Introduction
Key Teaching Points.
-
•
If the vein of Marshall (VOM) is thin and short in patients with perimitral flutter via a VOM, we recommend ablation in a branch of the VOM from the coronary sinus.
-
•
If the VOM is thin and short in patients with perimitral flutter via a VOM and attempting to pass a catheter through the VOM would be considered difficult, it would be useful to diagnose it by accurate activation mapping with 3-dimensional mapping.
-
•
If the VOM is thin and short in patients with perimitral flutter via a VOM and attempting to pass a catheter through the VOM would be considered difficult, it would be useful to diagnose it by a pacing study, such as with the post pacing interval.
Even though mitral isthmus block is completely achieved after pulmonary vein (PV) isolation for atrial fibrillation (AF) by adding a linear ablation, in some cases atrial tachycardias (ATs) can occur that break through between the left atrial appendage and left PVs. This type of perimitral flutter has been reported as a ridge-related reentry (RRR).1, 2 Jiang and colleagues2 speculated that one of the mechanisms for the appearance of this RRR tachycardia could be reentry through the vein of Marshall (VOM). In a previous report, an AT through the VOM was diagnosed by passing a thin catheter though the VOM and ablating it from the left atrial endocardium. In this report, we describe a novel method of diagnosing and ablating an AT through the VOM.
Case report
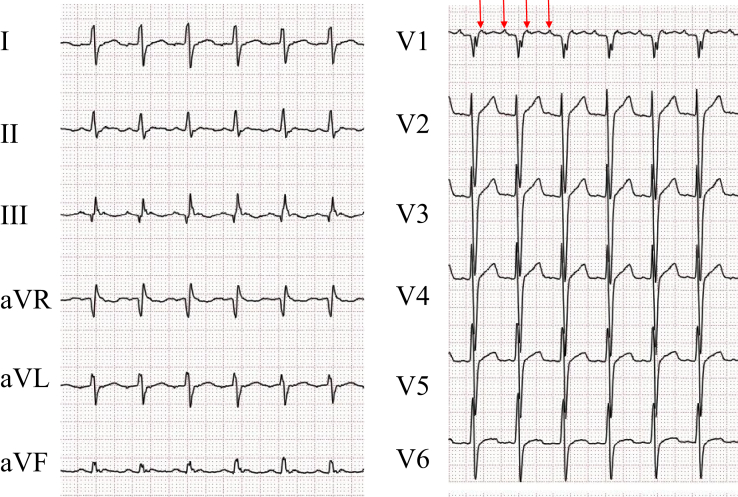
A 52-year-old man with heart failure owing to persistent AF for 5 years underwent multiple catheter ablation procedures for AF. He underwent a bilateral extensive PV isolation, roof line, mitral isthmus block line, superior vena cava isolation, cavotricuspid isthmus block line, and right atrial lateral line. When the last ablation procedure was performed, we confirmed bidirectional block of each isolation line. For the mitral isthmus block line we confirmed in particular that the time interval of the double potentials on the mitral isthmus block line was 120 ms and the coronary sinus (CS) potential sequence changed to a proximal-to-distal activation after pacing from the left atrial appendage (LAA). Therefore, it was considered that the mitral isthmus block line had been completed, and there was no recurrence of the AF, AT, or atrial flutter. However, 1 year after the ablation procedure the patient visited the emergency room because of palpitations. A 2:1 AT (heat rate 115 beats/min) appeared (Figure 1), and it was decided to perform a sixth procedure for the AT.
Figure 1.
Standard 12-lead electrocardiogram at the time of the atrial tachycardia. The P waves are negative in lead I and positive in leads II, III, aVF, and V1. The red arrow delineates the P wave.
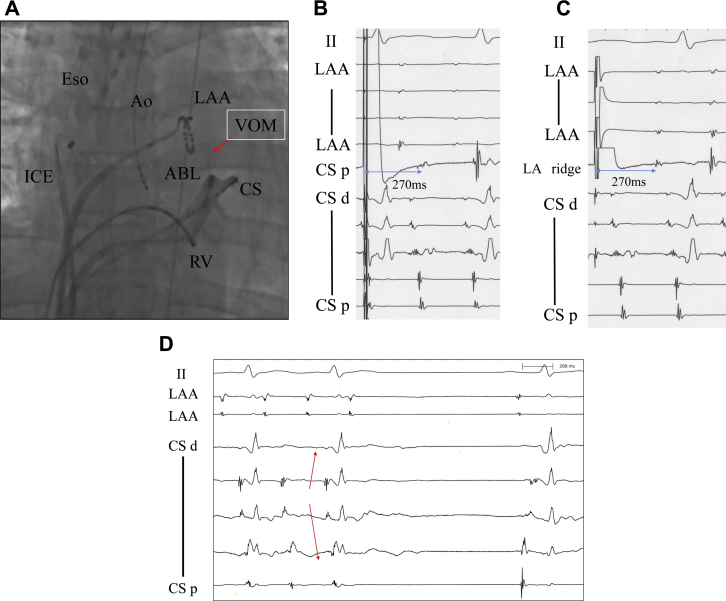
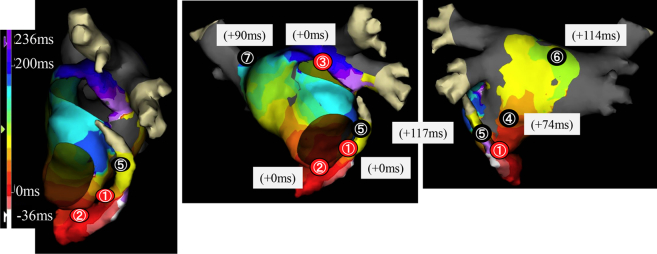
An 8.5F Agilis Steerable Introducer (St. Jude Medical, St. Paul, MN) for the ablation catheter and 10-pole catheter was inserted into the CS (Figure 2A). The tachycardia cycle length was 270 ms. An activation map during the AT using the EnSite Velocity NavX 3-dimensional (3D) mapping system (St. Jude Medical, St. Paul, MN) was constructed and fused with the 64-slice cardiac 3D computed tomography image obtained in advance. The earliest activation site of the AT was recorded by the catheter in the CS at the 4 o'clock position on the mitral annulus and no activation potentials earlier than those in the CS were observed in the left atrium (LA) (Figure 2D). According to the 3D mapping, the circuit of the AT conducted through the CS from the 4 o'clock position of the mitral valve annulus, turned to the anterior LA, ascended the left atrial anterior wall, and reached the front of the left PVs. Based on the EnSite activation map, the tachycardia activation time was 260 ms and the electrical activity on the CS mid electrodes continuously conducted through the LAA, and the difference in time between the LA ridge and CS mid was 10 ms (Figure 3).
Figure 2.
A: Fluoroscopic image (anterior-posterior view) of the coronary sinus (CS) and branch of the vein of Marshall (VOM), and the intracardiac electrocardiogram (ICE) recordings. By a contrast injection from the tip of the irrigated ablation catheter (ABL), the VOM was identified. Ao = electrode catheter as a reference in the aortic cusp; Eso = thermometer in the esophagus; LAA = ring catheter in the left atrial appendage; RV = right ventricle. B, C, D: The intracardiac electrograms for measuring the post pacing interval (PPI) at CS proximal (B) and left atrial (LA) ridge (C) and during the termination with ablation (D). The PPI at both sites (B and C) matched the tachycardia cycle length. D: Before the ablation the earliest activation site was at the CS mid (red arrow). After the atrial tachycardia terminated, the sequence of the CS potentials changed from proximal to distal.
Figure 3.
Activation map during the atrial tachycardia constructed with a NavX system. The numbers in the figure show the post pacing interval (PPI) at 7 typical points. The red circles represent the sites at which the PPI matched the tachycardia cycle length, and the black circles represent the sites at which the PPI did not match the tachycardia cycle length. ① 4 o'clock position on the mitral isthmus, ② 7 o'clock position on the mitral isthmus, ③ anterior ridge of the left superior pulmonary vein (PV), ④ posterior wall of the left atrium (LA), ⑤ near the mitral isthmus of the LA, ⑥ LA roof, ⑦ right superior PV.
When the post pacing interval was measured in the LA, it matched the tachycardia cycle length at the 4 o'clock (Figure 2B and Figure 3①) and 7 o'clock (Figure 3②) positions of the mitral isthmus and anterior ridge of the left superior PV (Figure 2C and Figure 3③), but it did not match the tachycardia cycle length on the posterior wall of the LA (Figure 3④), near the mitral isthmus (Figure 3⑤), LA roof, (Figure 3⑥), or right superior PV (Figure 3⑦). This tachycardia reached the CS after passing through the LA ridge according to the 3D mapping and pacing study. However, the RRR tachycardia was considered to have conducted to the CS without passing through the endocardium.
When contrast was injected from the tip of the irrigated ablation catheter located at the earliest activation site in the CS, the VOM could be imaged (Figure 2A). From the 3D map and post pacing interval findings, it was determined that the AT conducted via the VOM. A radiofrequency catheter ablation application was delivered in a branch of the VOM from the CS. With only 1 application at a maximum of 25 W for 23 seconds, the AT terminated. When pacing from the LAA, the sequence of the CS potentials changed to proximal-to-distal and complete mitral isthmus block was confirmed. The noninducibility of the AT was confirmed, and the procedure was ended. AF recurred 4 months after this procedure. We performed catheter ablation again. We confirmed mitral isthmus block and added an ablation of reconnection at an anterior site of the left inferior PV. After that there have been no recurrences of the AT for 6 months.
Discussion
In the present case, we could diagnose that the AT utilized the VOM by using 3D mapping and a pacing study, without inserting a catheter into the VOM. Further, we could ablate it only from a branch of the VOM from the CS.
Although the relationship between the VOM and genesis of AF have been previously reported,3, 4 there have been reports of catheter ablation of recurrent ATs via the VOM after AF ablation in a few cases.5, 6, 7, 8 In many of the previous reports, it could be confirmed that the VOM was the origin of the arrhythmia or a part of the reentry circuit, by inserting a thin electrode catheter into the Marshall vein.6, 7 However, the thickness of the VOM by autopsy is small and an average of only 1.1 mm in diameter, and a 3F probe can be inserted into it in only about 72% of the cases.9, 10 Finally, even if it can be accessed by a 3F probe, it can reach only an average of 9.3 mm from the ostium of the VOM. Therefore, cannulation with an electrode catheter is not always possible. Although we believed it would be useful to pass a thin catheter through the VOM to confirm the AT circuit, we did not attempt it, because in this case the VOM was very thin and short (Figure 2) and we considered that attempting to pass a catheter through the VOM would be difficult. Therefore, the circuit of the AT was estimated by using the information of the detailed potentials from the 3D mapping, and performing a conventional pacing study. As a result, the conduction to the CS mid electrodes from the ridge could not pass through the LA, and it was speculated that it passed through an epicardial site via the VOM.
Regarding the methods of catheter ablation, in a previous report of patients that underwent catheter ablation of AF with a VOM origin, it was reported that more than 90% could be isolated by ablation of the PV ridge, and in a subsequent case report they also performed ablation within the LA.7, 8 On the other hand, in a case report of 18 cases of ablation of AF with a VOM origin, it was reported that endocardial ablation alone was insufficient.4 There are mainly 3 conduction points between the VOM and LA: the ridge in front of the left PV, the body of the VOM, and the Marshall bundle (MB) attachment site of the CS, which can vary case by case.11, 12, 13 In a report that examined the MB of the bifurcation of the CS and VOM in detail, it was found that there is a connection of less than 10 mm from the CS.10 It is considered that it is possible to isolate the conduction of the CS musculature and MB by ablation from a branch of the VOM accessed from the CS. In this case, a single ablation application at that site could terminate the AT. From the above, the branch of the MB of the VOM and branch of the VOM within the CS were considered to be important sites in order to isolate the MB, and to the best of our knowledge, this case is the first report of the successful ablation at this site. This ablation method would be less invasive than any other method, and would therefore be useful. We also believed ablation at the anterior ridge of the PV or an ethanol injection would be useful.8 However, in such a case with a thin and short VOM, we recommend that an ablation in a branch of the VOM from the CS should be preferentially selected for the ablation of an AT via the VOM.
Conclusion
This case had an AT that recurred after multiple ablation procedures for AF and was diagnosed by a detailed construction of a 3D map and pacing study. This AT via the VOM could be treated by an isolation between the MB and CS musculature. This method was considered useful for treating reentrant tachycardias via the VOM.
References
- 1.Takatsuki S., Fukumoto K., Igawa O., Kimura T., Nishiyama N., Aizawa Y., Tanimoto Y., Tanimoto K., Miyoshi S., Fukuda K. Ridge-related reentry: a variant of perimitral atrial tachycardia. J Cardiovasc Electrophysiol. 2013;24:781–787. doi: 10.1111/jce.12120. [DOI] [PubMed] [Google Scholar]
- 2.Jiang C.X., Dong J.Z., Long D.Y. Ridge-related reentry despite apparent bidirectional mitral isthmus block. Heart Rhythm. 2016;13:1845–1851. doi: 10.1016/j.hrthm.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 3.Hwang C., Wu T.J., Doshi R.N., Peter C.T., Chen P.S. Vein of Marshall cannulation for the analysis of electrical activity in patients with focal atrial fibrillation. Circulation. 2000;101:1503–1505. doi: 10.1161/01.cir.101.13.1503. [DOI] [PubMed] [Google Scholar]
- 4.Katritsis D., Ioannidis J.P., Anagnostopoulos C.E., Sarris G.E., Giazitzoglou E., Korovesis S., Camm A.J. Identification and catheter ablation of extracardiac and intracardiac components of ligament of Marshall tissue for treatment of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2001;12:750–758. doi: 10.1046/j.1540-8167.2001.00750.x. [DOI] [PubMed] [Google Scholar]
- 5.Báez-Escudero J.L., Morales P.F., Dave A.S., Sasaridis C.M., Kim Y.H., Okishige K., Valderrábano M. Ethanol infusion in the vein of Marshall facilitates mitral isthmus ablation. Heart Rhythm. 2012;9:1207–1215. doi: 10.1016/j.hrthm.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hwang C., Chen P.S. Ligament of Marshall: why it is important for atrial fibrillation ablation. Heart Rhythm. 2009;6:S35–40. doi: 10.1016/j.hrthm.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto T., Maruyama M., Seino Y., Shimizu W. Marshall bundle reentry: a novel type of macroreentrant atrial tachycardia. Heart Rhythm. 2014;11:1229–1232. doi: 10.1016/j.hrthm.2014.03.051. [DOI] [PubMed] [Google Scholar]
- 8.Briceño D.F., Valderrábano M. Recurrent perimitral flutter due to vein of Marshall epicardial connections bypassing the mitral isthmus: response to ethanol infusion. Circ Arrhythm Electrophysiol. 2014;7:988–989. doi: 10.1161/CIRCEP.114.001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeSimone C.V., Noheria A., Lachman N., Edwards W.D., Gami A.S., Maleszewski J.J., Friedman P.A., Munger T.M., Hammill S.C., Packer D.L., Asirvatham S.J. Myocardium of the superior vena cava, coronary sinus, vein of Marshall, and the pulmonary vein ostia: gross anatomic studies in 620 hearts. J Cardiovasc Electrophysiol. 2012;23:1304–1309. doi: 10.1111/j.1540-8167.2012.02403.x. [DOI] [PubMed] [Google Scholar]
- 10.Makino M., Inoue S., Matsuyama T.A., Ogawa G., Sakai T., Kobayashi Y., Katagiri T., Ota H. Diverse myocardial extension and autonomic innervation on ligament of Marshall in humans. J Cardiovasc Electrophysiol. 2006;17:594–599. doi: 10.1111/j.1540-8167.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 11.Hwang C., Fishbein M.C., Chen P.S. How and when to ablate the ligament of Marshall. Heart Rhythm. 2006;3:1505–1507. doi: 10.1016/j.hrthm.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Rodríguez-Mañero M., Schurmann P., Valderrábano M. Ligament and vein of Marshall: a therapeutic opportunity in atrial fibrillation. Heart Rhythm. 2016;13:593–601. doi: 10.1016/j.hrthm.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han S., Joung B., Scanavacca M., Sosa E., Chen P.S., Hwang C. Electrophysiological characteristics of the Marshall bundle in humans. Heart Rhythm. 2010;7:786–793. doi: 10.1016/j.hrthm.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]