Abstract
Background/Aims
Brain natriuretic peptide (BNP) is secreted by cardiomyocytes under stretch condition. High blood levels are associated with decreased patient survival in heart failure patients and in hemodialysis (HD) patients. We report the monthly BNP change in the first months of HD therapy in incident patients and its relationship with fluid removal and cardiac history (CH).
Methods
All patients starting HD therapy in our unit from May 2008 to December 2012 were retrospectively analyzed. Every month (M1 to M6), BNP was assessed before a midweek dialysis session. CH, monthly pre- and postdialysis blood pressure, and postdialysis body weight were collected.
Results
A total of 236 patients were included in the analysis. The median BNP at HD start was 593 (175-1,433) pg/mL, with a significant difference between CH− and CH+ patients (291 vs. 731 pg/mL, p < 0.0001). Mortality was significantly higher in patients in the higher BNP tertile. BNP decreased significantly between M1 and M2 and then plateaued. The BNP change between M1 and M2 and between M1 and M6 was significantly correlated with the initial fluid removal. Applying stepwise multiple regression, the BNP change between M1 and M2 was significantly and independently related to fluid removal. The BNP level at M6 was also related to patient survival.
Conclusions
We confirm that in incident HD patients, BNP level is related to fluid excess and cardiac status. The BNP decrease in the first months of HD therapy is related to fluid excess correction. BNP appears as an important tool to evaluate hydration status correction after HD onset.
Keywords: Hemodialysis, Incident patients, Fluid overload, Brain natriuretic peptide, Survival
Introduction
Brain natriuretic peptide (BNP) is secreted by cardiomyocytes under stretch condition [1]. In heart failure patients, high BNP blood levels are associated with decreased patient survival [2]. This association has also been reported in hemodialysis (HD) patients, for whom it is difficult to know whether a BNP increase is related to the cardiac condition, fluid excess, or both. Zoccali et al. [3] found that BNP is a strong predictor of both left ventricular mass and ejection fraction. However, we have shown in a small cohort of incident HD patients that the initial fluid removal is significantly associated with BNP decrease. It is suggested that BNP is a surrogate of fluid excess and its consequences on the cardiac structure. However, BNP as an indicator of fluid status remains controversial [4]. In our unit, we perform monthly assessment of plasma BNP level in all patients. In this study, we report the BNP change in the first months of HD therapy in incident patients, its relationship with fluid excess correction, and its relationship with cardiac history (CH) and patient survival.
Subjects and Methods
From May 2008 to December 2012, all incident HD patients in our unit were included in this retrospective study. The end of the follow-up was September 2014. All dialysis parameters and biological data were integrated in an electronic heath record (Winlap®, Metz, France). The BNP level was assessed at dialysis onset, then averaged monthly in case of several monthly data from month 1 (M1) to month 6 (M6). BNP from predialysis midweek session blood samples was assessed routinely. The BNP level was determined using a chemiluminescent microparticle immunoassay on i8200 (Architect Abbott, Paris, France; The Grand Vallon's Biochemistry Laboratory, Cerballiance, Sainte-Foy-lès-Lyon, France). The normal value is <100 pg/mL. For each patient were collected the CH including coronary artery disease, valve disease, heart failure, left ventricular hypertrophy, and arrhythmia. A patient was considered to have a CH if he presented one of these conditions (presence, CH+; absence, CH-). In addition, were collected the monthly average of pre- and postdialysis blood pressure (BP) as well as the postdialysis prescribed body weight (BW). The initial BW decrease was calculated as the difference between the BW at the first HD session and that after 8 weeks, the nadir of BW change as previously reported [5].
Statistical Analysis
The data are presented as median and interquartile range. The patients were studied as a whole group as well as in the CH− and CH+ subgroups. Differences between the subgroups were compared using Mann-Whitney and χ2 tests. The BNP at M1 (M1BNP) was analyzed using stepwise multiple regression. Survival among M1BNP tertiles was studied using Kaplan-Meier and multivariate Cox analysis including age, gender, albumin, C-reactive protein, cancer and CH, diabetes, and M1BNP tertiles. The monthly BNP change was studied applying the Friedman test for repeated measures. The BNP difference between M1 and M2 and between M1 and M6 was consecutively calculated in the whole group as well as the CH− and CH+ subgroups and compared using the Wilcoxon test. Relationships between BNP and systolic BP changes were studied using Spearman rank correlation and multiple regression analysis. Survival according to M6BNP was evaluated with Kaplan-Meier and Cox analysis.
Results
Patient Cohort
Among 242 incident patients from May 2008 to December 2012, 236 were included after exclusion of 6 patients because of missing data. The patient characteristics are displayed in Table 1. All patients were under high-efficiency HD with high-flux polysulfone dialyzers and prescribed blood and dialysate flows of 300–350 and 500 mL/min, respectively, after several weeks. The average treatment time at 3 months was 4.8 h. The median follow-up was 2.2 years (range, 0.8-3.2). CH was present in 153 patients (64.8%) as coronary artery disease (21.2%), arrhythmia (12.8%), heart failure (13.2%), left ventricular hypertrophy (48%), valve disease (7.2%), or other cardiac disease (8.5%). These CH+ patients were older, with more catheters at HD onset. Diabetes, long-lasting hypertension history, and male gender prevalence were higher, but without reaching significance (Table 1).
Table 1.
Patients characteristics at hemodialysis start
| All | CH- | CH+ | |
|---|---|---|---|
| Number of patients | 236 | 83 | 153 |
| Age, years | 75.4 (62.2 – 82.1) | 65.4 (55.2 – 78.0) | 74.4 (67.5 – 83.5)** |
| Female gender, % | 34 | 42 | 29 |
| Body mass index | 25.4 (21.3 – 27.9) | 24.4 (20.4 – 27.1) | 25.9 (22.0 – 28.2) |
| Diabetes, % | 41 | 33 | 45 |
| Long-lasting hypertension, % | 67 | 59 | 71 |
| Catheter at start, % | 43 | 30 | 56* |
| Initial BNP value, pg/mL | 593 (175 – 1,433) | 291 (118 – 964) | 731 (230 – 1,799)* |
Data are presented as median (interquartile range) unless indicated otherwise. BNP, brain natriuretic peptide; CH, cardiac history. Comparisons between CH– and CH+ patients were run using Mann-Whitney and χ2 tests (* p < 0.001, ** p < 0.0001).
M1BNP
The median BNP level at M1 was 593 (175-1,433) pg/mL. It was significantly higher in CH+ patients (731 vs. 291 pg/mL). According to the multiple regression analysis, the M1BNP level was positively and independently associated with age (p = 0.0020), C-reactive protein (p = 0.0007), and CH (0.0026), but not with gender, serum albumin, or body mass index (BMI). The prognostic value of BNP at M1 in the whole group is displayed in Figure 1. Mortality was significantly higher in the higher M1BNP 3rd tertile when compared to the 1st and 2nd tertiles (p < 0.0001; 13, 18, and 39 deaths in the 1st, 2nd, and 3rd tertiles, respectively; 3rd-tertile hazard ratio = 3.9, 95% CI = 2.2-6.9). The Cox analysis found that only age (p = 0.0024) and the 3rd BNP tertile at M1 (p = 0.0050, 95% CI = 1.20-2.71) were associated with the patients' mortality, whereas serum albumin, BMI, C-reactive protein, gender, CH, and diabetes were not. The same analysis was performed for M1BNP in the CH− patients. There were 4, 4, and 7 deaths in the 1st, 2nd, and 3rd tertiles, respectively, and the log-rank test was marginally significant (p = 0.043). In CH+ patients 9, 14, and 32 deaths were observed in the 1st, 2nd, and 3rd tertiles, respectively (log-rank test p = 0.0004).
Fig. 1.
Survival curve according to the M1BNP tertiles in the whole group of incident hemodialysis patients. BNP, brain natriuretic peptide.
BNP Change during the First 6 Months
The median BNP level decreased significantly between M1 and M2 from 610 (191-1,361) to 291 (113-753) pg/mL (p < 0.0001). Figure 2 displays median BNP from M1 to M6 (125 patients with complete data). BNP declined significantly between M1 and M2 and then plateaued as shown in the figure (Friedman test analysis; p < 0.0001). From M2 to M6, BNP was significantly lower than at M1. M2BNP was significantly higher than M4BNP, M5BNP, and M6BNP. Between M1 and M2, the median BNP decreased from 382 (137-1,199) to 196 (76-567) pg/mL in CH− patients (p = 0.0001) and from 729 (221-1,561) to 395 (133-887) pg/mL in CH+ patients (p < 0.0001). The proportion of patients with a normal BNP value (<100 pg/mL) increased from 8.4% at M1 to 21% at M2 and 35% at M6 (Fig. 3 reports the paired comparison between M1 and M6 in the whole group). At M6 the BNP level was <100 pg/mL in 45% of CH− and 30% of CH+ patients.
Fig. 2.
Brain natriuretic peptide (BNP) decline after hemodialysis start (median ± 95% CI) according to the Friedman test. * p < 0.05 between M1BNP and M2-M6BNP; #p < 0.05 between M2BNP and M3-M6BNP.
Fig. 3.
Brain natriuretic peptide (BNP) levels at M1 and M6 (* p < 0.0001). The grey line represents the upper range for normal BNP value (<100 pg/mL). The number of patients with a normal BNP level increased from 20 to 60 patients in paired comparison (p < 0.0001).
The BNP change between M2 and M1 was related to the initial BW decrease in the whole group (r = 0.29, p = 0.0001; Fig. 4), in the CH− patients (r = 0.26, p = 0.041), and in the CH+ patients (r = 0.30, p = 0.008). The BNP change between M6 and M1 was also correlated with the initial BW change in the whole group (r = 0.33, p < 0.0001), in the CH− patients (r = 0.46, p < 0.0001), and in the CH+ patients (r = 0.26, p = 0.006). The correlation between the percent change in BNP between M1 and M6 and the percent change in predialysis systolic BP between M1 and M6 was of borderline significance (p = 0.068). Analyzed using multiple regression, the BNP change between M1 and M2 was significantly associated with initial fluid removal (p = 0.0014), but not with age, gender, diabetes, BMI, CH, or cancer history.
Fig. 4.
Relationship between the brain natriuretic peptide (BNP) change between M1 and M2 and the correction of fluid excess between in the first weeks of hemodialysis start (r = 0.29; p = 0.0001). BW, body weight.
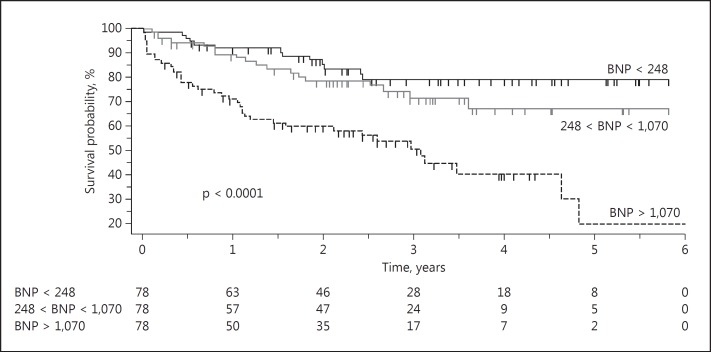
The survival analysis applying the Kaplan-Meier method displayed a significantly higher risk of death for the higher BNP tertile at M6 in the whole group (Fig. 5, with 7, 6, and 26 deaths in the 1st, 2nd, and 3rd tertiles, respectively; log-rank test p < 0.0001). In CH+ patients, the higher M6BNP tertile was also significantly associated with increased mortality (log-rank test p = 0.0002, with 5, 4, and 21 deaths in the 1st, 2nd, and 3rd tertiles, respectively), but it remained borderline for CH− patients (log-rank test p = 0.051, with 2, 2, and 5 deaths in the 1st, 2nd, and 3rd tertiles, respectively). In the multivariate Cox analysis applied to the whole group, age and M6BNP were independently associated with mortality, whereas BMI at M6, diabetes, CH, gender, and predialysis systolic BP at M6 were not. Receiver operating characteristic curve analysis of the whole group showed an area under the curve of 0.72 (p < 0.0001) and a BNP threshold of 359 pg/mL (Fig. 6). In CH− and CH+ patients the area under the curve was 0.71 in both subgroups, with a BNP threshold of 413 and 359 pg/mL, respectively.
Fig. 5.
Survival according to the brain natriuretic peptide (BNP) tertiles at M6. The log-rank test is significant (p < 0.0001; hazard ratio for being in the upper tertile = 4.09 [1.88-8.93]).
Fig. 6.
Receiver operating characteristic curve analyzing the sensitivity and specificity of M6BNP on survival in the whole group of patients.
Discussion
Our data confirm that BNP is a marker of fluid overload in incident dialysis patients, with a significant reduction associated with fluid removal. Probing for dry weight remains a cornerstone of the management of incident patients in our unit, with correction of high BP with a nadir of postdialysis BW at the end of M2 [5, 6]. We found a significant correlation between the initial fluid removal and the BNP change between M1 and M2, but also between M1 and M6. The high BNP levels at dialysis start underline the deleterious effect of fluid excess on the heart. Fluid accumulates early all along the progression of chronic kidney disease [7]. Fluid removal by probing alleviates this burden and besides hypertension improvement, the BNP decrease highlights the beneficial cardiac effect of fluid management. As previously reported in a small number of patients [8], we confirm here that the patients' CH influences the BNP level, but we found that even in these patients, fluid overload is a main explanatory parameter of the BNP level. BNP is a marker of cardiac stretch under the effect of fluid overload. It is used in emergency rooms to differentiate dyspnea from cardiac and pulmonary origins [9]. Recently, it has been recognized that beside fluid overload (“wet BNP”), BNP increase may occur independently of fluid overload (“dry BNP”) [10]. This explains why patients with cardiac disease (referred to as CH+ patients in our study) have higher BNP levels, complicating their interpretation in dialysis patients prone to fluid excess. However, our findings demonstrate that even though fluid removal normalizes BNP less frequently in CH+ than in CH− patients (30 vs. 45%), the significant BNP decrease under probing highlights the contribution of fluid excess even in CH+ patients.
Recently, direct measurement of fluid excess using bioelectrical impedance analysis (BIA) techniques clearly found an association of fluid excess and BNP increase. Tapolyai et al. [11] reported an exponential relationship between the BNP level and the fluid excess assessed using a multifrequency BIA device (BCM®), with a high specificity of BNP level increase in patients with relative fluid overload >15%, the level associated with increased mortality when patients' fluid status is assessed with this device [12, 13]. In another study and using another BIA multifrequency device, Sivalingam et al. [14] found a significantly higher BNP level and a much lower proportion of patients with normal BNP values (<100 pg/mL) in overhydrated patients. Moreover, Celik et al. [15] reported a significant BNP increase from baseline during the hospital stay in patients with acute complications requiring prolonged hospitalization. Concomitantly a significant decrease in postdialysis BW was associated with a BNP decrease within several weeks after discharge. Our hypothesis is that acute complications trigger catabolism and disrupt fluid balance reflected by the BNP increase. However, in that study we were not able to correlate the BNP and BW changes. The number of patients was small (42), and there was a large heterogeneity of clinical conditions. However, Ohashi et al. [16] recently pointed out the critical role in of body cell mass decrease dialysis patients, reflected by extracellular and intracellular fluid imbalance, and its relationship with BNP increase, confirming our hypothesis.
BNP as a marker of fluid overload has been disputed by Agarwal [4] based on an ancillary analysis of the DRIP study [17]. The DRIP study is the first and only randomized controlled trial showing that probing is efficient to decrease BP in hypertensive prevalent HD patients. A group of patients underwent additional ultrafiltration (UF) and was compared to a control group with no additional UF. Opposite to our data, in that study BNP change was not found to be associated with BW or BP changes. Surprisingly, BNP decreased also in the control group during the 8-week study period; the reason is not clear. Moreover, the authors found that the BNP change was associated with the baseline BNP value. The BNP median was low, at 93 pg/mL, whereas in our study, the median BNP at M1 was 501 pg/mL. It is possible that the fact that 50% of the patients had normal BNP values introduced a bias in catching BNP changes associated with additional UF. Also, no analysis regarding CH, which can influence the BNP level (“dry BNP” as referred to above), was mentioned in that study. Last but not least, the BW change was not reported. If we refer to the DRIP study [17], the BW change in the UF group was 1.0 kg after 8 weeks, representing 1.2% of the baseline BW. This BW decrease is much lower than that in our study, where it was 5.2% of the initial BW. It is also possible that a number of patients had to prematurely stop the additional UF because of adverse symptoms (endpoint of the study), impairing the possibility of a significant BNP change.
Regarding BNP as a prognostic factor, we confirmed that the BNP value in the first month of HD therapy is associated with patient survival, as previously reported [3]. It was largely significant in the whole group and in the CH+ patients. However, it was of borderline significance in CH− patients. The difference between the 2 subgroups (CH− and CH+) may be interpreted by the fact that the BNP increase in incident CH− patients is related to fluid overload that is reversible, and that it reflects a temporary marker of reversible cardiac impairment, efficiently corrected by probing.
Our study has important limitations. It is observational, not interventional, and we cannot ascertain that probing applied in a controlled study would confirm the reduction of BNP level. The DRIP study [17] was controlled, but its own limitations have been discussed above. However, studying incident patients at this very specific moment of the patient pathway reduces the cross-sectional limitations of prevalent cohorts. Moreover, we do not have data of blood volume monitoring (BVM) in our patients. This tool assessing the plasma volume changes during the session related to UF is an indirect marker of fluid overload related to patient survival [18]. A low plasma volume change reflects a higher refilling rate from interstitial spaces. BNP data have been rarely analyzed with BVM. Both van de Pol et al. [19] and De Mauri et al. [20] reported that a higher refilling rate with a flatter BVM slope was associated with higher BNP level, reflecting fluid overload. No comparison exists between these tools in managing fluid in chronic HD patients. Also, we do not have BIA data, as discussed previously. There is urgent need to evaluate prospectively in an interventional study the combination of the direct quantification of fluid overload from BIA, BVM, and BNP as the marker of cardiac impairment related to fluid excess. Finally, urine output and residual renal function were not routinely recorded in our unit, and these data are not available for the current study. The BNP level is not influenced by glomerular filtration rate compared to N-terminal pro-B-type natriuretic peptide [21]. However, we cannot exclude that patients with higher urine volume at M6 may have lower fluid excess or easier fluid management with lower BNP values.
In conclusion, BNP appears as an important tool to assess both fluid overload and fluid excess correction in incident dialysis patients. Its interpretation regarding cardiac status is of primary importance. In a significant proportion of patients, even with cardiac disease, normal BNP can be achieved. Further studies are needed to evaluate the importance of longitudinal BNP follow-up and to determine at which frequency it is needed with a positive benefit/cost ratio. Regarding the high financial burden of fluid overload-related hospitalizations [22], it may represent a very important tool for the clinician and the community.
Statement of Ethics
At admission to the unit, the patients sign a consent form, allowing their data to be computerized and analyzed for clinical research purposes. The study was approved by the internal ethical board of the NephroCare Tassin-Charcot unit.
Disclosure Statement
No conflict of interest applies for any of the authors regarding this study. No funding or grant were used or obtained for this study.
References
- 1.Joffy S, Rosner MH. Natriuretic peptides in ESRD. Am J Kidney Dis. 2005;46:1–10. doi: 10.1053/j.ajkd.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Anand IS, Fisher LD, Chiang YT, Latini R, Masson S, Maggioni AP, Glazer RD, Tognoni G, Cohn JN, Val-HeFT Investigators Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the Valsartan Heart Failure Trial (Val-HeFT) Circulation. 2003;107:1278–1283. doi: 10.1161/01.cir.0000054164.99881.00. [DOI] [PubMed] [Google Scholar]
- 3.Zoccali C, Mallamaci F, Benedetto F, Tripepi G, Parlongo S, Cataliotti A, Cutrupi S, Giacone G, Bellanuova I, Cottini E, Malatino L, Creed Investigators Cardiac natriuretic peptides are related to left ventricular mass and function and predict mortality in dialysis patients. J Am Soc Nephrol. 2001;12:1508–1515. doi: 10.1681/ASN.V1271508. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal R. B-type natriuretic peptide is not a volume marker among patients on hemodialysis. Nephrol Dial Transplant. 2013;28:3082–3089. doi: 10.1093/ndt/gft054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chazot C, Vo-Van C, Deleaval P, Lorriaux C, Hurot JM, Mayor B, Jean G. Predialysis systolic blood pressure evolution in incident hemodialysis patients: effects of the dry weight method and prognostic value. Blood Purif. 2012;33:275–283. doi: 10.1159/000337101. [DOI] [PubMed] [Google Scholar]
- 6.Charra B, Laurent G, Chazot C, Calemard E, Terrat JC, Vanel T, Jean G, Ruffet M. Clinical assessment of dry weight. Nephrol Dial Transplant. 1996;11:16–19. doi: 10.1093/ndt/11.supp2.16. [DOI] [PubMed] [Google Scholar]
- 7.Essig M, Escoubet B, de Zuttere D, Blanchet F, Arnoult F, Dupuis E, Michel C, Mignon F, Mentre F, Clerici C, Vrtovsnik F. Cardiovascular remodelling and extracellular fluid excess in early stages of chronic kidney disease. Nephrol Dial Transplant. 2008;23:239–248. doi: 10.1093/ndt/gfm542. [DOI] [PubMed] [Google Scholar]
- 8.Chazot C, Vo-Van C, Lorriaux C, Mayor B, Hurot JM, Deleaval P, Zaoui E, Jean G. Brain natriuretic peptide in incident hemodialysis patients: effects of fluid removal, cardiac status and prognostic value. World Congress of Nephrology, Cape Town. 2015 [Google Scholar]
- 9.Maisel AS, McCord J, Nowak RM, Hollander JE, Wu AH, Duc P, Omland T, Storrow AB, Krishnaswamy P, Abraham WT, Clopton P, Steg G, Aumont MC, Westheim A, Knudsen CW, Perez A, Kamin R, Kazanegra R, Herrmann HC, McCullough PA, Breathing Not Properly Multinational Study Investigators Bedside B-type natriuretic peptide in the emergency diagnosis of heart failure with reduced or preserved ejection fraction. Results from the Breathing Not Properly Multinational Study. J Am Coll Cardiol. 2003;41:2010–2017. doi: 10.1016/s0735-1097(03)00405-4. [DOI] [PubMed] [Google Scholar]
- 10.Francis GS, Felker GM, Tang WH. A test in context: critical evaluation of natriuretic peptide testing in heart failure. J Am Coll Cardiol. 2016;67:330–337. doi: 10.1016/j.jacc.2015.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tapolyai M, Faludi M, Reti V, Lengvarszky Z, Szarvas T, Fulop T, Beko G, Berta K. Volume estimation in dialysis patients: the concordance of brain-type natriuretic peptide measurements and bioimpedance values. Hemodial Int. 2013;17:406–412. doi: 10.1111/hdi.12023. [DOI] [PubMed] [Google Scholar]
- 12.Chazot C, Wabel P, Chamney P, Moissl U, Wieskotten S, Wizemann V. Importance of normohydration for the long-term survival of haemodialysis patients. Nephrol Dial Transplant. 2012;27:2404–2410. doi: 10.1093/ndt/gfr678. [DOI] [PubMed] [Google Scholar]
- 13.Wizemann V, Wabel P, Chamney P, Zaluska W, Moissl U, Rode C, Malecka-Masalska T, Marcelli D. The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transplant. 2009;24:1574–1579. doi: 10.1093/ndt/gfn707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sivalingam M, Vilar E, Mathavakkannan S, Farrington K. The role of natriuretic peptides in volume assessment and mortality prediction in haemodialysis patients. BMC Nephrol. 2015;16:218. doi: 10.1186/s12882-015-0212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Celik G, Silinou E, Vo-Van C, Jean G, Chazot C. Plasma BNP, a useful marker of fluid overload in hospitalized hemodialysis patients. Hemodial Int. 2012;16:47–52. doi: 10.1111/j.1542-4758.2011.00627.x. [DOI] [PubMed] [Google Scholar]
- 16.Ohashi Y, Saito A, Yamazaki K, Tai R, Matsukiyo T, Aikawa A, Sakai K. Brain natriuretic peptide and body fluid composition in patients with chronic kidney disease: a cross-sectional study to evaluate the relationship between volume overload and malnutrition. Cardiorenal Med. 2016;6:337–346. doi: 10.1159/000447024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal R, Alborzi P, Satyan S, Light RP. Dry-weight reduction in hypertensive hemodialysis patients (DRIP): a randomized, controlled trial. Hypertension. 2009;53:500–507. doi: 10.1161/HYPERTENSIONAHA.108.125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwal R. Hypervolemia is associated with increased mortality among hemodialysis patients. Hypertension. 2010;56:512–517. doi: 10.1161/HYPERTENSIONAHA.110.154815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van de Pol AC, Frenken LA, Moret K, Baumgarten R, van der Sande FM, Beerenhout CM, Kooman JP, Leunissen KM. An evaluation of blood volume changes during ultrafiltration pulses and natriuretic peptides in the assessment of dry weight in hemodialysis patients. Hemodial Int. 2007;11:51–61. doi: 10.1111/j.1542-4758.2007.00154.x. [DOI] [PubMed] [Google Scholar]
- 20.De Mauri A, Bellomo G, Navino C, David P, Chiarinotti D, Capurro F, Brustia M, De Maria M, Ruva CE, Ciardi L, De Leo M. Plasma B-type natriuretic peptide in dialyzed patients: marker of cardiovascular disease or link to plasma refilling. J Nephrol. 2011;24:507–514. doi: 10.5301/JN.2011.6240. [DOI] [PubMed] [Google Scholar]
- 21.Bednarek-Skublewska A, Zaluska W, Ksiazek A. The relationship between serum level of N-terminal pro-B-type natriuretic peptide and nutritional status, and inflammation in chronic hemodialysis patients. Clin Nephrol. 2010;73:14–20. doi: 10.5414/cnp73014. [DOI] [PubMed] [Google Scholar]
- 22.Arneson TJ, Liu J, Qiu Y, Gilbertson DT, Foley RN, Collins AJ. Hospital treatment for fluid overload in the Medicare hemodialysis population. Clin J Am Soc Nephrol. 2010;5:1054–1063. doi: 10.2215/CJN.00340110. [DOI] [PMC free article] [PubMed] [Google Scholar]