Abstract
Aims
The aim of this study was to evaluate the efficacy of levocarnitine injection for renal anemia in hemodialysis patients.
Methods
In this randomized controlled clinical trial, we randomly assigned patients on maintenance hemodialysis at our hospital to receive levocarnitine injections (n = 30) or no injection (n = 30) and monitored the patients during 12 months of treatment. In the treatment group, patients received an injection of levocarnitine 1,000 mg 3 times weekly after hemodialysis sessions. All patients received recombinant human erythropoietin as an erythropoiesis-stimulating agent (ESA). Response to ESA therapy was determined by calculating the erythropoietin responsiveness index (ERI; ESA dose·kg−1·g−1· dL−1·week−1).
Results
(1) The target levels of hemoglobin and hematocrit were maintained during the study period in both the levocarnitine group and the control group. (2) The dose of ESAs required to maintain these levels decreased gradually in the levocarnitine group and was significantly lower at 6 and 12 months than at study initiation. Furthermore, the dose of ESAs was significantly lower than that in the control group at 12 months. (3) The ERI showed a significant decrease at 6 and 12 months in the levocarnitine group, with a significant difference between the 2 groups at 12 months.
Conclusion
Our results suggest that levocarnitine administration can reduce the dose of ESAs required in patients with renal anemia on hemodialysis and improve the response to ESA therapy.
Keywords: Erythropoiesis-stimulating agents, Erythropoietin responsiveness index, Hemodialysis, Levocarnitine, Renal anemia
Introduction
Renal anemia is a common complication of chronic kidney disease that negatively affects the patients' quality of life but also increases the risk of cardiovascular events and is associated directly and indirectly with various infections [1, 2, 3]. Thus, renal anemia clearly has a strong influence on prognosis. However, there are concerns about the adverse effects of treatment with erythropoiesis-stimulating agents (ESAs), including an increased risk of cardiovascular events and death [4, 5, 6]. Therefore, it is desirable to minimize the use of ESAs.
Carnitine is a derivative of the essential amino acids lysine and methionine (molecular weight 161). Biosynthesized primarily in the liver and kidney, carnitine is present in almost all tissues, particularly in skeletal and cardiac muscles. When renal function is normal, carnitine is filtered by the glomerulus and then reabsorbed in the renal tubule, resulting in stable carnitine levels in the body. Carnitine deficiency is a clinical condition wherein the body is unable to use long-chain fatty acids in tissues as an energy source because the interstitial carnitine content is low. A wide variety of symptoms can result from carnitine deficiency, including fat accumulation in the organs, hyperammonemia due to liver dysfunction, skeletal muscle weakness, and cardiomyopathy [7]. Apart from hemodialysis, certain anticonvulsants and antibiotics can also cause secondary carnitine deficiency [7]. In hemodialysis patients, carnitine imbalance or deficiency can result from insufficient intake, a decrease in carnitine biosynthesis, or removal of carnitine from the body during dialysis [8]. Carnitine is also thought to be associated with the maintenance of red blood cells, including maintenance of their numbers, substrate storage capacity, membrane lipid turnover, and protein construction.
Previous studies have explored whether carnitine supplementation in hemodialysis patients results in better function and longevity of the red blood cells, thus decreasing the need for ESA [9, 10, 11]. We previously reported that anemia, atherosclerosis, and cardiac activity were improved by oral levocarnitine therapy in hemodialysis patients [12].
In the present study, we administered levocarnitine injections to hemodialysis patients, recorded the ESA dose per week, and calculated the erythropoietin responsiveness index (ERI) in order to examine whether carnitine was effective in correcting renal anemia.
Methods
Patients and Study Protocol
This randomized controlled clinical trial included patients (1) who underwent hemodialysis at our hospitals; (2) whose medical decisions were made at our hospitals; and (3) who were aged 20–85 years. The study excluded (1) patients who had previously taken levocarnitine in either oral or injected form; (2) patients who were taking any carnitine preparation as a supplement; (3) patients who had difficulty communicating owing to dementia or other factors; (4) patients with acute inflammation; (5) patients taking an immunosuppressive drug, steroid, or antibiotic; and (6) patients with a history of blood transfusion within the past 6 months. This prospective, open-label, randomized, parallel, controlled, multicenter trial screened 208 patients who were undergoing maintenance hemodialysis at our hospital. Sixty of these patients were selected to participate in the study and were randomly assigned to the levocarnitine group, which received levocarnitine injections (n = 30), or to the control group, which did not receive injections (n = 30). Randomization was carried out by dynamic allocation based on age, sex, hemodialysis vintage, hemoglobin level, and presence or absence of diabetes mellitus. Thus, we ensured that there were no significant differences in baseline characteristics between the 2 groups. An independent investigator with no prior knowledge of the participants monitored the randomization of participants based on entry order. Details of the assignments were then given to 6 independent investigators.
All patients underwent hemodialysis or hemodiafiltration therapy 3 times per week in 4-h sessions at 3 Japanese blood purification centers. All patients included in the study received recombinant human erythropoietin (rHuEPO) as an ESA. The patients in the treatment group additionally received levocarnitine 1,000 mg by injection 3 times weekly after dialysis. The patients were monitored for 12 months. According to the Japanese Society for Dialysis Therapy (JSDT) guidelines, iron deficiency is diagnosed according to the following criteria: transferrin saturation ≤20% and serum ferritin level ≤100 ng/mL [13]. In such patients, if there were no contraindications for iron preparations, 13 doses of 40 mg of saccharated ferric oxide solution were administered. At the end of dialysis, these agents were slowly administered via the venous side of the dialysis circuit.
Study Evaluations
We monitored the monthly average dosage of ESAs and assessed the relationship between the ESA dose per week and the responsiveness index of renal anemia. ERI (ESA dose·kg−1·g−1·dL−1·week−1) was defined as the average weekly units of ESA divided by clinical dry weight (in kg) and average blood hemoglobin (in g/dL) to normalize the amount of required ESA to the severity of anemia [14]. At the beginning of each week, a blood sample was obtained from each patient before dialysis began. Blood cell counts and levels of serum creatinine, serum urea nitrogen, albumin, electrolytes, total cholesterol, low-density lipoprotein cholesterol, triglyceride, serum iron, total iron binding capacity, and serum ferritin were measured by routine clinical chemistry procedures using commercial kits. High-sensitivity C-reactive protein and serum β2-microglobulin levels were measured by latex agglutination. Intact parathyroid hormone was measured by radioimmunoassay. These variables were evaluated at baseline and at 12 months (at the end of the study). N-terminal pro-brain natriuretic peptide levels were measured by the electro-chemiluminescence immunoassay method. The cardiothoracic ratio was measured by assessing the chest X-ray at baseline and at 12 months. In addition, we set the following endpoints: death, severe cardiovascular lesion, incapacity for oral intake, or the onset of a serious adverse event.
Echocardiography was conducted immediately after the midweek hemodialysis session to minimize any influence of the patient's hydration state at the beginning and end of the study. Echocardiography was performed using Vivid T7® (GE Healthcare, Tokyo, Japan). All patients were examined by a single trained cardiologist who was blinded to the documentation of the participants' clinical characteristics; examinations were conducted at baseline and after 12 months of levocarnitine treatment (or equivalent times for the control group) to determine the ejection fraction.
Statistical Analysis
Data were expressed as means ± standard deviations or medians (interquartile ranges) as appropriate. Continuous variables were compared by the Student t test or the Mann-Whitney U test, and categorical variables were compared by the χ2 test or the Fisher exact test as appropriate to the data distribution. A repeated-measures analysis of variance was used to compare the values at baseline and at 6 and 12 months. Statistical significance was set at p < 0.05. All analyses were performed using the JMP version 12 software (SAS Institute Ltd., Cary, NC, USA).
Results
Baseline Demographic and Clinical Data
All enrolled patients remained in the study until the end of the trial. The patients' characteristics are described in Table 1. The levocarnitine group included 21 men and 9 women, while the control group included 17 men and 13 women. The average duration of dialysis was 48 ± 77 months for the levocarnitine group and 52 ± 54 months for the control group. Diabetic nephropathy was the primary disease in 53% of the patients in the levocarnitine group and in 57% of the patients in the control group. There were no significant differences in the comorbidities, medications, and laboratory data between the 2 groups at baseline. At the start of the experiment, the mean phosphorus, corrected calcium, and intact parathyroid hormone values were all within the target management value of the chronic kidney disease-mineral and bone disorder guidelines from the JSDT [15].
Table 1.
Patient characteristics and laboratory data at baseline
| Levocarnitine group | Control group | p value | |
|---|---|---|---|
| Patients | 30 | 30 | |
| Male/female | 21/9 | 17/13 | 0.291 |
| Age, years | 70 ± 10 | 69 ± 11 | 0.781 |
| Duration of dialysis, months | 48 ± 77 | 52 ± 54 | 0.761 |
| Diabetes mellitus, % | 53 | 57 | 0.799 |
| Dry weight, kg | 57.8 ± 8.9 | 57.4 ± 8.0 | 0.805 |
| Cardiothoracic ratio, % | 49.8 ± 4.5 | 49.6 ± 5.2 | 0.865 |
| Comorbidities, % | |||
| Ischemic heart disease | 17 | 13 | 0.723 |
| Cerebrovascular disease | 10 | 13 | 0.693 |
| Peripheral artery disease | 3 | 7 | 0.561 |
| Medications, % | |||
| RAS inhibitors | 83 | 80 | 0.743 |
| Calcium channel blockers | 73 | 80 | 0.549 |
| (3-Blockers | 27 | 33 | 0.581 |
| Vitamin D | 90.0 | 87 | 0.693 |
| Phosphate binders | 100 | 100 | – |
| Statins | 47 | 50 | 0.800 |
| Iron supplementation | 0 | 0 | – |
| Laboratory data | |||
| Hemoglobin, g/dL | 11.0 ± 1.1 | 10.8 ± 1.2 | 0.491 |
| ESA dose, units/week | 6,747 ± 4,562 | 6,241 ± 5,226 | 0.691 |
| ERI | 10.7 ± 7.3 | 10.0 ± 7.9 | 0.717 |
| Distribution of ERI, n | 0.502 | ||
| <5.0 | 5 | 9 | |
| 5.0±10.0 | 11 | 8 | |
| 10.1±15.0 | 5 | 5 | |
| >15.0 | 9 | 8 | |
| Serum urea nitrogen, mg/dL | 59.2 ± 13.4 | 60 ± 13.4 | 0.885 |
| Creatinine, mg/dL | 8.2 ± 2.3 | 8.5 ± 1.9 | 0.601 |
| Albumin, g/dL | 3.7 ± 0.4 | 3.7 ± 0.4 | 0.805 |
| Total cholesterol, mg/dL | 149 ± 25 | 157 ± 27 | 0.252 |
| LDL cholesterol, mg/dL | 83 ± 24 | 88 ± 17 | 0.342 |
| Triglyceride, mg/dL | 127 ± 63 | 131 ± 61 | 0.811 |
| C-reactive protein, mg/dL | 0.28 ± 0.14 | 0.31 ± 0.14 | 0.412 |
| Corrected calcium, mg/dL | 9.1 ± 0.4 | 9.1 ± 0.4 | 0.792 |
| Phosphate, mg/dL | 5.5 ± 0.7 | 5.4 ± 0.7 | 0.574 |
| Intact PTH, pg/mL | 142 ± 80 | 153 ± 98 | 0.643 |
| Iron, µg/mL | 66 ± 28 | 61 ± 27 | 0.554 |
| TSAT, % | 25.2 ± 7.0 | 24.0 ± 8.1 | 0.472 |
| Ferritin, ng/mL | 111 ± 49 | 99 ± 49 | 0.328 |
| (β2-Microglobulin, mg/L | 24.7 ± 7.0 | 26.9 ± 4.7 | 0.173 |
| Median NT-proBNP (IQR), pg/mL | 9,310 (4,855 – 14,050) | 7,990 (2,995 – 14,200) | 0.596 |
Values are means ± standard deviations unless otherwise indicated. ERI, erythropoiesis resistance index; ESA, erythropoiesis-stimulating agent; IQR, interquartile range; LDL, low-density lipoprotein; NT-proBNP, N-terminal pro-brain natriuretic peptide; PTH, parathyroid hormone; RAS, renin-angiotensin system; TSAT, transferrin saturation.
All enrolled patients completed the trial. During the study period, angiotensin receptor blocker treatment was interrupted in 1 patient, the angiotensin receptor blocker dose was reduced in 1 patient, and the calcium channel blocker dose was reduced in 2 patients in the levocarnitine group. In the control group, angiotensin receptor blocker treatment was initiated and interrupted in 1 patient each, while the calcium channel blocker dose was increased in 1 patient and reduced in 1 patient. During the study period, none of the patients took β-blockers in the levocarnitine group or control group. In 1 patient in the levocarnitine group and 2 patients in the control group, the dose of vitamin D was increased during the study period because of secondary hyperparathyroidism. None of the patients required newly initiated vitamin D therapy. The dose of levocarnitine was 17.8 ± 2.9 mg/kg in a single dose, being 53.6 ± 8.9 mg/kg weekly in the levocarnitine group. None of the patients in the control group took any carnitine preparation as a supplement during the study.
Changes in Hemoglobin, Required ESA Dose, and ERI during the Study
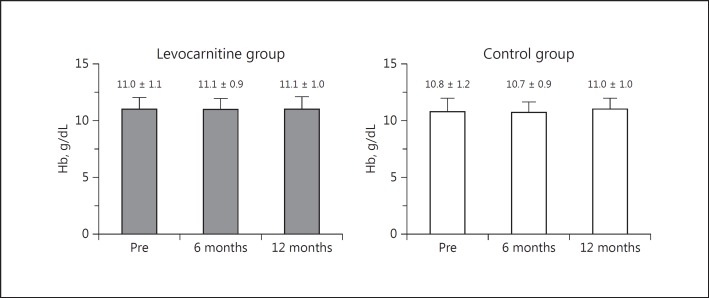
Before treatment was initiated, there were no significant differences between the levocarnitine group and the control group in hemoglobin, ESA dose, or ERI (Table 1). Hemoglobin values were within the target values specified in the JSDT guidelines on anemia in chronic kidney disease treatment [13]. There was no significant difference in the distribution of ERI between the 2 groups. As shown in Figure 1, there was no significant change in the hemoglobin level in each group during the study period, and no significant difference was noted between the 2 groups during the study period.
Fig. 1.
Changes in Hb values from baseline to 6 and 12 months in the treatment and control groups with a comparison between the 2 groups. Hb, hemoglobin; Pre, before treatment.
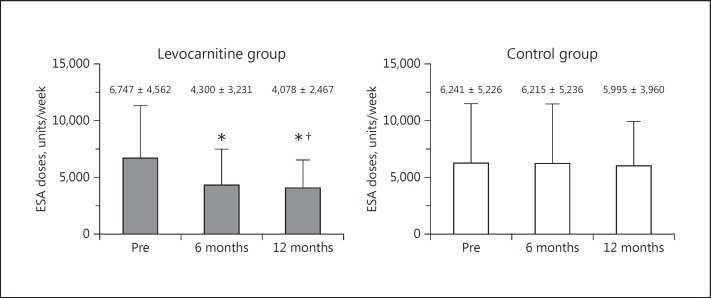
Figure 2 shows the required ESA doses per week. In the levocarnitine group, the administration of ESA per week significantly decreased from 6,747 ± 4,562 units/week to 4,300 ± 3,231 units/week at 6 months (p < 0.0001). A further significant decrease to 4,078 ± 2,467 units/week at 12 months was observed (p < 0.0001). The control group showed no significant difference in the administration of ESA doses per week throughout the study period. At the 12-month time point, the required ESA dose per week was significantly lower in the levocarnitine group than in the control group (p < 0.05).
Fig. 2.
Changes in the total ESA dose per week from baseline to 6 and 12 months in the treatment and control groups with a comparison between the 2 groups. * p < 0.0001 versus Pre. †p < 0.05 versus ESA dose at the same time point in the control group. ESA, erythropoiesis-stimulating agent; Pre, before treatment.
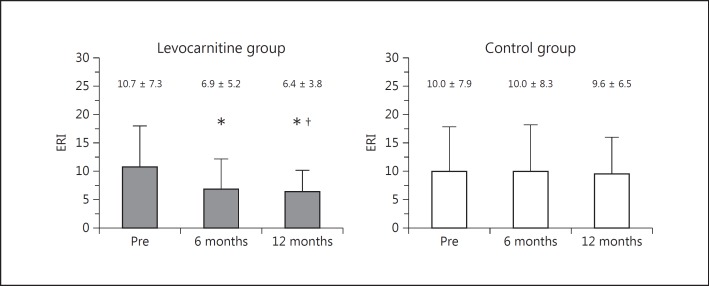
In the levocarnitine group, ERI was 10.7 ± 7.3 at the beginning of the study and decreased significantly to 6.9 ± 5.2 at 6 months (p < 0.0001) and to 6.4 ± 3.8 (p < 0.0001) at 12 months (Fig. 3). In contrast, there was no significant change in ERI in the control group during the study period. ERI in the treatment group was significantly lower than that in the control group at 12 months (p < 0.05) after the start of therapy.
Fig. 3.
Changes in ERI from baseline to 6 and 12 months in the treatment and control groups with a comparison between the 2 groups. * p < 0.0001 versus Pre. †p < 0.05 versus ERI at the same time point in the control group. ERI, erythropoiesis resistance index; Pre, before treatment.
Other Clinical Variables and Adverse Effects
Iron supplementation was administered in 17 patients in the levocarnitine group and 13 patients in the control group during the study (p = 0.309). There were no significant differences in the serum iron, transferrin saturation, and ferritin values within the groups or between the 2 groups after 12 months. Furthermore, there were no significant changes in lipid profiles, electrolytes, or intact parathyroid hormone levels within the groups or between the 2 groups after 12 months. There were no significant changes in systolic and diastolic blood pressures, heart rate, dry weight, cardiothoracic ratio, and ejection fraction within the groups or between the 2 groups after 12 months (Table 2). During the observation period, no patients in either group exhibited a significant increase in the occurrence of adverse effects, such as skin rash or gastrointestinal dysfunction.
Table 2.
Changes in patients' hemodynamics and related parameters before and after 12 months of levocarnitine treatment
| Levocarnitine group (n = 30) |
Control group (n = 30) |
|||||
|---|---|---|---|---|---|---|
| Pre | 12 months | p value | Pre | 12 months | p value | |
| Systolic BP, mm Hg | 149 ± 20 | 148 ± 20 | 0.243 | 148 ± 23 | 149 ± 22 | 0.439 |
| Diastolic BP, mm Hg | 79 ± 13 | 79 ± 14 | 0.699 | 81 ± 15 | 80 ± 15 | 0.107 |
| Heart rate, bpm | 78 ± 12 | 77 ± 11 | 0.246 | 78 ± 14 | 78 ± 13 | 0.821 |
| Dry weight, kg | 57.8 ± 8.9 | 57.5 ± 8.9 | 0.367 | 57.4 ± 8.0 | 57.9 ± 8.2 | 0.884 |
| Cardiothoracic ratio, % | 49.8 ± 4.5 | 49.9 ± 4.3 | 0.897 | 49.6 ± 5.2 | 49.8 ± 5.1 | 0.301 |
| Ejection fraction, % | 57.6 ± 8.5 | 58.7 ± 8.1 | 0.052 | 57.4 ± 9.2 | 57.3 ± 9.6 | 0.864 |
Values are means ± standard deviations. BP, blood pressure; Pre, before treatment.
Discussion
To assess the effects of levocarnitine injections on renal anemia, we examined ESA requirements and ERI for 12 months. In the present study, ERI was significantly reduced in the levocarnitine group without increased hemoglobin levels. ERI, which is an index of resistance to ESAs, decreased to approximately half its initial value at 12 months after the start of therapy. The control group showed no significant changes in either ESA administration or ERI. Levocarnitine injection improved the response to ESAs in patients undergoing hemodialysis by maintaining hemoglobin levels and decreasing the required ESA dose.
The introduction of ESAs has led to great improvements in treating renal anemia. However, some patients with renal anemia have a low response to ESAs, meaning that they fail to reach the target hemoglobin or hematocrit values despite the administration of high doses of ESA. Various parameters are used to measure the response to ESAs. The JSDT 2008 guidelines for anemia in chronic kidney disease patients recommended the rHuEPO dose per week to be an index of ESA resistance [13]. European guidelines and a report that used recent data from the JSDT have examined the relationship between ESA dose and mortality [16, 17]. Other reports use a comparison of the rHuEPO dose per week with the hemoglobin or hematocrit values (rHuEPO doses·hemoglobin−1·week−1 or rHuEPO doses·hematocrit−1·week−1) as an index of anemia [18, 19]. Other indices include the rHuEPO doses·kg−1·week−1, which is used in the European guidelines [16, 20]. In recent years, rHuEPO·kg−1·g−1·dL−1·week−1 [18, 21, 22] has been used more frequently. In our clinical trials, we examined ERI using ESA doses·kg−1·g−1·dL−1·week−1.
The association between carnitine and renal anemia has been investigated since before ESA therapy became available. Hurot et al. [9] and Trovato et al. [23] reported that the improvement of anemia was significantly better in a group treated with carnitine than in a placebo group, although the number of cases was small. Labonia [24] reported that the need for ESAs decreased as a result of carnitine administration. Matsumoto et al. [10] reported that when carnitine was administered to a group of patients with ESA-resistant anemia, anemia showed a tendency to improve. Matsumura et al. [11] reported that the dose of ESAs required was inversely proportional to serum carnitine levels. Wanic-Kossowska et al. [25] reported that a group that received a combination of carnitine and ESA showed improvement in anemia, reduction in ESA requirement, and improvement in red blood cell function as compared to groups that received only carnitine or only ESA. In addition, we have conducted a relatively large-scale study of hemodialysis patients in which 113 patients took oral levocarnitine [14]. We found that the required ESA dose had a tendency to decrease after the study began and decreased significantly in the treatment group compared with the control group at 12 months after the start of the therapy.
Previous studies have reported that long-term supplementation with levocarnitine improves myocardial contractility and decreases left ventricular volume, accompanied by the amelioration of uremic anemia or improvements in uremic anemia by the administration of ESAs, which reduced the incidence of left ventricular mass index [26]. On the other hand, in the present study, ejection fraction values were not changed by levocarnitine therapy. This was possibly because many patients in the present study might have had preserved ejection fraction at baseline, and there were few patients with an increased cardiothoracic ratio at baseline. However, further studies are needed to clarify the efficacy of levocarnitine injection therapy with respect to cardiac function in patients with left ventricular hypertrophy or lowered cardiac function, since we could not assess left ventricular mass index in the present study.
In our previous study in which oral levocarnitine was administered, adverse reactions included fish odor syndrome and gastrointestinal intolerance; these were suspected to be associated with levocarnitine treatment, and a few patients were withdrawn from the study due to poor adherence to oral levocarnitine therapy. However, in the present study, none of the patients had adverse reactions to levocarnitine injection therapy. Trimethylamine N-oxide (TMAO) is a circulating organic compound produced by the metabolism of dietary levocarnitine and choline, which was recently found to directly induce atherosclerosis in rodents [27, 28]. Both levocarnitine and choline are metabolized by interstitial bacteria to trimethylamine, a metabolite which is absorbed from the intestine and subsequently oxidized via hepatic flavin monooxygenase enzymes to form TMAO [29]. Under normal physiological conditions, circulating TMAO is rapidly cleared from the bloodstream, almost exclusively by urinary excretion [30, 31]. Therefore, increased TMAO concentrations are correlated with coronary atherosclerosis burden and may be associated with long-term mortality in patients with chronic kidney disease undergoing coronary angiography [32, 33]. However, no reports have demonstrated that oral levocarnitine treatment would lead to the acceleration of atherosclerosis due to accumulation of TMAO in hemodialysis patients. Therefore, further studies are needed to clarify whether injection therapy of levocarnitine would be useful and safe as compared to oral therapy with regard to the accumulation of TMAO or acceleration of atherosclerosis in these populations.
Our study has several limitations. First, we did not measure serum carnitine concentrations, and the levocarnitine injection used was 1,000 mg in all patients and was administered after hemodialysis 3 times a week. Therefore, the appropriate dose of levocarnitine injection could not be determined in this study. In the future, an examination of the impact of the dose of the levocarnitine injection is necessary. Second, the number of patients included in the present study was relatively small, and we did not attain a ceiling of improvement in hemodialysis patient outcomes. Therefore, interventions such as levocarnitine therapy that specifically target measurable global outcomes, such as mortality, morbidity, and health care costs, require further consideration. Finally, this study was not double blinded. Therefore, an adequately powered, high-quality clinical trial, such as a prospective double-blind, randomized, controlled trial, is needed to clarify whether improvements in ESA responsiveness due to levocarnitine treatment lead to an improved prognosis.
In conclusion, this study showed that levocarnitine injection was useful for treating hemodialysis patients with renal anemia. Such patients might benefit from levocarnitine therapy because of the amelioration of ESA responsiveness and reduction in ESA dose. Large clinical studies are necessary to ascertain whether this therapy significantly impacts mortality and morbidity in patients undergoing hemodialysis.
Statement of Ethics
The study protocol was approved by the Ethics Committee of Keiai Hospital, and all patients provided written informed consent (Clinical Trial Registration No. UMIN000025327). The study protocol was designed in accordance with the Declaration of Helsinki.
Disclosure Statement
M.A. has received honoraria from Otsuka Pharmaceuticals Co. Ltd. The other authors have no conflicts of interest to declare.
References
- 1.Johansen KL, Finkelstein FO, Revicki DA, et al. Systematic review of the impact of erythropoiesis-stimulating agents on fatigue in dialysis patients. Nephrol Dial Transplant. 2012;27:2418–2425. doi: 10.1093/ndt/gfr697. [DOI] [PubMed] [Google Scholar]
- 2.Inaba M, Hayashino Y, Shoji T, et al. Disappearance of association in diabetic patients on hemodialysis between anemia and mortality risk: the Japan dialysis outcomes and practice pattern study. Nephron Clin Pract. 2012;120:c91–c100. doi: 10.1159/000335979. [DOI] [PubMed] [Google Scholar]
- 3.Akizawa T, Saito A, Gejyo F, et al. JET Study Group Low hemoglobin levels and hypo-responsiveness to erythropoiesis-stimulating agent associated with poor survival in incident Japanese hemodialysis patients. Ther Apher Dial. 2014;18:404–413. doi: 10.1111/1744-9987.12155. [DOI] [PubMed] [Google Scholar]
- 4.Parfrey PS, Foley RN, Wittreich BH, Sullivan DJ, Zagari MJ, Frei D. Double-blind comparison of full and partial anemia correction in incident hemodialysis patients without symptomatic heart disease. J Am Soc Nephrol. 2005;16:2180–2189. doi: 10.1681/ASN.2004121039. [DOI] [PubMed] [Google Scholar]
- 5.Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 6.Palmer SC, Navaneethan SD, Craig JC, et al. Meta-analysis: erythropoiesis-stimulating agents in patients with chronic kidney disease. Ann Intern Med. 2010;153:23–33. doi: 10.7326/0003-4819-153-1-201007060-00252. [DOI] [PubMed] [Google Scholar]
- 7.Pons R, De Vivo DC. Primary and secondary carnitine deficiency syndromes. J Child Neurol. 1995;10((suppl 2)):S8–S24. [PubMed] [Google Scholar]
- 8.Fornasini G, Upton RN, Evans AM. A pharmacokinetics model for L-carnitine in patients receiving hemodialysis. Br J Clin Pharmacol. 2007;64:335–345. doi: 10.1111/j.1365-2125.2007.02926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hurot JM, Cucherat M, Haugh M, Fouque D. Effects of L-carnitine supplementation in maintenance hemodialysis patients: a systematic review. J Am Soc Nephrol. 2002;13:708–714. doi: 10.1681/ASN.V133708. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto Y, Amano I, Hirose S, Tsuruta Y, Hara S, Murata M, Imai T. Effects of L-carnitine supplementation on renal anemia in poor responders to erythropoietin. Blood Purif. 2001;19:24–32. doi: 10.1159/000014474. [DOI] [PubMed] [Google Scholar]
- 11.Matsumura M, Hatakeyama S, Koni I, Hara S, Mabuchi H, Muramoto H. Correlation between serum levels and erythrocyte osmotic fragility in hemodialysis patients. Nephron. 1996;72:574–578. doi: 10.1159/000188942. [DOI] [PubMed] [Google Scholar]
- 12.Higuchi T, Abe M, Yamazaki T, Mizuno M, Okawa E, Ando H, Oikawa O, Okada K, Kikuchi F, Soma M. Effects of levocarnitine on brachial-ankle pulse wave velocity in hemodialysis patients: a randomized controlled trial. Nutrients. 2014;6:5992–6004. doi: 10.3390/nu6125992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsubakihara Y, Nishi S, Akiba T, Hirakata H, Iseki K, Kubota M, Kuriyama S, Komatsu Y, Suzuki M, Nakai S, Hattori M, Babazono T, Hiramatsu M, Yamamoto H, Bessho M, Akizawa T. 2008 Japanese Society for Dialysis Therapy: guidelines for renal anemia in chronic kidney disease. Ther Apher Dial. 2010;14:240–275. doi: 10.1111/j.1744-9987.2010.00836.x. [DOI] [PubMed] [Google Scholar]
- 14.Higuchi T, Abe M, Yamazaki T, Okawa E, Ando H, Hotta S, Oikawa O, Kikuchi F, Okada K, Soma M. Levocarnitine improves cardiac function in hemodialysis patients with left ventricular hypertrophy: a randomized controlled trial. Am J Kidney Dis. 2016;67:260–270. doi: 10.1053/j.ajkd.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Fukagawa M, Yokoyama K, Koiwa F, Taniguchi M, Shoji T, Kazama JJ, Komaba H, Ando R, Kakuta T, Fujii H, Nakayama M, Shibagaki Y, Fukumoto S, Fujii N, Hattori M, Ashida A, Iseki K, Shigematsu T, Tsukamoto Y, Tsubakihara Y, Tomo T, Hirakata H, Akizawa T, CKD-MBD Guideline Working Group; Japanese Society for Dialysis Therapy Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther Apher Dial. 2013;17:247–288. doi: 10.1111/1744-9987.12058. [DOI] [PubMed] [Google Scholar]
- 16.Locatelli F, Aljama P, Bárány P, Canaud B, Carrera F, Eckardt KU, Hörl WH, Macdougal IC, Macleod A, Wiecek A, Cameron S, European Best Practice Guidelines Working Group Revised European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrol Dial Transplant. 2004;19((suppl 2)):ii1–ii47. doi: 10.1093/ndt/gfh1032. [DOI] [PubMed] [Google Scholar]
- 17.Fukuma S, Yamagichi T, Hashimoto S, Nakai S, Iseki K, Tsubakihara Y, Fukuhara S. Erythropoiesis-stimulating agent responsiveness and mortality in hemodialysis patients: results from a cohort study from the dialysis registry in Japan. Am J Kidney Dis. 2012;59:108–116. doi: 10.1053/j.ajkd.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Kalantar-Zadeh K, McAllister CJ, Lehn RS, Lee GH, Nissenson AR, Kopple JD. Effect of malnutrition-inflammation in maintenance hemodialysis patients. Am J Kidney Dis. 2003;42:761–773. doi: 10.1016/s0272-6386(03)00915-6. [DOI] [PubMed] [Google Scholar]
- 19.Kaysen GA, Muller HG, Ding J, Chetow GM. Challenging the validity of the EPO index. Am J Kidney Dis. 2006;47:157–166. doi: 10.1053/j.ajkd.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 20.KDOQI; National Kidney Foundation KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis. 2006;47((suppl 3)):S11–S145. doi: 10.1053/j.ajkd.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Locatelli F, Andrulli S, Memoli B, Maffei C, Del Vecchio L, Aterini S, De Simone W, Mandalari A, Brunori G, Amato M, Cianciaruso B, Zoccali C. Nutritional-inflammation status and resistance to erythropoietin therapy in hemodialysis patients. Nephrol Dial Transplant. 2006;21:991–998. doi: 10.1093/ndt/gfk011. [DOI] [PubMed] [Google Scholar]
- 22.Mallick S, Rafiroiu A, Kanthety R, Iqbal S, Malik R, Rahman M. Factors predicting erythropoietin resistance among maintenance hemodialysis patients. Blood Purif. 2012;33:238–244. doi: 10.1159/000335256. [DOI] [PubMed] [Google Scholar]
- 23.Trovato GM, Ginardi V, Di Marco V, Dell'Aira AE, Corsi M. Long-term L-carnitine treatment of chronic anemia of patients with end-stage renal failure. Curr Ther Res. 1982;31:1042–1049. [Google Scholar]
- 24.Labonia WD. L-Carnitine effects on anemia in hemodialyzed patients treated with erythropoietin. Am J Kidney Dis. 1995;26:757–764. doi: 10.1016/0272-6386(95)90439-5. [DOI] [PubMed] [Google Scholar]
- 25.Wanic-Kossowska M, Kazmierski M, Pawliczak E, Kobelski M. Combined therapy with L-carnitine and erythropoietin of anemia in chronic kidney failure patients undergoing hemodialysis. Pol Arch Med Wewn. 2007;117:1–5. [PubMed] [Google Scholar]
- 26.Trovato GM, Iannetti E, Murgo AM, Carpinteri G, Catalano D. Body composition and long-term levo-carnitine supplementation. Clin Ther. 1998;149:209–214. [PubMed] [Google Scholar]
- 27.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lang DH, Yeung CK, Peter RM, Ibarra C, Gasser R, Itagaki K, Philpot RM, Rettie AE. Isoform specificity of trimethylamine N-oxygenation by human flavin-containing monooxygenase (FMO) and P450 enzymes: selective catalysis by FMO3. Biochem Pharmacol. 1998;56:1005–1012. doi: 10.1016/s0006-2952(98)00218-4. [DOI] [PubMed] [Google Scholar]
- 30.Al-Waiz M, Mitchell SC, Idle JR, Smith RL. The metabolism of 14C-labelled trimethylamine and its N-oxide in man. Xenobiotica. 1987;17:551–558. doi: 10.3109/00498258709043962. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell SC, Zhang AQ, Noblet JM, Gillespie S, Jones N, Smith RL. Metabolic disposition of [14C]-trimethylamine N-oxide in rat: variation with dose and route of administration. Xenobiotica. 1997;27:1187–1197. doi: 10.1080/004982597239949. [DOI] [PubMed] [Google Scholar]
- 32.Wilson WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116:448–455. doi: 10.1161/CIRCRESAHA.116.305360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stubbs JR, House JA, Ocque AJ, Zhang S, Johnson C, Kimber C, Schmidt K, Gupta A, Wetmore JB, Nolin TD, Spertus JA, Yu AS. Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J Am Soc Nephrol. 2016;27:305–313. doi: 10.1681/ASN.2014111063. [DOI] [PMC free article] [PubMed] [Google Scholar]