Abstract
Objective:
Postmenopausal osteoporosis is characterized by increased fracture risk. However, each approved treatment has specific side effects. Therefore, foods with plant origins have increasingly attracted attention as an alternative treatment. Studies have shown that Elaeagnus angustifolia (EA) has antioxidant properties. The present study aimed to investigate the effects of EA hydroalcoholic extract on ovariectomy-induced osteoporosis in rats using stereological methods.
Material and Methods:
55 female Sprague-Dawley rats were randomly assigned to control, sham operated (normal saline), ovariectomized (OVX), OVX + EA fruit extract (600 mg/kg BW/day), and OVX + estradiol benzoate (3 mg/kg BW) for 16 weeks. Blood samples were collected to measure calcium, phosphorus, and alkaline phosphatase (ALP) plasma levels. Then, specimens from tibia and fifth lumbar vertebra (L5) bones were prepared and stereological analysis was done.
Results:
Ovariectomy significantly decreased the calcium level and increased the ALP level in the OVX group. In spite of improvement in calcium hemostasis in groups treated with estrogen and EA fruit extract (p<0.05), only treatment with estrogen was able to reduce ALP levels. Moreover, treatment with EA fruit extract and estrogen caused a significant increase in the number of osteoblasts in vertebra and tibia compared to the OVX group (p<0.05). Estrogen and EA fruit extract were also able to reduce the number of osteoclasts in tibia of the treated OVX rats (p<0.05).
Conclusion:
The results showed that EA extract exerted more effects, markedly, on osteoblastogenesis in the OVX rats. Thus, it could be considered as a potential agent to treat patients with osteoporosis.
Key Words: Elaeagnus angustifolia, Stereology, Osteoporosis, Tibia bone, ALP, Rat
Introduction
Osteoporosis is characterized by low bone mineral density, structural deterioration of bone microarchitecture, and increased fracture risk (Jeddi et al., 2013 ▶). Hypoestrogenemia is an important cause of osteoporosis, which induces loss of bone mass in long bones and lumbar vertebrae (Gatta et al., 2015 ▶). Bone continually undergoes remodeling mediated by coordinated activities of bone resorbing cells (osteoclasts) and bone forming cells (osteoblasts). Loss of bone mass in osteoporosis is caused by an imbalance in bone remodeling, such a way that the rate of osteoclast-mediated bone resorption is higher than that of osteoblast-mediated bone formation (Bidwell et al. 2013 ▶,).
The majority of studies have shown oxidative stress as another important predisposing factor for osteoporosis (Manolagas, 2010 ▶). Estrogen deficiency lowers antioxidant levels and reduced antioxidant levels enhance bone loss (Sánchez-Rodríguez et al., 2007 ▶). In postmenopausal women, this acceleration of bone loss due to lack of estrogens can be associated with an increase in cytokine production by peripheral blood monocytes concomitant with generation of Reactive Oxygen Species (ROS) (Kim et al., 2012 ▶).
Estrogens can also stimulate bone formation. Many studies have also indicated that Hormone Replacement Therapy (HRT) was associated with a reduction of hip, spine, and wrist fractures (Saleh et al., 2011 ▶). Although HRT has been proven efficacious in preventing bone loss, concerns about its side effects have led to interest in treatment modalities that not only decrease morbidity, but also have minimal side effects. Antioxidant nutrients might reduce the production of free radicals that contribute to bone resorption and enhance bone formation (Puel et al., 2004 ▶).
Herbs or medicinal plants have been used for treatment of numerous diseases for thousands of years. Elaeagnus angustifolia (EA), a plant native to western Asia, is a traditional herbal medicine. EA extract is rich in phytoestrogens and flavonoids that are known as natural estrogens and are capable to induce the transcriptional activity of the human estrogen receptor expressed in cultured cells by transient transfection (Bucur et al., 2008 ▶). Some studies have also shown anti-inflammatory effects of the fruits of this plant on rheumatoid arthritis and osteoarthritis (Nikniaz et al., 2014 ▶). This herbal medicine contains several chemical compounds, which usually exert their beneficial effects through multiple pathways and cover several targets. This property may help the treatment of the multifactorial pathogenesis of osteoporosis.
To date, evidence has demonstrated that a cocktail of different antioxidants might be more efficient compared to supplementation with a single active ingredient (Greenwald et al., 2007 ▶).
Since histomorphology of the bone may be altered in different sample preparation conditions, well-grounded stereological examinations provide quantitative morphological data on the most important characteristics. For the first time, the present study aims to quantitatively evaluate the effects of EA hydroalcoholic extract consumption on amelioration of ovariectomy-induced osteoporosis in rats.
Materials and Methods
Drugs and chemical agents
Estradiol benzoate was purchased from Jaber Ebne Hayan Pharmaceutical Company, Tehran, Iran. Ketamine 10% and Xylazine 2% were also purchased from Alfasan Company (Netherlands).
Preparation of the hydroalcoholic extract
EA fruits were purchased from Keshtvasanat Company, Beyza, Fars, Iran. Then, a plant voucher specimen (No. SoP UoG21001) was kept in the herbarium of Shiraz University. The pericarp was separated from the seed, dried in shadow, and grounded. After that, the pericarp powder (100 g) was added to 500 ml ethanol 70%. The obtained solution was left in the percolator at room temperature for 72 hr. Afterwards, the solvent was completely removed from the hydroalcoholic extracts by rotavapor at 40 ºC and dried in a vacuum desiccator (The efficiency of this method was 23.5%) (D’Amelio, 1999 ▶).
Animal grouping and experimental design
This work was approved by the Ethics Committee (No. 2225b125) of Shiraz University of Medical Sciences, Shiraz, Iran. In this study, 55 adult female Sprague-Dawley rats (12-14 weeks old and weighing 200±20 g) were purchased from the Laboratory Animal Center of Shiraz University of Medical Sciences. The rats were preserved under standard housing laboratory conditions (room temperature with relative humidity of 60±5%, temperature of 23±2 ºC, and 12 hr/12 hr light/dark cycles) and were fed with a standard pellet diet and water ad libitum. After one week of adaptation to the diet and the new environment, the animals were screened and those with body weight extremes were excluded from the study. Then, the rats were randomly divided into five groups each containing 11 animals. The animals in group 1 were considered as the control group, group 2 animals were sham operated, and those in groups 3 to 5 underwent ovariectomy. The rats in all the ovariectomized (OVX) groups were fed for 60 days to lose bone. Subsequently, the animals in groups 1, 2, and 3 received normal saline, those in group 4 were treated with oral EA fruit extract (600 mg/kg body weight daily), and group 5 received subcutaneous injection of estradiol benzoate (3 mg/kg body weight weekly) for four months. According to the results of other studies, the best suggested dose for EA fruit extract in regard with improving inflammatory condition was 600 mg/kg BW. Therefore, we decided to use this dose (Khodakarm-Tafti et al, 2015 ▶).
Oophorectomy
The adult female rats were bilaterally ovariectomized or sham-operated under anesthesia by ketamine 10% (100 mg/kg, Alfasan, Netherlands) and xylazine 2% (10 mg/kg, Alfasan, Netherlands). After ligation of the uterine horn through a midline longitudinal incision, both ovaries were surgically removed in all the groups, except for the control group. The sham-operated control rats had their ventral incision, but manipulation of their ovaries was performed without excising them.
Collection of specimens and biochemical tests
Eight and sixteen weeks after treatment with EA fruit extract, blood samples were collected in chilled non-heparinized tubes to clot at room temperature by cardiocentesis. The blood samples were centrifuged at 3500 rpm at 4˚C for 20 min and the separated sera were evaluated for biochemical markers, including calcium, phosphorus, and alkaline phosphatase (ALP). Serum creatinine and BUN levels were also quantified by calorimetric methods using kits supplied by Biosystems S.A, Spain. It should be mentioned that biochemical assessment was done 2, 4, and 6 months after the beginning of the study.
All rats were anesthetized with ketamine and xylazine solution intraperitoneally and sacrificed by using thiopental (100 mg/kg) at the end of the experiment. Then, each rat’s body weight and uterus weight were determined. Afterwards, the fifth lumbar vertebral body (L5) and the right tibia were isolated, cleaned of connective tissue, fixed in 10% buffered formalin, and decalcified in formic acid for stereological studies.
Estimation of total phenolic compounds
The Total Phenol Content (TPC) of the plant extract was evaluated using the modified Folin-Ciocalteu spectrophotometric method described previously by Waterhouse (2002). ▶ This method involves reduction of Folin-Ciocalteu reagent by phenolic compounds, with a concomitant formation of a blue complex (Waterhouse, 2002 ▶). In this study, an aliquot of 40 µL solution of the extract was mixed with 3.16 ml water and 200 µL Folin-Ciocalteu reagents. The mixture was incubated for 8 min. Then, 600 µl of 0.25% sodium carbonate was added to this solution. The obtained solution was further incubated at room temperature for 2 hr and its absorbance was measured at 765 nm against the blank sample. The same procedure was repeated for gallic acid standard solution, and the calibration standard curve was construed. The measurement was then compared with the standard curve prepared for gallic acid solution. The concentration of the total phenol was expressed as milligrams of Gallic Acid Equivalents (GAE) per gram of dry extract (mg of GA/g of dE) (Noorafshan et al., 2015 ▶). Based on the calibration curve’s equation, the TPC in 80% ethanol extract was 8.75 mg GAE/g dry extract.
Estimation of bone volume
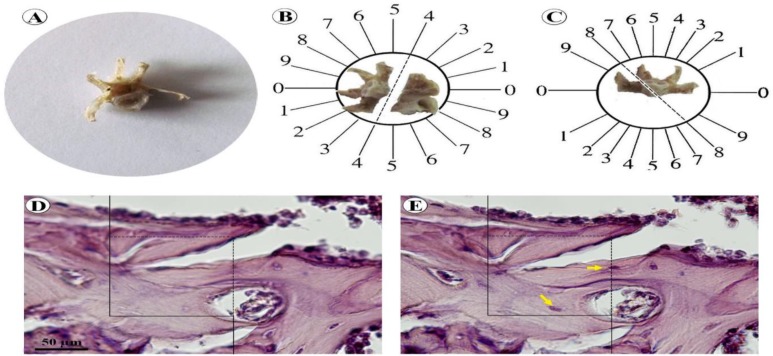
Sherle’s method was used to estimate the bones’ volume (Karbalay-Doust et al., 2012 ▶). Subsequently, the vertebra and tibia were decalcified and processed histologically. The orientator technique was applied to obtain the isotropic random sections of the bones (Mattfeldt et al., 1990 ▶) (Figures 1 A, B and C). Briefly, the bones were placed on two circles (an equidistance divided circle and a non-equidistance divided circle) and were sectioned using a blade. In this way, 10-12 slabs were sampled from each bone. An aspherical piece of about 1 mm diameter was punched out from a random slab using a trocar. Thereafter, the diameter and area of the circular pieces were calculated. The sampled slabs and the circular pieces of each animal’s bone were embedded in one paraffin block. Then, 5 and 25 µm sections were obtained. After staining the tissue sections with hematoxylin and eosin, the area of the circular pieces were measured again to approximate the global degree of bone tissue shrinkage. These methods allowed us to estimate the Degree of shrinkage (Dsh), which could be induced by tissue processing and staining procedures. Finally, the following formula was used (Nyengaard, 1999 ▶; Gundersen et al., 1988 ▶):
Figure 1.
The stereological methods. A lumbar vertebra was dissected out (A). Orientator method was used to obtain isotropic uniform random sections of the bone (B, C).Optical dissector method (D). An unbiased counting frame was lied on the images (E). The cell nuclei, which were not in contact with the forbidden lines (bold lines), were counted (arrows
where “area after” and “area before” were the areas of each circular piece of the bones after and before processing and staining, respectively.
The final volume of the bone was also calculated using the below formula:
V (final) = (1-Dsh) × V (primary)
Estimation of the volume density of the bones’ trabeculae
The volume density of the trabeculae “Vv (trabeculae)” refers to the proportion of the unit volume of the osseous tissue that is filled by trabeculae. Briefly, volume density of the trabeculae was evaluated on 4 µm thickness sections through the point-counting method and using Delesse’s formula (Karbalay-Doust et al., 2012 ▶, Nyengaard, 1999 ▶):
Where “P (trabeculae)” was the number of the test points falling on the trabeculae and “P (reference)” was the number of the points falling on the reference tissues (trabeculae plus cavities) of L5 and tibia bones. The following formula was used to estimate the absolute trabecular volume (Noorafshan et al., 2012 ▶):
V (bone) = V (final) × Vv (bone)
Estimation of the numerical density and absolute number of bone cells
Numerical density “NV (cells/bone)” refers to the number of cells per unit volume of the trabeculae and was evaluated using Disector method (Figures 1 D and E). Briefly, a 25-µm section was evaluated by moving down through the z-axis optical plane of the tissue using a microscope (Nikon E-200, Japan) linked to a monitor. An unbiased counting frame was lied on the tissue’s live image to sample the bone cells (Noorafshan et al., 2012 ▶). Upper and lower guard zones of the osseous tissue were set to be 5 µm using a microcator (MT 12, Heidenhain, Germany). The average final section thickness “t” was also measured on the tissue using the microcator (20 µm). Besides, an optical section (10 µm) was considered as the disector’s height. The numerical density of the cells was measured using the following formula:
Where “ΣQ” was the number of the cells counted in all the Disectors, “h” was the height of the optical Disector, “a/f” was the area of the counting frame, “ΣP” was the total number of the counted frames, “BA” or block advance was the setting of the microtome to cut the paraffin block, and “t” was the mean of the final section thickness.
To estimate the total number of the bone cells, the following formula was applied:
N (bone cells) =NV (cells/bone) V (final)
Statistical analysis
The SPSS statistical software (v. 18) was used to carry out statistical analysis. First, normal distribution of the data was assessed. Then, the data were analyzed by Mann-Whitney U test. The results were presented as mean ± standard deviation, and significance level was set as p≤0.05.
Results
Body weight and uterus weight
The mean body weight was 225±32 g in all study groups at the beginning of the experiment. After 6 months, all rats were weighed again and no noticeable increase was observed in their body weight. However, the weight of the uterus significantly decreased in the OVX rats compared to those with intact ovaries (p=0.001).
Biochemical parameters
Serum calcium, phosphorus, and ALP levels in the experimental groups are shown in Table 1. As the Table depicts, no significant difference was found among the experimental groups regarding serum calcium levels at the beginning of the experiment. However, OVX caused a significant decrease in the calcium levels in the untreated OVX group compared to the sham, control, and EA fruit extract-treated groups at the end of the study (p=0.001). Nonetheless, no significant change in serum phosphorus level was observed in the control, sham, and extract-treated animals at all stages of the study.
Table 1.
Biochemical parameters in the experimental and control groups
| Blood parameters |
Time
(month) |
Control | Sham | OVX | OVX + EA | OVX + estrogen |
|---|---|---|---|---|---|---|
| Ca (mg/dl) | 2 4 6 |
9.7 ± 0.5 9.8 ± 0.8*† 10.2 ± 0.5* |
9.6 ± 0.25 10.1 ± 0.3*† 9.6 ± 0.6* |
9.8±0.3 8.1±1.0 8.2±0.6 |
9.6±0.2 8.9±0.7 9.1±0.2* |
9.5±0.2 9.8±1*† 10.9±0.6*‡ |
| P (mg/dl) | 2 4 6 |
5.1 ± 1.0 5.9 ± 0.8 6.6 ± 1.9 |
4.6 ± 0.8 5 ± 0.8 6.6 ± 0.8 |
4.2 ± 0.70 6.5 ± 1.0 5.2 ± 0.8 |
4.5±0.9 6.7±0.2 6.6±0.8 |
4.1±0.4 6.7±.6 6.6±0.8 |
| ALP (mg/dl) | 2 4 6 |
333± 143*†‡ 311± 151*† 324± 146*† |
391 ± 94*†‡ 313 ± 124*† 295 ± 144*† |
465±85 410 ± 82 466± 101 |
504±100 433±102 511±83 |
518±132 265±110† 379±118† |
CA: Calcium, P: Phosphorus, ALP: Alkaline Phosphatase
p< 0.05, OVX + EA vs. control or sham-operated groups or estrogen.
p<0.05, control or sham vs. estrogen.
P<0.05, OVX vs. control or sham or estrogen or EA extract groups
The results showed a significant increase in plasma ALP level in the OVX rats compared to the sham-operated and control groups in the first stage of the study (p=0.001). Yet, administration of estradiol resulted in a decrease in bone turnover marker during the treatment. Nevertheless, no significant difference was seen between the estrogen-treated rats and the sham-operated and control groups with respect to ALP levels. In spite of improvement in calcium hemostasis, no significant decrease in bone turnover marker (ALP) was observed in the EA fruit extract-treated group compared with the estrogen-treated group. In addition, no significant differences were found between the EA fruit extract-treated rats and control and sham groups in this regard. Furthermore, no significant change was found in serum creatinine and BUN levels in any of the experimental groups before and after the treatment.
Stereological study
The weight of the vertebrae and tibia, volume of the vertebrae and tibia, total volume of the bones’ trabeculae, and total number of the osteocytes, osteoblasts, and osteoclasts are presented in Tables 2 and 3. Additionally, the qualitative microscopic evaluation of the L5 was shown in Figure 2.
Table 2.
Stereological parameters of vertebra in control, sham-operated, and ovariectomized (OVX) rats with or without treatment with Elaeagnus angustifolia extract (600 mg/kg/day) and estrogen (3 mg/kg/week
| Weight (mg) | Volume (mm 3 ) |
Number (×10
6
)
|
||||
|---|---|---|---|---|---|---|
| Groups | Vertebra | Vertebra | Trabeculae | Osteocytes | Osteoblasts | Osteoclasts |
| Control | 272±23 | 180±35 | 118±20 | 1.67±0.40 | 0.60±0.27 | 8.0±4.8 |
| Sham-operated | 292±45 | 179±17 | 120±21 | 1.84±0.46 | 0.61±0.09 | 7.4±2.7 |
| OVX | 201±10 † | 125±6 † | 67±12† | 0.98±0.17 † | 0.19±0.10† | 23.6±11† |
| OVX+estrogen | 248±22 | 199±9* | 82±10 | 1.61±0.54 | 0.61±0.21 * | 17.7±9.4 |
| OVX+ EA | 245±30 | 196±15* | 72±6 | 1.23±0.37 | 0.63±0.21 * | 18.04±8.3 |
EA: Elaeagnus angustifolia.
p< 0.05, OVX vs. control or sham-operated groups.
p<0.05, OVX vs. OVX+ estrogen or EA extract groups.
Table 3.
Stereological parameters of tibia in the control, sham-operated, and ovariectomized (OVX) rats with or without treatment with Elaeagnus angustifolia extract (600 mg/kg/day) and estrogen (3mg/kg/week
| Weight (mg) | Volume (mm 3 ) |
Number (×10
6
)
|
||||
|---|---|---|---|---|---|---|
| Groups | Tibia | Tibia | Trabeculae | Osteocytes | Osteoblasts | Osteoclasts |
| Control | 372±31 | 233±20 | 158±31 | 26.55±7.93 | 5.15±2.85 | 90.5±7.8 |
| Sham-operated | 364±41 | 210±20 | 156±31 | 23.32±3.57 | 5.33±1.54 | 83.5±12.3 |
| OVX | 219±35† | 139±36 † | 86±16 † | 11.20±4.82 † | 1.27±0.61 † | 399.7±106.2 † |
| OVX+estrogen(3mg/kg/week) | 372±61* | 243±32* | 110±22 | 27.62±11.95* | 5.51±4.47* | 154.6±100.4* |
| OVX+EA(600mg/kg) | 376±57* | 236±17* | 99±9 | 20.29±3.73 | 7.86±2.56* | 255.1±154*# |
p< 0.05, OVX vs. control or sham-operated groups.
p<0.05, OVX vs. OVX+ estrogen or EA extract groups.
p<0.05, OVX+ EA extract vs. control or sham groups
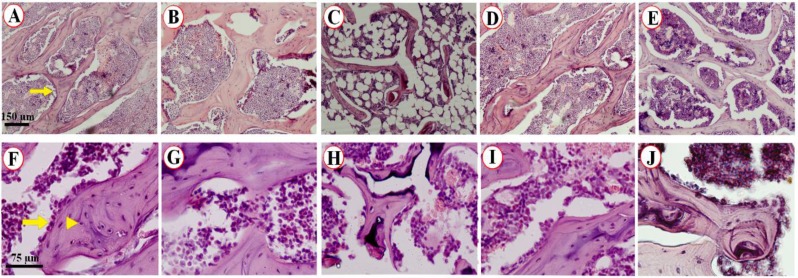
Figure 2.
The qualitative microscopic evaluation of the L5. The trabeculae showed normal appearance in the control (A) and sham-operated (B) rats. The trabecular atrophic changes could be seen obviously in the OVX rats (C). Treatment of the OVX animals with estrogen (D) and Elaeagnus angustifolia (E) showed protective changes in the bone. Survey of the number of osteoblasts and osteoclasts indicated a normal population in the control (F) and sham-operated (G) animals. A massive cell reduction could be observed in the OVX animals (H). The osteoblasts and osteocytes were protected in the animals treated with estrogen (I) and Elaeagnus angustifolia (J). The scale bar was 150 µm for the upper row Figures and 75 µm for the lower row. The arrow in A indicates bone trabeculae. The arrow and arrowhead in F indicate the osteoblast and osteocyte, respectively
The weight of the vertebrae and tibia
According to Table 2, the weight of L5 significantly decreased by 31% in the OVX group in comparison to the control and sham-operated groups (p<0.05).
However, the weight of L5 increased in the EA fruit extract- and estrogen-treated groups compared to the OVX groups, but the difference was not statistically significant.
Moreover, the weight of the tibia was significantly lower in the OVX group compared to the sham and control groups (p<0.05) (Table 3). Besides, the mean weight of the tibia significantly increased in both OVX groups receiving EA fruit extract and estrogen supplement compared to the OVX group (p<0.05). Nevertheless, no significant differences were found among the control, sham-operated, and treated groups regarding the weight of the vertebrae and tibia.
The volume of the vertebrae and tibia
On the average, the volume of L5 and tibia decreased by 30% in the OVX group in comparison to the sham and control groups (p<0.05). However, treatment of the OVX rats with 600 mg/kg EA fruit extract and estrogen significantly increased the mean volume of L5 and tibia (p<0.05). Nonetheless, no statistically significant difference was observed among the control, sham, estrogen, and EA fruit extract-treated rats regarding the volume of the vertebrae and tibia.
Total volume of the bones’ trabeculae
The volume of the trabeculae of L5 and tibia decreased by averagely 44% in the OVX group compared to the sham and control groups (p<0.05). Nonetheless, no significant differences were observed between the OVX and EA fruit extract-treated OVX groups in this regard.
Total number of osteocytes
The results of our study showed a 41% decline in the total number of osteocytes in the total volume of the L5 trabeculae in the OVX group compared to the control and sham-operated groups (p<0.05). However, an increase was observed in the total number of osteocytes in the EA fruit extract- and estrogen-treated groups in comparison to the OVX group, but the difference was not statistically significant. On the other hand, no significant difference was found among the OVX + EA fruit extract-treated, estrogen-treated, sham, and control groups concerning the number of osteocytes.
In the tibia, the total number of osteocytes was 57% and 51% lower in the OVX group compared to the control and sham-operated animals, respectively (both p<0.05). Nonetheless, an increase was observed in the total number of osteocytes in the EA fruit extract- and estrogen-treated groups in comparison to the OVX group, but the difference was only significant in the group receiving estrogen (p=0.002). Nevertheless, no significant difference was found among the EA fruit extract-treated, control, and sham-operated groups with respect to the number of osteocytes.
Total number of osteoblasts
In the tibia, the total number of osteoblasts was 57% and 51% lower in the OVX group compared to the control and sham-operated animals, respectively. The number of osteoblasts in the L5 and tibia were also 70% and 75% lower in the OVX group compared to the control and sham groups, respectively (p<0.05 for both). However, a significant increase in the number of osteoblasts was seen in the EA fruit extract- and estrogen-treated groups compared to the OVX group (p<0.05). Yet, no significant difference was seen among the EA fruit extract-treated, control, and sham-operated groups in this regard.
Total number of osteoclasts
The number of osteoclasts in the L5 was 3-fold higher in the OVX rats compared to the sham and control groups (p<0.05 for both). However, the number of osteoclasts decreased in the L5 of the EA fruit extract- and estrogen-treated groups compared to the OVX groups although the difference was not statistically significant. In addition, no significant differences was found among the control, sham-operated, and treated groups regarding the total number of osteoclasts.
Furthermore, the number of osteoclasts in the tibia significantly increased by 4.5 folds in the OVX rats compared to the sham and control groups. On the other hand, the number of osteoclasts significantly decreased in the EA fruit extract- and estrogen-treated groups compared to the OVX group (p<0.05 for both). Additionally, the results showed a significant difference between the EA fruit extract-treated, but not estrogen-treated, and control and sham groups in this regard.
Discussion
In osteoporosis, an imbalance between bone resorption and formation is due to extension of lifespan of osteoclasts and shortening of lifespan of osteoblasts and possibly intestinal absorption of calcium is impaired (Ashouri et al., 2015 ▶). Estrogen deficiency-induced bone loss is a complex interaction of estrogen, the immune system, and the bones. Estrogen maintains bone homeostasis through regulatory effects on the immune system and oxidative stress and direct effects on bone cells (Noorafshan et al., 2015 ▶). Treatment of postmenopausal osteoporosis involves use of drugs and hormone therapy, but each approved treatment has specific side effects. In general, two types of drugs are used in treatment of osteoporosis, antiresorptive and anabolic agents. Most drugs, such as estrogen, are antiresorptive agents (Choi, 2015 ▶). As an alternative treatment, foods with plant origins attracted increased attention because of their potential benefits and reduced adverse effects. It is noticeable that many plants have the potential to prevent and treat osteoporosis. However, only a restricted number of these plants have been meticulously investigated up to now (Jia et al., 2012 ▶). EA, a commonly prescribed herb in Western Asia for management of bone diseases (Ebrahimi et al., 2014 ▶; Zargari, 1990 ▶), is known for its effects on strengthening the bone. It was demonstrated that peel, fruit, and seed of this fruit contains different flavonoids, polyphenols, and terpenoids (Farzaei et al., 2015 ▶).
In the present study, OVX led to a significant decrease in calcium concentration compared to the other groups, but serum levels of phosphorus remained unchanged. Finally, however, no significant change was observed among the control, sham, estrogen-treated, and EA extract-treated animals regarding serum calcium level. Menopause is linked to decreased intestinal calcium absorption and increased renal excretion of calcium (Hohman et al., 2015 ▶). Calcium deficiency leads to worsening of bone deposition. Some studies demonstrated that phenol compounds in plants were able to enhance intestinal absorption of calcium, deposition of calcium ions in osteoblastic MC3T3-E1 cells, and inhibition of osteoclast formation (García-Villalba et al., 2014 ▶).
Bone is metabolically active and is continually repaired and remodeled throughout an individual’s life. Bone cells activity can be evaluated through biochemical markers. Most biochemical indices of bone resorption are related to collagen breakdown products or osteoclast-specific enzymes. Markers of bone formation are either by-products of collagen neosynthesis or osteoblast-related proteins, such as ALP (Garnero, 2014 ▶). Generally, all bone biochemical markers markedly increase at the beginning of menopause. Bone loss is accompanied by a significant increase in bone remodeling, as evidenced by increased biochemical bone turnover markers, such as serum ALP (Mukaiyama et al., 2015 ▶). ALP plasma concentration is an indicator of bone formation and a major index of osteoblastic activity (Xufeng et al. 2014 ▶). In the current study, plasma ALP concentration significantly increased in the OVX rats in comparison to the sham-operated and control groups. Administration of estrogen significantly reduced bone turnover, which was accompanied by a decrease in serum ALP level. However, at the end of the study, the results indicated that ALP level was significantly higher in the EA-treated animals compared to the control, sham, and estrogen-treated groups. EA contains phenolic compounds, based on an in vitro study, a mixture of phenolic acids significantly upregulated ALP gene expression and stimulated osteoblast differentiation, resulting in significantly increased bone mass (Chen et al., 2010 ▶).
Based on the literature, this was the first study investigating the anti-osteoporotic effects of EA stereologically. Assessment of tibia and L5 showed that OVX rats had a lower total volume of the bone trabecular and higher trabecular separation compared to the other groups, indicating increased bone fragility (Peel, 2009 ▶). In agreement with the previous studies, the increased number of osteoclasts and decreased number of osteoblasts and osteocytes in the OVX rats pointed toward increased bone resorption, while these changes were modified in EA- and estrogen-treated groups (Nishide et al. 2013 ▶). In our study, administration of estrogen or EA to osteoporotic rats decreased the number of osteoclasts and significantly increased the number of osteoblasts. After the onset of menopause, drop in the blood level of estrogen results in bone loss and increases the incidence of osteoporosis (Khosla et al., 2012 ▶). Many studies have acknowledged the role of pro-inflammatory cytokines in the etiology and pathogenesis of osteoporosis. Some evidence has also linked bone loss to ROS. Estrogen deficiency provokes oxidative stress, impairs bone antioxidant defense, increases lipid peroxidation and H2O2 and diminishes enzymatic antioxidants, such as super oxygen dehydrogenase and glutathione peroxidase (Goldring et al., 2015 ▶). Estrogen deficiency also upregulates the formation of osteoclasts and osteoblasts by induction of the production and activity of cytokines, including IL-6, TNF, IL-1, and Macrophage Colony Stimulating Factors (M-CSF) (Callaway et al., 2015 ▶). Two phytosterols have been detected in EA, namelyβ-sitosterol and stigmasterol (Si et al., 2011 ▶, Bekker et al., 1997 ▶). Phytosterols, which have estrogen-like activity, enhance differentiation and proliferation of primary osteoblasts by increasing the mRNA expression of ALP and might modulate osteoclastogenesis via regulation of Osteoprotegerin (OPG) and RANKL mRNA expression in bone cells (Mok et al., 2010 ▶).
It has also been shown that EA has anti-inflammatory effects in animal models (Nikniaz et al., 2014 ▶). Besides, ethanolic extract of EA possesses antioxidant activity (Chen et al., 2014 ▶, Wang et al., 2013 ▶). The antioxidant activity of this extracts was linearly related to polyphenols, but non-linearly related to flavonoids (Bucur et al., 2008 ▶).
In contrast to the effect of other antiremodeling plant extracts on bone that mainly modulate and inhibit osteoclastogenesis demonstrated with decrease in the bone turnover markers (decrease in serum ALP levels compared to the OVX group) (Noorafshan et al., 2015 ▶), the current study findings demonstrated that EA exerted an uncoupling bone formation with a significant increase in osteoblasts count in the EA extract-treated group compared to the OVX group, which was shown biochemically with increased ALP levels in the EA extract-treated group.
Recent studies have suggested that postmenopausal osteoporosis might be due not only to augmented osteoclast formation and to activity, but also to an increase in osteoblastic inhibition and a decrease in osteoblastic activity (D’Amelio et al., 2011 ▶). Over the last years, anabolic treatment has been anticipated as the therapy for postmenopausal osteoporosis. These drugs significantly diminish the risk of vertebral and non-vertebral fragility fractures (Greenspan et al., 2007 ▶). Although suppressed osteoclastogenesis might be considered in determining increased bone mass in EA-fed animals, increased bone mass in these animals was associated with increased ALP level, osteoblast number, bone mineralization, and bone volume.
This is the first stereological and a preliminary study to evaluate the possible use of EA in treatment of osteoporosis. However, considering the results of this study, future research should focus on isolated or mixture of active constituents to determine the mechanisms underlying the bone effects and to reveal the beneficial therapeutic and safety properties of its phytochemicals, as a complementary and alternative medicine for management of osteoporosis.
The results of this study provided a basis for clinical evaluation and demonstrated the potential effects of EA extract, as a herbal drug. The findings suggested that EA extract could be an effective natural anabolic alternative for treatment of postmenopausal osteoporosis. Yet, further research are needed to be conducted in this area.
Acknowledgment
The present manuscript was adapted from project No.33-9518 approved by Shiraz University of Medical Sciences, Shiraz, Iran. The authors would like to thank Ms. A. Keivanshekouh at the Research Improvement Center of Shiraz University of Medical Sciences for improving the English level of the manuscript.
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
References
- Ashouri E, Meimandi EM, Saki F, Dabbaghmanesh MH, Omrani GR, Bakhshayeshkaram M. The impact of LRP5 polymorphism (rs556442) on calcium homeostasis, bone mineral density, and body composition in Iranian children. J Bone Miner Metab. 2015;33:651–657. doi: 10.1007/s00774-014-0624-4. [DOI] [PubMed] [Google Scholar]
- Bekker NP, Glushenkova AI. Lipids of the leaves of Elaeagnusangustifolia. Chem Nat Compd. 1997;33:543–544. [Google Scholar]
- Bidwell JP, Alvarez MB, Hood M Jr, Childress P. Functional impairment of bone formation in the pathogenesis of osteoporosis: the bone marrow regenerative competence. Curr Osteoporos Rep. 2013;11:117–125. doi: 10.1007/s11914-013-0139-2. [DOI] [PubMed] [Google Scholar]
- Bucur L, Negreanu-Pîrjol T, Giurginca M, Istudor V. Some new Elaeagnus angustifolia l Extracts and the pharmaceutical products` antioxidant activities determined by the chemiluminiscence method. Revue. Roumaine. Du Chimie. 2008;53:961–964. [Google Scholar]
- Callaway DA, Jiang JX. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J Bone Miner Metab. 2015;33:359–370. doi: 10.1007/s00774-015-0656-4. [DOI] [PubMed] [Google Scholar]
- Chen JR, Lazarenko OP, Wu X, Kang J, Blackburn ML, Shankar K, Badger TM, Ronis MJ. Dietary-induced serum phenolic acids promote bone growth via p38 MAPK/β-catenin canonical Wnt signaling. J Bone Miner Res. 2010;25:2399–2411. doi: 10.1002/jbmr.137. [DOI] [PubMed] [Google Scholar]
- Chen Q, Chen J, Du H, Li Q, Chen J, Zhang G, Liu H, Wang J. Structural characterization and antioxidant activities of polysaccharides extracted from the pulp of Elaeagnus angustifolia L. Int J Mol Sci. 2014;15:11446–11455. doi: 10.3390/ijms150711446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi , HJ New antiresorptive therapies for postmenopausal osteoporosis. J Menopausal Med. 2015;21:1–11. doi: 10.6118/jmm.2015.21.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amelio P, Roato I, D’Amico L, Veneziano L, Suman E, Sassi F, Bisignano G, Ferracini R, Gargiulo G, Castoldi F, Pescarmona GP, Isaia GC. Bone and bone marrow pro-osteoclastogenic cytokines are up regulated in osteoporosis fragility fractures. Osteoporos Int. 2011;22:2869–2877. doi: 10.1007/s00198-010-1496-7. [DOI] [PubMed] [Google Scholar]
- D’Amelio Sr FS. Botanicals. USA: CRC Press LLC; 1999. pp. 39–41. [Google Scholar]
- Ebrahimi AA, Nikniaz Z, Ostadrahimi A, Mahdavi R, Nikniaz L. "The effect of Elaeagnus angustifolia L whole fruit and medulla powder on women with osteoarthritis of the knee: A randomized controlled clinical trial. Eur J Integ Med. 2014;6:672–679. [Google Scholar]
- Farzaei MH, Bahramsoltani R, Abbasabadi Z, Rahimi RA. Comprehensive review on phytochemical and pharmacological aspects of Elaeagnus angustifolia L. J Pharm Pharmacol. 2015;67:1467–1480. doi: 10.1111/jphp.12442. [DOI] [PubMed] [Google Scholar]
- Farrier AJ, Sanchez Franco LC, Shoaib A, Gulati V, Johnson N, Uzoigwe CE, Choudhury MZ. New anti-resorptives and antibody mediated anti-resorptive therapy. Bone Joint J. 2016;98-B:160–165. doi: 10.1302/0301-620X.98B2.36161. [DOI] [PubMed] [Google Scholar]
- Garnero P. New developments in biological markers of bone metabolism in osteoporosis. Bone. 2014;66:46–55. doi: 10.1016/j.bone.2014.05.016. [DOI] [PubMed] [Google Scholar]
- García-Villalba R, Larrosa M, Possemiers S, Tomás-Barberán FA, EspínJC Bioavailabilityof phenolics from an oleuropein-rich olive (Oleaeuropaea) leaf extract and its acute effect on plasma antioxidant status: comparison between pre-and postmenopausal women. Eur J Nutr. 2014;53:1015–1027. doi: 10.1007/s00394-013-0604-9. [DOI] [PubMed] [Google Scholar]
- Gatta LA, Jiang X, Schnatz PF. Hormone therapy in women with primary ovarian insufficiency or early menopause. Menopause. 2015;22:923–925. doi: 10.1097/GME.0000000000000518. [DOI] [PubMed] [Google Scholar]
- Goldring SR. Inflammatory signaling induced bone loss. Bone. 2015;80:143–149. doi: 10.1016/j.bone.2015.05.024. [DOI] [PubMed] [Google Scholar]
- Greenwald P, Anderson D, Nelson SA, Taylor PR. Clinical trials of vitamin andmineral supplements for cancer prevention. Am J clinNutr. 2007;85:314S–317S. doi: 10.1093/ajcn/85.1.314S. [DOI] [PubMed] [Google Scholar]
- Greenspan SL, Bone HG, Ettinger MP, Hanley DA, Lindsay R, Zanchetta JR, Blosch CM, Mathisen AL, Morris SA, Marriott TB. Effect of recombinant human parathyroid hormone (1–84) on vertebral fracture and bone mineral density in postmenopausalwomen with osteoporosis: a randomized trial. Ann Intern Med. 2007;146:326–339. doi: 10.7326/0003-4819-146-5-200703060-00005. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sørensen FB, Vesterby A. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Hohman EE, Weaver CM. A grape-enriched diet increases bone calcium retention and cortical bone properties in ovariectomized rats. J Nutr. 2015;145:253–259. doi: 10.3945/jn.114.198598. [DOI] [PubMed] [Google Scholar]
- Jeddi M, Roosta MJ, Dabbaghmanesh MH, Omrani GR, Ayatollahi SM, Bagheri Z, Showraki AR, Bakhshayeshkaram M. Normative data and percentile curves of bone mineral density in healthy Iranian children aged 9-18 years. Arch Osteoporos. 2013;8:114. doi: 10.1007/s11657-012-0114-z. [DOI] [PubMed] [Google Scholar]
- Jia M, Nie Y, Cao DP, Xue YY, Wang JS, Zhao L, Rahman K, Zhang QY, Qin LP. 2012. Evid Based Complement Alternat Med. Potential antiosteoporotic agents from plants: a comprehensive review;2012:1–29. doi: 10.1155/2012/364604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbalay-Doust S, Noorafshan A, Pourshahid SM. Taxol and Taurine Protect the Renal Tissue of Rats after Unilateral Ureteral Obstruction: A Stereological Survey. Korean J Urol. 2012;53:360–367. doi: 10.4111/kju.2012.53.5.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodakarm-Tafti A, Mehrabani D, Homafar L, Farjanikish G. Healing Effects of Elaeagnus angustifolia Extract in Experimentally Induced Ulcerative Colitis in Rats. J Pharmacol Toxicol. 2015;10:29–35. [Google Scholar]
- Khosla S, Oursler MJ, Monroe DG. Estrogen and the skeleton. Trends Endocrinol Metab. 2012;23:576–581. doi: 10.1016/j.tem.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim OY, Chae JS, Paik JK, Seo HS, Jang Y, Cavaillon JM, Lee JH. Effects of aging and menopause on serum interleukin-6 levels and peripheral blood mononuclear cell cytokine production in healthy nonobese women. Age (Dordr) 2012;34:415–425. doi: 10.1007/s11357-011-9244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattfeldt T, Mall G, Gharehbaghi H, Möller P. Estimation of surface area and length with the orientator. J Microsc. 1990;159:301–17. doi: 10.1111/j.1365-2818.1990.tb03036.x. [DOI] [PubMed] [Google Scholar]
- Manolagas SC. from estrogencentric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010;31:266–300. doi: 10.1210/er.2009-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok SK, Chen WF, Lai WP, Leung PC, Wang XL, Yao XS, Wong MS. Icariin protects against bone loss induced by oestrogen deficiency and activates oestrogen receptor-dependent osteoblastic functions in UMR 106 cells. Br J Pharmacol. 2010;159:939–949. doi: 10.1111/j.1476-5381.2009.00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaiyama K, Kamimura M, Uchiyama S, Ikegami S, Nakamura Y, Kato H. Elevation of serum alkaline phosphatase (ALP) level in postmenopausal women is caused by high bone turnover. Aging Clin Exp Res. 2015;27:413–8. doi: 10.1007/s40520-014-0296-x. [DOI] [PubMed] [Google Scholar]
- Nikniaz Z, Ostadrahimi A, Mahdavi R, Ebrahimi AA, Nikniaz L. Effects of Elaeagnus angustifolia L supplementation on serum levels of inflammatory cytokines and matrix metalloproteinases in females with knee osteoarthritis. Complement Ther Med. 2014;22:864–9. doi: 10.1016/j.ctim.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Nishide Y, Tadaishi M, Kobori M, Tousen Y, Kato M, Inada M, Miyaura C, Ishimi Y. Possible role of S-equol on bone loss via amelioration of inflammatory indices in ovariectomizedmice. J Clin Biochem Nutr. 2013;53:41–8. doi: 10.3164/jcbn.12-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorafshan A, Dabbaghmanesh MH, Tanideh N, Koohpeyma F, Rasooli R, Hajihoseini M, Bakhshayeshkaram M, Hosseinabadi OK. Stereological study of the effect of black olive hydroalcoholic extract on osteoporosis in vertebra and tibia in ovariectomized rat. Osteoporos Int. 2015;26:2299–2307. doi: 10.1007/s00198-015-3126-x. [DOI] [PubMed] [Google Scholar]
- Noorafshan A, Hoseini L, Karbalay-Doust1 S, Nadimi E. A Simple Stereological Method for Estimating the Number and the Volume of the Pancreatic Beta Cells. JOP. 2012;13:427–432. doi: 10.6092/1590-8577/802. [DOI] [PubMed] [Google Scholar]
- Noorafshan A, Dabbaghmanesh MH, Tanideh N, Koohpeyma F, Rasooli R, Hajihoseini M, Bakhshayeshkaram M, Hosseinabadi OK. Stereological study of the effect of black olive hydroalcoholic extract on osteoporosis in vertebra and tibia in ovariectomized rat. Osteoporos Int. 2015;26:2299–2307. doi: 10.1007/s00198-015-3126-x. [DOI] [PubMed] [Google Scholar]
- Nyengaard JR. Stereologic methods and their application in kidney research. J Am Soc Nephrol. 1999;10:1100–23. doi: 10.1681/ASN.V1051100. [DOI] [PubMed] [Google Scholar]
- Peel N. Bone remodelling and disorders of bone metabolism. Surgery. 2009;27:470–482. [Google Scholar]
- Puel C, Quintin A, Agalias A, Mathey J, Obled C, Mazur A, Davicco MJ, Lebecque P, Skaltsounis AL, Coxam V. Olive oil and its main phenolic micronutrient (oleuropein) prevent inflammation-induced bone loss in the ovariectomised rat. Br J Nutr. 2004;92:119–127. doi: 10.1079/BJN20041181. [DOI] [PubMed] [Google Scholar]
- Saleh NK, Saleh HA. Olive Oil effectively mitigates ovariectomy-induced osteoporosis in rats. BMC Complement Altern Med. 2011;11:10. doi: 10.1186/1472-6882-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Rodríguez MA, Ruiz-Ramos M, Correa-Muñoz E, Mendoza- Núñez VM. Oxidative stress as a risk factor for osteoporosis in elderly Mexicans as characterized by antioxidant enzymes. BMC Musculoskelet Disord. 2007;8:124. doi: 10.1186/1471-2474-8-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si CL, Qin PP, Lu YY, Wu L, Wang HH, HuiLF , LiuZ , NiYH GC-MS analysis of chemical composition and free radical scavenging activity of Elaeagnus angustifolia bark. Adv Mater Res. 2011;183–185:854–858. [Google Scholar]
- Waterhouse AL. “Determination of total phenolics,”. In: R. E. Wrolstad., editor. Current Protocols in Food Analytical Chemistry. New York, NY, USA: John Wiley & Sons; 2002. units I, pp. I1.1.1–I1.1.8. [Google Scholar]
- Wang Y, Guo T, Li J, Zhou S, Zhao P, Fan M. Four flavonoidglycosides from the pulps of Elaeagnus angustifolia and theirantioxidant activities. Adv Mater Res. 2013;756-759:16–20. [Google Scholar]
- Xufeng He, Qiang Shen. Salvianolic acid B promotes bone formation by increasing activity of alkaline phosphatase in a rat tibia fracture model: a pilot study. doi: 10.1186/1472-6882-14-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zargari A, editor. Medicinal plants. Tehran: Tehran University Press; 1990. [Google Scholar]