Abstract
Background
Chronic kidney disease is a frequent comorbidity among patients with acute coronary syndrome (ACS). We aimed to evaluate treatment characteristics in ACS patients according to their renal function and to assess the effect of differences in therapy on clinical outcomes.
Methods
Included were patients with ACS enrolled in the biennial Acute Coronary Syndrome Israeli Surveys (ACSIS) during 2000-2013. Excluded were patients with cardiogenic shock at presentation. The estimated glomerular filtration rate (eGFR) was calculated using the simplified Modification of Diet in Renal Disease (MDRD) formula. The distribution of the eGFRs was divided into 4 categories (<45, 45-59.9, 60-74.9, and ≥75 mL/min/1.73 m2). The primary endpoint was all-cause mortality at 1 year.
Results
A total of 13,194 patients with ACS were included. Patients with a reduced eGFR were less likely to be admitted to a coronary care unit and had lower rates of coronary angiograms and subsequent percutaneous coronary interventions. Furthermore, as the eGFR was lower, the patients were less frequently treated with aspirin, clopidogrel, β-blockers, and ACE inhibitors/angiotensin receptor blockers. We demonstrated an inverse association between renal function and 1-year mortality, with the highest mortality rates observed in the group with the lowest eGFR (HR = 3.8, 95% CI 2.9-4.9, p < 0.0001). Differences in mortality remained significant following a multivariate analysis for all the baseline characteristics as well as for invasive and medical treatment (HR = 2.7, 95% CI 1.9-3.7, p < 0.0001).
Conclusions
ACS patients with chronic kidney disease represent a high-risk group with an increased mortality risk. Despite this high risk, these patients are less frequently selected for an invasive treatment strategy and are less commonly treated with guideline-based medications. However, reduced renal function was associated with higher mortality regardless of the variations in therapy.
Keywords: Renal function, Acute coronary syndrome, Outcome
Introduction
Chronic kidney disease is a frequent comorbidity among patients with acute coronary syndrome (ACS) [1, 2, 3]. Several studies have demonstrated well that even mild renal disease is an independent risk factor for cardiovascular complications and death after a myocardial infarction. Among the patients included in the VALIANT study, each reduction of the estimated glomerular filtration rate (eGFR) by 10 units was associated with a 10% increase in the risk for death and nonfatal cardiovascular outcomes [1]. Different factors associated with impaired renal function are believed to contribute to adverse outcomes of patients with ACS. These factors include insulin resistance [4, 5], oxidative stress [6], inflammation [7], endothelial dysfunction [8], vascular calcifications [9], and hypercoagulability [10]. Furthermore, the presence of chronic kidney disease is associated with a higher prevalence of baseline cardiovascular comorbidities including diabetes, heart failure, previous myocardial infarction, and stroke [1, 2, 11].
Despite the clear association of renal dysfunction with adverse cardiovascular outcomes, little is known about how ACS patients with impaired renal function are managed. Several studies have demonstrated that ACS patients with chronic kidney disease, compared to patients with normal renal function, are more commonly selected for a conservative rather than an invasive strategy approach with an early coronary angiogram and subsequent angioplasty [1, 2, 12]. It has also been demonstrated that guideline-based medications such as β-blockers, ACE inhibitors, statins, and antiplatelets are underutilized in ACS patients with chronic kidney disease [1, 2, 12]. However, the majority of the available data is related to patients with advanced chronic kidney disease, and it is not clear whether these differences in treatment remain in patients with mild or moderate chronic kidney disease. It is also unclear whether the differences in therapy contribute to the adverse outcomes of patients with renal dysfunction.
The current study aims to evaluate invasive and medical therapy in ACS patients according to their renal function and to assess the effect of differences in therapy on the clinical outcomes of ACS patients.
Subjects and Methods
Study Population
The Acute Coronary Syndrome Israeli Survey (ACSIS) is a biennial, 2-month survey that has been carried out since 1992 in all intensive coronary care units and cardiology departments in Israel. The study population consisted of those patients with ACS (ST- and non-ST-segment elevation myocardial infarction and unstable angina pectoris) included in the ACSIS during 2000-2013. Excluded were patients with cardiogenic shock at presentation. Demographic, historical, and clinical data were recorded by the study physicians on prespecified forms for consecutive participants. The diagnosis of ACS was based on clinical, electrocardiographic, and enzymatic criteria, and eligibility for the study was validated before discharge from the hospital. The patients were managed at the discretion of each center.
Renal Function Assessment
Serum creatinine levels were recorded at presentation to the hospital. The eGFR was calculated using the simplified Modification of Diet in Renal Disease (MDRD) formula [13]:
For women, the product of this equation was multiplied by a factor of 0.742.
The distribution of the eGFRs was divided into 4 categories (<45, 45-59.9, 60-74.9, and ≥75 mL/min/1.73 m2), incorporating the guidelines of the National Kidney Foundation [14].
Outcomes
The primary outcome of our study was all-cause mortality at 1 year. Mortality rates were determined for all participants from hospital charts and by matching the identification numbers of the patients with the Israeli National Population Registry. Secondary outcomes included in-hospital mortality and the occurrence of Thrombolysis in Myocardial Infarction (TIMI) major bleeding.
Statistical Analysis
Categorical variables are expressed as percentage and continuous variables are expressed as mean ± SD. The study cohort was stratified into 4 groups according to the renal function assessment (groups 1-4). The comparison of population characteristics was performed by χ2 test or Fisher exact test for categorical variables and by the Student t test or Wilcoxon rank tests, as appropriate, for continuous variables and secondary outcomes. Kaplan-Meier survival curves with the Mantel-Haenszel log-rank test were used to compare survival. We conducted a Cox proportional-hazards analysis to estimate the HRs and 95% CIs for all-cause mortality at 1 year.
To adjust for differences in baseline clinical characteristics and comorbidities, invasive coronary procedures during hospitalization, and medical therapy at discharge, a step-wise multivariable logistic regression analysis (for age, body mass index, gender, diabetes mellitus, hypertension, smoking status, prior myocardial infarction, prior percutaneous coronary intervention [PCI], prior coronary artery bypass graft, congestive heart failure, cerebrovascular accident or transient ischemic attack, peripheral vascular disease, cholesterol levels, coronary angiography and revascularization during hospitalization, and medical therapy with aspirin, clopidogrel, β-blockers, ACE inhibitors or angiotensin receptor blockers [ARBs], and statins at hospital discharge) was used to examine prognostic factors for the outcomes.
A p value of <0.05 was considered to indicate statistical significance. All statistical analyses were performed with the use of SAS statistical software version 9.1.
Results
Baseline Characteristics
The 13,194 patients that were included in the study had a mean age of 63.5 ± 13 years and included 25.8% females. The mean (±SD) eGFR was 82.83 ± 51 mL/min/1.73 m2. A total of 5,506 (41.7%) of the patients had an eGFR of ≥75 mL/min/1.73 m2, 2,444 (18.6%) had an eGFR of 60-74.9 mL/min/1.73 m2, 1,639 (12.4%) had an eGFR of 45-59.9 mL/min/1.73 m2, and 3,605 (27.3%) had an eGFR of <45 mL/min/1.73 m2. Patients with reduced renal function were older and more frequently female. The prevalence of most of the coexisting conditions at baseline - including hypertension, diabetes, and prior cardiovascular disease including prior myocardial infarction, congestive heart failure, and coronary revascularization, as well as cerebrovascular and peripheral arterial disease - increased with decreasing eGFRs (Table 1). Accordingly, the proportion of patients who were receiving cardiovascular pharmacotherapies (antiplatelets, statins, β-blockers, and ACE inhibitors/ARBs) at baseline increased with decreasing eGFRs.
Table 1.
Baseline characteristics of the study population according to eGFR (mL/min/1.73 m2)
| Group 1 ≥75 (n = 5,506) | Group 2 60 – 74.9 (n = 2,444) | Group 3 45 – 59.9 (n = 1,639) | Group 4 <45 (n = 3,605) | p value | |
|---|---|---|---|---|---|
| Creatinine, mg/dL | 0.9 ± 0.15 | 1.1 ± 0.13 | 1.3 ± 0.19 | 3.2 ± 1.9 | <0.0001 |
| eGFR, mL/min/1.73 m2 | 112.6 ± 44.4 | 67.9 ± 4.3 | 53.2 ± 4.3 | 30.2 ± 11.4 | 0.00044 |
| Age, years | 57.9 ± 11.61 | 65.3 ± 11.5 | 70.8 ± 10.8 | 68.2 ± 13.3 | <0.0001 |
| BMI | 28.2 ± 13.2 | 27.7 ± 7.3 | 27.8 ± 8.6 | 28.4 ± 16.2 | 0.65226 |
| Female gender | 15.4 | 23 | 33.3 | 31.1 | <0.0001 |
| Diabetes | 30.2 | 31.7 | 40.5 | 41.4 | <0.0001 |
| Hypertension | 48.2 | 61 | 74.8 | 63.2 | <0.0001 |
| Current smoker | 49.88 | 31.32 | 22.33 | 26.92 | <0.0001 |
| Myocardial infarction | 24 | 28.4 | 37.1 | 35.7 | <0.0001 |
| PCI | 25 | 28.9 | 31.7 | 26.4 | <0.0001 |
| CABG | 6.2 | 10.9 | 15.4 | 13.4 | <0.0001 |
| CHF | 2.9 | 5.6 | 11.8 | 15.1 | <0.0001 |
| CVA/TIA | 5 | 7.6 | 11.5 | 11.1 | <0.0001 |
| PVD | 4.8 | 7.6 | 10.7 | 14.4 | <0.0001 |
| Cholesterol, mg/dL | 189.1 ± 45.7 | 184.7 ± 42.1 | 180 ± 48 | 187.2 ± 47.4 | <0.0001 |
| HDL, mg/dL | 39.8 ± 12.4 | 41.6 ± 12.4 | 42 ± 13.4 | 41.2 ± 13.1 | <0.0001 |
| LDL, mg/dL | 115.6 ± 39 | 112.9 ± 37.1 | 106.6 ± 39.5 | 101.76 ± 39 | <0.0001 |
| Triglycerides, mg/dL | 174.1 ± 129.5 | 240.2 ± 360.1 | 150.5 ± 104.2 | 149.8 ± 111.5 | <0.0001 |
| Medicationsa | |||||
| Aspirin | 39.7 | 49.9 | 56.1 | 61.7 | <0.0001 |
| Clopidogrel | 7 | 7.8 | 10.3 | 12.4 | <0.0001 |
| Anticoagulants | 1.6 | 3.4 | 5.4 | 8.2 | <0.0001 |
| β-Blockers | 28.2 | 36.6 | 45.2 | 51.2 | <0.0001 |
| ACE-Is/ARBs | 28.9 | 37.2 | 49.5 | 50 | <0.0001 |
| Statins | 38.3 | 44 | 48.9 | 51.9 | <0.0001 |
Values are presented as percentage or mean ± SD. eGFR, estimated glomerular filtration rate; BMI, body mass index; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; CHF, congestive heart failure; CVA, cerebrovascular accident; TIA, transient ischemic attack; PVD, peripheral vascular disease; HDL, high-density lipoprotein; LDL, low-density lipoprotein; ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
Chronic prehospitalization medical therapy.
Treatment Characteristics
Table 2 compares the treatment characteristics of the patients according to their renal function. Patients with a reduced eGFR were less likely to be admitted to a coronary care unit or a cardiology ward and less commonly underwent an echocardiographic assessment for left ventricular ejection fraction. As the eGFR was lower, the patients were more frequently selected for a conservative approach with significantly lower rates of coronary angiograms and subsequent PCIs during the index hospitalization. Conversely, patients with lower eGFRs were more commonly referred for surgical revascularization with coronary artery bypass grafting. Interestingly, patients with chronic kidney disease were less frequently treated with guideline-based cardiovascular medications including antiplatelets, statins, and β-blockers. In contrast, these patients more commonly received antianginal medication and anticoagulants.
Table 2.
In-hospital treatment according to eGFR (mL/min/1.73 m2)
| Group 1 ≥75 (n = 5,506) | Group 2 60 – 74.9 (n = 2,444) | Group 3 45 – 59.9 (n = 1,639) | Group 4 <45 (n = 3,605) | p value | |
|---|---|---|---|---|---|
| Procedure | |||||
| Coronary angiography | 91 | 88.2 | 82.3 | 66.7 | <0.0001 |
| PCI | 74 | 73.8 | 66.1 | 53.6 | <0.0001 |
| CABG | 4.3 | 4.3 | 5.7 | 5.7 | 0.00391 |
| Echocardiography | 79 | 77.5 | 78.2 | 74.3 | <0.0001 |
| Admission ward | |||||
| CCU/cardiology | 86.11 | 85.24 | 80.72 | 80.67 | <0.0001 |
| Internal | 12.56 | 13.28 | 17.5 | 17.5 | <0.0001 |
| Other | 1.33 | 1.48 | 1.78 | 1.84 | <0.0001 |
| Medical therapya | |||||
| Aspirin | 96.8 | 95.4 | 92.3 | 89.9 | <0.0001 |
| Clopidogrel | 76.5 | 75.1 | 65.3 | 47.3 | <0.0001 |
| Anticoagulants | 2.6 | 4.2 | 6.3 | 7.6 | <0.0001 |
| β-Blockers | 81.4 | 81.1 | 78.8 | 73.9 | <0.0001 |
| Nitrates | 9.9 | 16.4 | 19.5 | 34.9 | <0.0001 |
| ACE-Is/ARBs | 74.6 | 77.5 | 77.7 | 59.6 | <0.0001 |
| Diuretics | 11.2 | 20.7 | 33.6 | 34 | <0.0001 |
| Statins | 89.6 | 87.6 | 84.2 | 65.7 | <0.0001 |
Values are presented as percentage. eGFR, estimated glomerular filtration rate; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; CCU, coronary care unit; ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
Medical therapy at discharge from hospital.
Outcomes
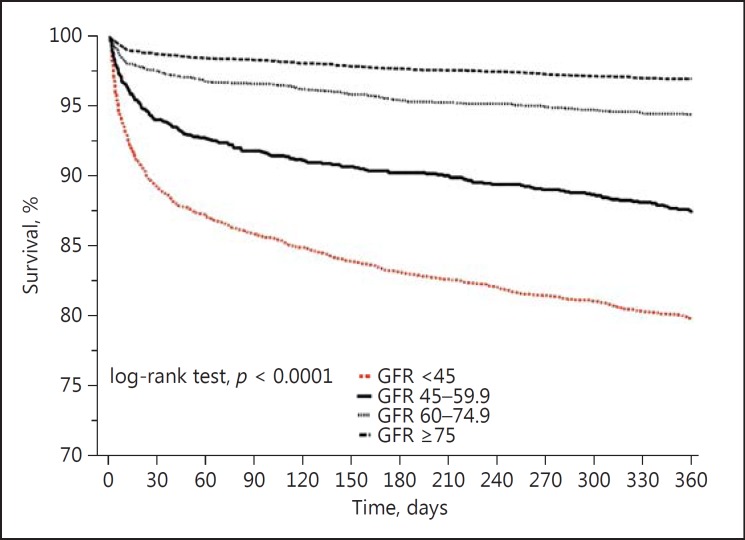
Lower eGFRs were associated with higher mortality rates (Fig. 1). The unadjusted Kaplan-Meier estimates of survival at 1 year were 97% in the group with an eGFR of ≥75 mL/min/ 1.73 m2, 94.5% in the group with an eGFR of 60-74.9 mL/min/1.73 m2, 87.3% in the group with an eGFR of 45-59.9 mL/min/1.73 m2, and 80% in the group with an eGFR <45 mL/min/1.73 m2.
Fig. 1.
Kaplan-Meier curves for 1-year all-cause mortality according to renal function. GFR, glomerular filtration rate.
Using the group with an eGFR of ≥75 mL/min/1.73 m2 as the reference group yielded unadjusted HRs for all-cause death that increased as the degree of renal impairment increased (Table 3). In the adjusted model (for all the baseline characteristics listed in Table 1), groups with a lower eGFR had worse outcomes than the reference group, with the worst outcomes in the group with the lowest eGFR (HR = 3.8, 95% CI 2.9-4.9, p < 0.0001) (Table 3). In order to evaluate the effect of differences in therapy on the outcome, we conducted a second multivariate analysis with adjustment for all the baseline characteristics with the addition of coronary angiograms and PCIs during hospitalization and medical therapy at discharge with aspirin, clopidogrel, β-blockers, and ACE inhibitors/ARBs until hospital discharge. Following this analysis, the 1-year mortality risk of patients with low eGFRs dropped slightly but still remained significantly higher than in the reference group (HR = 2.7, 95% CI 1.9-3.7, p < 0.0001) (Table 3).
Table 3.
HRs for 1-year all-cause mortality according to renal function (eGFR, mL/min/1.73 m2)
| Group 1 ≥75 | Group 2 60 – 74.9 | Group 3 45 – 59.9 | Group 4 <45 | |
|---|---|---|---|---|
| Unadjusted HR (95% CI] | 1 | 2 (1.6 – 2.5) | 4.4 (3.6 – 5.4) | 7.6 (6.4 – 9) |
| p value | <0.0001 | <0.0001 | <0.0001 | |
| HR adjusted for baseline characteristicsa (95% CI] | 1 | 1.3 (0.9 – 1.8) | 2 (1.5 – 2.7) | 3.8 (2.9 – 4.9) |
| p value | 0.07 | <0.0001 | <0.0001 | |
| HR adjusted for baseline characteristicsa and treatmentb (95% CI] | 1 | 1.4 (1 – 1.9) | 1.9 (1.4 – 2.7) | 2.7 (1.9 – 3.7) |
| p value | 0.05 | 0.0002 | <0.0001 | |
Age, body mass index, gender, diabetes mellitus, hypertension, smoking status, prior myocardial infarction, prior percutaneous coronary intervention, prior coronary artery bypass graft, congestive heart failure, cerebrovascular accident or transient ischemic attack, peripheral vascular disease, and cholesterol levels.
Coronary angiography and revascularization during hospitalization, and medical therapy with aspirin, clopidogrel, (3-blockers, ACE inhibitors or angiotensin receptor blockers, and statins at hospital discharge.
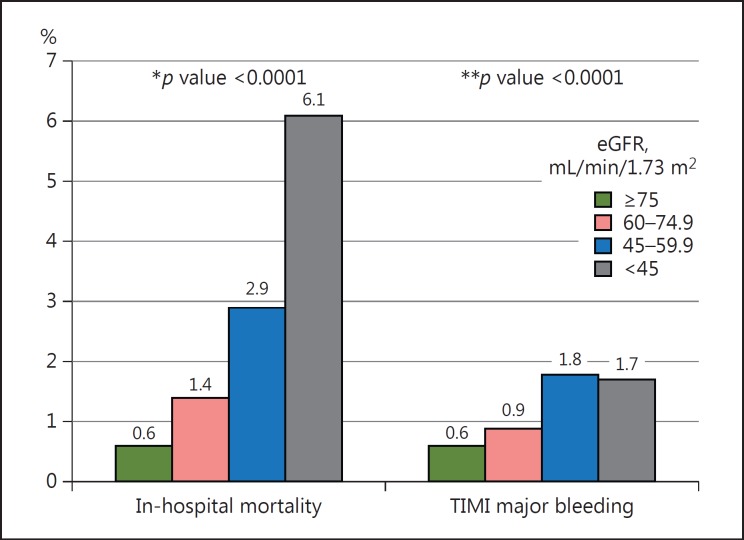
Similar to the primary endpoint, decreasing eGFRs were associated with increased in-hospital mortality. An eGFR <60 mL/min/1.73 m2 was associated with a 3-fold increased risk for TIMI major bleeding during hospitalization (Fig. 2).
Fig. 2.
Secondary outcomes of the study population according to renal function. * p value for the trend of in-hospital mortality according to renal function. ** p value for the trend of Thrombolysis in Myocardial infarction (TIMI) major bleeding. eGFR, estimated glomerular filtration rate.
Discussion
The current study demonstrated an inverse association between the eGFR and 1-year mortality risk of patients admitted with ACS. While the association of impaired renal function with adverse clinical outcome in ACS patients has been demonstrated previously, our study is the first to include a comprehensive analysis of treatment characteristics according to renal function. We demonstrated that despite their high risk for adverse outcomes, patients with chronic kidney disease were less frequently referred for coronary angiography and subsequent angioplasty and were less commonly treated with guideline-based medical therapy. However, differences in outcomes between the 4 renal function groups remained significant even following multivariate adjustment for all clinical and demographic baseline characteristics as well as for coronary angiography and revascularization during hospitalization and medical therapy at discharge.
Chronic kidney disease is a very strong predictor of adverse clinical outcomes in patients with ACS [2, 3, 4]. The mechanism of this association is not fully understood and seems to be multifactorial [5, 6, 7, 8, 9, 10, 15, 16, 17, 18, 19]. Several studies have demonstrated significant differences in the treatment of ACS patients according to their renal function. A Canadian cohort of 5,549 consecutive patients admitted with ACS between 1997 and 1999 demonstrated that medical interventions with β-blockers, acetylsalicylic acid, lipid-lowering therapy, and thrombolysis were significantly less likely to be used in patients with eGFRs <60 mL/min/1.73 m2[15]. Similar findings were made in another study on 3,106 patients with acute myocardial infarction. Patients with moderate or severe chronic kidney disease received adjunctive and reperfusion therapies less frequently than those with normal renal function [2]. Not surprisingly, postdischarge death was less likely in patients who received acute reperfusion therapy, aspirin, and β-blocker therapy. These findings were supported by several other studies on patients with ACS [12, 20].
However, most of these studies were conducted almost 2 decades ago, when fibrinolytic therapy was commonly used and treatment with several guideline-based medications such as ACE inhibitors, statins, and clopidogrel was not well established. Moreover, most of the available data are related to patients with advanced chronic kidney disease, and it is not clear whether these differences in treatment remain in patients with mild or moderate renal dysfunction. It is also unclear whether the differences in therapy contribute to the adverse outcomes of patients with renal dysfunction. In the current study, we demonstrated that differences in treatment are present even among patients with moderate chronic kidney disease (eGFR <60 and >45 mL/min/1.73 m2). Furthermore, following adjustment for in-hospital coronary procedures and medical therapy at discharge, the 1-year mortality risk decreased but remained significant.
Despite their increased mortality risk, patients with chronic kidney disease were less frequently selected for an invasive strategy with an early coronary angiogram and subsequent angioplasty and were less commonly treated with guideline-based medications. This observation, referred to as the “treatment risk paradox,” has been described before in different populations of ACS patients that underwent early risk stratification using various risk scores [21, 22]. In these studies, rates of coronary angiography, revascularization, and medical treatment decreased with increasing patient risk. Both patient-related factors (frailty, mental and functional status, and patient preference) and physician-related factors (misjudgment of a patient's risk at baseline) appear to contribute substantially to this phenomenon. Other factors that may explain differences in therapy among patients with chronic kidney disease include the risk of contrast-induced nephropathy and the potential risk of bleeding. It was previously shown that the risk of contrast-induced nephropathy is higher in patients with chronic kidney disease even among nondiabetics and may occur in up to 40% of patients with an eGFR <60 mL/min/1.73 m2 [23, 24]. Consequently, contrast-induced nephropathy was strongly associated with in-hospital complications and increased mortality risk.
ACS patients who develop major bleeding are a well-recognized high-risk population for death and cardiovascular complications [25, 26]. A large study on 17,421 ACS patients who were included in the ACUITY and the HORIZON MI studies demonstrated that even moderate chronic kidney disease is a strong and independent predictor of major bleeding during the first year [27]. These findings, which were supported by further reports [28, 29], may partially explain the more conservative approach that was more frequently selected for patients with chronic kidney disease. Furthermore, a meta-analysis of 9 trials involving 9,969 ACS patients with chronic kidney disease demonstrated that the benefits from antiplatelet therapy among these patients are potentially outweighed by bleeding hazards [30]. Similarly, in the current study we demonstrated that an eGFR <60 mL/min/1.73 m2 was associated with a 3-fold increased risk for TIMI major bleeding. Patients with advanced chronic kidney disease are usually excluded from major ACS clinical trials, and therefore data regarding the efficacy and safety of the different medications and interventions are frequently derived from post hoc analyses of trials of broader populations. Therefore, the clinical evidence is often unsatisfactory and in some cases even contradictory. For example, while several studies have demonstrated the clinical benefit of primary PCI to patients with STEMI (ST-segment elevation myocardial infarction) regardless of their renal function [31, 32], data from the GRACE registry demonstrated similar in-hospital mortality rates for patients with STEMI and severe chronic kidney disease, regardless of whether coronary revascularization was achieved [33]. Other possible explanations for the lower utilization of guideline-based pharmacotherapy in ACS patients with chronic kidney disease may include the higher prevalence of comorbidities and, as a consequence, more contraindications and side effects of medications, such as the increased risk of statin induced-myopathy [34] or renal functional deterioration and hyperkalemia with ACE inhibitors and ARBs.
The present study has several limitations. First, previous serum creatinine levels were not available to us, and therefore some patients may have presented with acute rather than chronic kidney disease. Second, all the equations that are used for GFR estimation - including the MDRD formula - are based on serum creatinine levels. Since creatinine is secreted in the renal tubules, these equations may overestimate the measured GFR. Nevertheless, the creatinine-based eGFR is still the most common mode for renal function assessment in clinical practice. Cystatin C is an alternative serum measure of kidney function that approximates direct measures of GFR and is less influenced by age, sex, or muscle mass. Therefore, the association between renal function and cardiovascular outcomes of patients with ACS may be more accurately assessed using cystatin C measurements rather than the eGFR. However, cystatin C was not measured in our study. Third, the primary endpoint of our study was all-cause mortality, and specific causes of death were not available. Nevertheless, it is reasonable to assume that in a population of patients with ACS, the majority of fatalities during the first year following hospital discharge would be due to cardiovascular causes. Finally, given the extreme differences in baseline characteristics between the 4 groups, even the most appropriate multivariate analysis may fail to isolate baseline renal function.
In conclusion, our findings support the available data regarding the association of chronic kidney disease with adverse outcomes in ACS patients. We demonstrated that despite their high risk, patients with chronic kidney disease are less frequently selected for an invasive strategy and are less commonly treated with guideline-based medical therapy. However, reduced renal function was associated with higher mortality regardless of the variations in therapy. The question whether a more invasive strategy and a more frequent administration of the conventional medical therapy can improve the outcomes of these patients should be investigated in future studies.
Disclosure Statement
The authors have no conflict of interest regarding this paper.
Statement of Ethics
The study has been approved by the local ethics committee. The authors have no ethical conflicts regarding this paper.
References
- 1.Anavekar NS, McMurray JJ, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 2.Wright RS, Reeder GS, Herzog CA, et al. Acute myocardial infarction and renal dysfunction: a high-risk combination. Ann Intern Med. 2002;137:563–570. doi: 10.7326/0003-4819-137-7-200210010-00007. [DOI] [PubMed] [Google Scholar]
- 3.El-Menyar A, Zubaid M, Sulaiman K, et al. In-hospital major clinical outcomes in patients with chronic renal insufficiency presenting with acute coronary syndrome: data from a registry of 8,176 patients. Mayo Clin Proc. 2010;85:332–340. doi: 10.4065/mcp.2009.0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caccamo G, Bonura F, Bonura F, et al. Insulin resistance and acute coronary syndrome. Atherosclerosis. 2010;211:672–675. doi: 10.1016/j.atherosclerosis.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 5.Schauer IE, Snell-Bergeon JK, Bergman BC, et al. Insulin resistance, defective insulin-mediated fatty acid suppression, and coronary artery calcification in subjects with and without type 1 diabetes. The CACTI Study. Diabetes. 2011;60:306–314. doi: 10.2337/db10-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taki K, Takayama F, Tsuruta Y, Niwa T. Oxidative stress, advanced glycation end product, and coronary artery calcification in hemodialysis patients. Kidney Int. 2006;70:218–224. doi: 10.1038/sj.ki.5000330. [DOI] [PubMed] [Google Scholar]
- 7.Muntner P, Hamm LL, Kusek JW, et al. The prevalence of nontraditional risk factors for coronary heart disease in patients with chronic kidney disease. Ann Intern Med. 2004;140:9–17. doi: 10.7326/0003-4819-140-1-200401060-00006. [DOI] [PubMed] [Google Scholar]
- 8.Zoccali C. The endothelium as a target in renal diseases. J Nephrol. 2007;20((suppl 12)):S39–S44. [PubMed] [Google Scholar]
- 9.Russo D, Palmiero G, De Blasio AP, et al. Coronary artery calcification in patients with CRF not undergoing dialysis. Am J Kidney Dis. 2004;44:1024–1030. doi: 10.1053/j.ajkd.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Cetin O, Bekpinar S, Unlucerci Y, et al. Hyperhomocysteinemia in chronic renal failure patients: relation to tissue factor and platelet aggregation. Clin Nephrol. 2006;65:97–102. doi: 10.5414/cnp65097. [DOI] [PubMed] [Google Scholar]
- 11.Hanna EB, Chen AY, Roe MT, Saucedo JF. Characteristics and in-hospital outcomes of patients presenting with non-ST-segment elevation myocardial infarction found to have significant coronary artery disease on coronary angiography and managed medically: stratification according to renal function. Am Heart J. 2012;164:52–57. doi: 10.1016/j.ahj.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Schiele F, Legalery P, Didier K, et al. Impact of renal dysfunction on 1-year mortality after acute myocardial infarction. Am Heart J. 2006;151:661–667. doi: 10.1016/j.ahj.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 14.National Kidney Foundation Kidney Disease Outcome Quality Initiative (K/DOQI) clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39((suppl 1)):S1–S266. [PubMed] [Google Scholar]
- 15.Keough-Ryan TM, Kiberd BA, Dipchand CS, et al. Outcomes of acute coronary syndrome in a large Canadian cohort: impact of chronic renal insufficiency, cardiac interventions, and anemia. Am J Kidney Dis. 2005;46:845–855. doi: 10.1053/j.ajkd.2005.07.043. [DOI] [PubMed] [Google Scholar]
- 16.Pai AS, Giachelli CM. Matrix remodeling in vascular calcification associated with chronic kidney disease. J Am Soc Nephrol. 2010;21:1637–1640. doi: 10.1681/ASN.2010040349. [DOI] [PubMed] [Google Scholar]
- 17.Schiele F. Renal dysfunction and coronary disease: a high-risk combination. J Nephrol. 2009;22:39–45. [PubMed] [Google Scholar]
- 18.Ochodnicky P, Vettoretti S, Henning RH, et al. Endothelial dysfunction in chronic kidney disease: determinant of susceptibility to end-organ damage and therapeutic response. J Nephrol. 2006;19:246–258. [PubMed] [Google Scholar]
- 19.Hage FG, Venkataraman R, Zoghbi GJ, et al. The scope of coronary heart disease in patients with chronic kidney disease. J Am Coll Cardiol. 2009;53:2129–2140. doi: 10.1016/j.jacc.2009.02.047. [DOI] [PubMed] [Google Scholar]
- 20.Bae EH, Lim SY, Cho KH, et al. GFR and cardiovascular outcomes after acute myocardial infarction: results from the Korea Acute Myocardial Infarction Registry. Am J Kidney Dis. 2012;59:795–802. doi: 10.1053/j.ajkd.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Yan AT, Yan RT, Tan M, et al. Management patterns in relation to risk stratification among patients with non-ST elevation acute coronary syndromes. Arch Intern Med. 2007;167:1009–1016. doi: 10.1001/archinte.167.10.1009. [DOI] [PubMed] [Google Scholar]
- 22.Rozenbaum Z, Elis A, Shuvy M, et al. CHA2DS2-VASc score and clinical outcomes of patients with acute coronary syndrome. Eur J Intern Med. 2016;36:57–61. doi: 10.1016/j.ejim.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Marenzi G, Lauri G, Assanelli E, et al. Contrast-induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2004;44:1780–1785. doi: 10.1016/j.jacc.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 24.Rihal CS, Textor SC, Grill DE, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259–2264. doi: 10.1161/01.cir.0000016043.87291.33. [DOI] [PubMed] [Google Scholar]
- 25.Vavalle JP, Clare R, Chiswell K, et al. Prognostic significance of bleeding location and severity among patients with acute coronary syndromes. JACC Cardiovasc Interv. 2013;6:709–717. doi: 10.1016/j.jcin.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manoukian SV, Feit F, Mehran R, et al. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol. 2007;49:1362–1368. doi: 10.1016/j.jacc.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 27.Mehran R, Pocock SJ, Nikolsky E, et al. A risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol. 2010;55:2556–2566. doi: 10.1016/j.jacc.2009.09.076. [DOI] [PubMed] [Google Scholar]
- 28.Khan R, Lopes RD, Neely ML, et al. Characterising and predicting bleeding in high-risk patients with an acute coronary syndrome. Heart. 2015;101:1475–1484. doi: 10.1136/heartjnl-2014-307346. [DOI] [PubMed] [Google Scholar]
- 29.Sabbag A, Guetta V, Fefer P, et al. Temporal trends and outcomes associated with major bleeding in acute coronary syndromes: a decade-long perspective from the Acute Coronary Syndrome Israeli Surveys 2000-2010. Cardiology. 2015;132:163–171. doi: 10.1159/000430838. [DOI] [PubMed] [Google Scholar]
- 30.Medi C, Montalescot G, Budaj A, et al. Reperfusion in patients with renal dysfunction after presentation with ST-segment elevation or left bundle branch block: GRACE (Global Registry of Acute Coronary Events) JACC Cardiovasc Interv. 2009;2:26–33. doi: 10.1016/j.jcin.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 31.Steg PG, James SK, Atar D, et al. Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC) Eur Heart J. 2012;33:2569–2619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 32.Keeley EC, Kadakia R, Soman S, et al. Analysis of long-term survival after revascularization in patients with chronic kidney disease presenting with acute coronary syndromes. Am J Cardiol. 2003;92:509–514. doi: 10.1016/s0002-9149(03)00716-1. [DOI] [PubMed] [Google Scholar]
- 33.Palmer SC, Di Micco L, Razavian M, et al. Effects of antiplatelet therapy on mortality and cardiovascular and bleeding outcomes in persons with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med. 2012;156:445–459. doi: 10.7326/0003-4819-156-6-201203200-00007. [DOI] [PubMed] [Google Scholar]
- 34.Armitage J, Bowman L, Wallendszus K, et al. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a doubleblind randomised trial. Lancet. 2010;376:1658–1669. doi: 10.1016/S0140-6736(10)60310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]