Okuyama T. Structures of two new fibrinolytic saponins from the seed of Luffa cylindrica Roem. Chem Pharm Bull (Tokyo) 1991;39:1185–1188. doi: 10.1248/cpb.39.1185. [DOI] [PubMed] [Google Scholar]
- Zhang R, Huang B, Du D, Guo X, Xin G, Xing Z et al. Anti-thrombosis effect of diosgenyl saponins in vitro and in vivo. Steroids. 2013;78:1064–1070. doi: 10.1016/j.steroids.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Zolfaghari B, Shokoohinia Y, Ramezanlou P, Sadeghi A, Mahmoudzadeh M, Minaiyan M. Effects of methanolic and butanolic fractions of Allium elburzense Wendelbo bulbs on blood glucose level of normal and STZ-induced diabetic rats. Res Pharm Sci. 2012;7:201–207. [PMC free article] [PubMed] [Google Scholar]
- Zolfaghari B, Kazemi M, Nematbakhsh M. The effects of unripe grape extract on systemic blood pressure and serum levels of superoxide dismutase, malondialdehyde and nitric oxide in rat. Adv Biomed Res. 2015;4:109. doi: 10.4103/2277-9175.157822. [DOI] [PMC free article] [PubMed] [Google Scholar]