Abstract
Background
Chronic kidney disease (CKD) is associated with a high prevalence of atrial fibrillation (AF), but in this population the risk/benefit ratio of anticoagulant therapy with vitamin K antagonists (VKA) for thromboprophylaxis is uncertain.
Summary
In end-stage renal disease (ESRD) patients undergoing hemodialysis, VKA seem less effective in stroke prevention than in the general population, with an increased risk of major bleeding. Recently, novel oral anticoagulant agents (NOACs) have proven to be effective for stroke prevention in AF and have demonstrated an improved safety profile compared to VKA. Limited data from post hoc analyses of controlled clinical trials suggest the safe and effective use of NOACs in patients with moderate renal impairment (i.e., estimated glomerular filtration rate, eGFR, between 30 and 50 mL/min). The question still remains whether NOACs can be used in patients with an eGFR <30 mL/min, since there are no studies addressing this subject. In fact, patients with CKD stage 4 and 5 were excluded from controlled clinical trials on anticoagulation therapy for stroke prevention in AF. Left atrial appendage (LAA) occlusion represents a nonpharmacological alternative for stroke prevention in patients with AF who are difficult to manage medically. Preliminary data indicate a similar efficacy and safety profile in patients with CKD compared to patients with normal renal function.
Key Messages
Stroke prevention in patients with ESRD and AF represents a clinical challenge with poor evidence. LAA occlusion may become the standard of care for stroke prevention in patients with ESRD and AF.
Keywords: Atrial fibrillation, Stroke, Chronic kidney disease, End-stage renal disease, New oral anticoagulants, Left atrial appendage occlusion
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia in the general population with an estimated prevalence of 1-2% and is associated with an increased risk of thromboembolic complications, particularly stroke [1, 2, 3, 4]. Chronic kidney disease (CKD), regardless of severity, is associated with a higher prevalence of AF compared to patients with preserved renal function [5, 6] and is an independent risk factor for increased mortality [7] and stroke in patients with AF [8]. The overall prevalence of AF is approximately 12% among dialysis patients [9]. In patients with AF and preserved renal function, anticoagulation therapy markedly reduces the risk of thromboembolism, but is associated with an increased risk of bleeding. In patients with CKD (not end-stage renal disease [ESRD]), the risk of bleeding due to vitamin K antagonists (VKA) is even greater, but the reduced risk of thromboembolic events is preserved [10]. Patients with ESRD undergoing hemodialysis represent a particular subgroup of patients in which anticoagulation therapy with warfarin seems to have an unfavorable risk/benefit ratio as it is less effective at preventing strokes and carries an increased risk of major bleeding [11, 12]. Recently, novel oral anticoagulant agents (NOACs) have proven to be effective in preventing strokes in AF and have demonstrated an improved safety profile, but these drugs are not effectively cleared in renal failure. Moreover, patients with CKD stage 4 and 5 were excluded from clinical trials on anticoagulation therapy for stroke prevention in AF. Left atrial appendage (LAA) occlusion represents a nonpharmacological alternative for stroke prevention in patients with AF who are difficult to manage medically.
Novel Oral Anticoagulants and CKD
Non-vitamin K-dependent oral anticoagulant agents, also known as NOACs, have been adopted in the last few years as alternative therapy to warfarin for thromboembolism risk reduction in patients with AF. These drugs have been shown to be equal or more effective than VKA at preventing thromboembolism, have an improved safety profile, and do not require continuous level monitoring. An important limitation of NOACs, besides their increased cost and our limited experience with their antidotes, is the fact that they all depend on renal elimination to a varying degree. There is a risk of drug accumulation in the presence of renal disease, particularly in patients with advanced CKD. Patients with an estimated glomerular filtration rate (eGFR) <30 mL/min were excluded in all 4 randomized trials comparing NOACs with warfarin [13, 14, 15, 16], contributing to the lack of evidence regarding their use in patients with advanced CKD and ESRD. In fact, current cardiology guidelines for the management of patients with AF suggest not using NOACs in the setting of severe CKD [17, 18]. The 2016 guidelines of the European Society of Cardiology confirm this position with 1 caveat, apixaban can be administered in patients with an eGFR as low as 25 mL/min.
Dose adjustments are suggested in patients with eGFR between 50 and 30 mL/min for rivaroxaban and edoxaban as well as in patients with creatinine >1.5 mg/dL plus age ≥80 years or weight ≤60 kg for apixaban [19]. These indications are derived from post hoc analysis of the large trials comparing warfarin with the new drugs (see below). Despite this, data from the Fresenius Medical Care records show there is a considerable number of patients with AF and eGFR<30 mL or on hemodialysis taking NOACs (23.5 and 11.6%, respectively) [20]. The kidney, primarily through renal filtration, eliminates these drugs, so in patients with CKD the pharmacokinetics are altered and their half-life is increased. Dosage and frequency of administration must be corrected to avoid problems with accumulation and toxicity.
Renal function is estimated by a variety of equations. Historically, these calculations have been based on the value of plasma creatinine. Currently, however, the most validated equations to estimate GFR are the Modification of Diet in Renal Disease (MDRD) [21] and Chronic Kidney Disease Epidemiology (CKD-EPI) [22]. Patients enrolled in the trials that compared NOACs with warfarin were included or excluded from the studies based on eGFR values calculated by the older Cockcroft-Gault formula instead of the more current equations [23]. Furthermore, these formulas can be used only in patients with stable creatinine values. They lose reliability in patients with fluctuating creatinine as in the case of patients in acute renal failure. In contrast to warfarin, which is exclusively hepatically metabolized, all NOACs have some level of renal elimination. For example, renal elimination accounts for 80% of the drug elimination for dabigatran, 36% for rivaroxaban, 27% for apixaban, and 50% for edoxaban. Furthermore, the binding of different NOACs with plasma proteins varies greatly. High protein binding makes some of these drugs virtually nondialysable. The levels of protein binding range from 35% for dabigatran, 55% for edoxaban, 87% for apixaban, and up to 95% for rivaroxaban. In the absence of reversal agents (the only one currently available is idarucizamab, a reversal agent of dabigatran) or the ability to dialyze, accumulation of these drugs can cause severe bleeding.
Currently, 4 NOACs have been approved by the European Medicines Agency (EMA) and the Food and Drug Administration (FDA): dabigatran, rivaroxaban, apixaban, and edoxaban. The only direct thrombin inhibitor is dabigatran, while the other 3 act by inhibiting factor Xa activation. Randomized trials have been performed using these 4 agents, comparing their safety and efficacy to warfarin [13, 14, 15, 16]. Post hoc analyses were also performed, analyzing the subgroup of patients with eGFR <50 mL/min.
The RE-LY study [13], which excluded patients with an eGFR <30 mL/min, demonstrated the superiority of dabigatran (150 mg twice daily) compared to warfarin in terms of protection from thromboembolism, with an equal risk of hemorrhage. Using a lower dose of dabigatran (110 mg twice a day), provided the same level of protection from thromboembolism when compared to warfarin with a decreased risk of bleeding. A subgroup analysis comparing warfarin with dabigatran in relation to baseline renal function showed that the efficacy of dabigatran was maintained irrespective of eGFR, but both dabigatran dosages displayed significantly lower rates of major bleeding in patients with eGFR ≥80 mL/min [24]. However, although the eGFR decreased gradually over time in both patients treated with warfarin or dabigatran, the decline in eGFR was significantly lower in the high- and low-dose dabigatran group [25], suggesting a possible nephroprotective effect of this NOAC.
The ROCKET-AF [14] study demonstrated a noninferiority of rivaroxaban against warfarin in the prevention of thromboembolic events, with no difference in bleeding. This study excluded patients with an eGFR <30 mL/min and adjusted doses based on eGFR. Patients with eGFR >50 mL/min took 20 mg/day of rivaroxaban, while those with eGFR between 30–50 mL/min took 15 mg/day. There was no significant difference in rivaroxaban's efficacy and risk of hemorrhage in both groups [26].
The ARISTOTLE study [15] compared the use of apixaban with warfarin and included patients with a plasma creatinine up to 2.5 mg/dL. Patients were randomized to a low-dose (2.5 mg twice daily) and high-dose (5 mg twice daily) apixaban group. Patients who had at least 2 risk factors: creatinine between 1.25 and 2.5 mg/dL, age >80 years old or body weight <60 kg were placed in the low dose group, while the rest of the patients were placed in the higher dose group. The study demonstrated a superiority of apixaban in the protection of thromboembolism, compared with warfarin, with a lower incidence of bleeding events. A subgroup analysis, performed in patients with eGFR <50 mL/min, showed a similar trend with a nonsignificant reduction of thromboembolic events and a significant decrease in bleeding risk [27]. When the efficacy and safety as a function of the deterioration of renal function over time were investigated, apixaban maintained its advantage compared to warfarin, both in terms of protection from thromboembolic events and reduction of bleeding [28].
The ENGAGE-AF study [16] included patients with eGFR >30 mL/min and had a different edoxaban dosing regimen for patients with a eGFR between 30 and 50 mL/min versus eGFR >50 ml/min. Subjects with eGFR between 30 and 50 mL/min were prescribed 30 mg of edoxaban twice a day versus 60 mg twice a day for patients with an eGFR >50 mL/min. The study showed no evidence of inferiority with edoxaban compared to warfarin in the prevention of stroke and systemic thromboembolism and a simultaneous reduction of bleeding events. Similar results were obtained in the subgroup analysis of patients with an eGFR between 30 and 50 mL/min [29].
The information about the use of NOACs in ESRD patients is very scarce. Chan et al. [30] recently published data from Fresenius Medical Care records regarding the use of dabigatran and rivaroxaban in hemodialysis patients. They identified an increased risk of hospitalization or death from bleeding in patients receiving NOACs compared to those taking warfarin. Another study showed similar drug levels in 18 hemodialysis patients taking 10 mg/day of rivaroxaban compared to healthy volunteers taking 20 mg/day. There was, however accumulation of the drug after a few days despite receiving hemodialysis which had no effect on plasma concentrations of the drug [31]. Given its high rate of renal elimination, dabigatran accumulates when administered to patients with ESRD [32]; however, it is the only of the 4 NOACs that is quickly eliminated by hemodialysis because of its low rate of binding to plasma proteins.
While EMA has not approved the use of any of the 4 NOACs in ESRD patients, the FDA approved apixaban in this patient population. This controversial decision was made due to the results of 2 small studies suggesting that a dose adjustment of apixaban is not required on the basis of renal function alone [33, 34, 35]. In 2014, the FDA approved a labeling change for apixaban, indicating that the full dosage of 10 mg/day can be prescribed to ESRD/dialysis patients. A reduced dose of 5 mg/day is only indicated with the presence of 1 of the following clinical characteristics: age ≥80 years or weight ≤60 kg. Moreover, recently FDA stated that administration of rivaroxaban 15 mg once daily will result in concentrations and pharmacodynamic activity similar to those observed in the ROCKET AF study [31]. However, clinical efficacy and safety studies with apixaban and rivaroxaban did not enroll patients with ESRD on dialysis or patients with a CrCl <15 mL/min; therefore, dosing recommendations are based on pharmacokinetic and pharmacodynamic (anti-FXa activity) data in subjects with ESRD maintained on dialysis.
In conclusion, there are limited data to suggest the safe and effective use of NOACs in patients with moderate renal impairment (i.e., eGFR between 30 and 50 mL/min). The question still remains whether NOACs can be used in patients with an eGFR <30 mL/min since there are no published studies addressing this subject. End-stage renal disease patients on hemodialysis with a high thromboembolic risk and demonstrated intolerance to vitamin K inhibitors could conceivably use apixaban (2.5 or 5 mg twice daily). Other options include using rivaroxaban at a reduced dose. There is a paucity of data to support the use of these agents in this patient population, so alternative, non-drug-related therapies, such as LAA closure, should be taken into consideration [36].
In a recent clinical update published in the European Heart Journal, Piccini et al. [37] stated that LAA closure may be an alternative to oral anticoagulation for stroke prevention in patients with advanced CKD and AF, demonstrating the growing interest and attention of the clinical community to this particular population.
Percutaneous LAA Occlusion
Rationale and Clinical Evidence
Anticoagulation therapy represents the standard of care to prevent thrombus formation and cardioembolic events in patients with AF. European Society of Cardiology (ESC), American Heart Association (AHA) and American College of Cardiology (ACC) Guidelines recommend using the CHA2DS2-VASc Score to assess the risk of stroke in patients with AF. ESC Guidelines [19] recommend patients with risk factor(s) ≥1 to receive effective stroke prevention therapy. Antithrombotic therapy is not recommended in patients with AF (irrespective of gender) who are <65 years old and with lone AF, as they have very low absolute event rates. AHA/ACC Guidelines [38] recommend oral anticoagulation for patients with CHA2DS2-VASc ≥2. For patients with nonvalvular AF and a CHA2DS2-VASc score of 1, no antithrombotic therapy or treatment with an oral anticoagulant or aspirin may be considered. HAS-BLED is the most validated bleeding score for patients with AF in whom antithrombotic therapy is indicated. When the HAS-BLED score is ≥3, caution and regular review are appropriate, as well as efforts to correct the potentially reversible risk factors for bleeding.
Unfortunately, about 13-20% of patients at risk for thromboembolic events have an absolute or relative contraindication to oral anticoagulation therapy due to an increased risk of bleeding [39, 40, 41]. The majority of these patients are often those that are also at high risk for thromboembolic events. In fact, some clinical parameters assessed in the thromboembolic risk scores (arterial hypertension, advanced age, and previous stroke), are also predictors of risk of hemorrhagic events. A balance between risks and benefits must be assessed before starting patients on anticoagulation.
The LAA is the site of thrombus formation in at least 90% of cases of cardio-embolic events (stroke and acute embolic arterial ischemia), in patients with nonrheumatic AF [42]. The pathophysiology of thrombus formation in the LAA during AF includes: endothelial dysfunction, decreased blood flow, and activation of inflammatory/hemostatic cascade – a mechanism described by Virchow about 150 years ago known as “Virchow's triad” [43, 44]. The hypothesis that anatomical exclusion of the most common site of thrombus formation in patients with AF could prevent a large proportion of cardiac embolism is the rationale for considering LAA occlusion as an alternative to anticoagulation therapy. Surgical resection of the LAA was performed in patients with AF for the first time in the 1940s [45]. Multiple studies over the years have evaluated the safety and efficacy of LAA exclusion during cardiac surgery with equivocal results. One meta-analysis which included 3,653 patients from 7 relevant studies, suggested LAA occlusion effectively reduced the risk of stroke peri- and post-operatively, without a significant increase in complications [46]. Nevertheless, surgical removal of the LAA was never widely adopted due to a lack of consistent clinical evidence from large clinical randomized trials. Technical issues, such as bleeding during the procedure due to fragility of the LAA wall or incomplete closure of the LAA, contributed to the premature abandonment of the first randomized surgical trial to evaluate the effectiveness and safety of surgical exclusion of the LAA [47]. A randomized control trial is currently underway consisting of 4,700 patients with AF undergoing heart surgery (for other reasons) to have the LAA removed or not to establish the safety and efficacy of LAA exclusion [48].
A variety of devices have recently been designed to percutaneously occlude the LAA. The PLAATO (percutaneous LAA transcatheter occlusion) was the first percutaneous LAA occlusion device and demonstrated an approximately 61% risk reduction for strokes when studied in 210 consecutive patients [49]. This device, however, is no longer being developed.
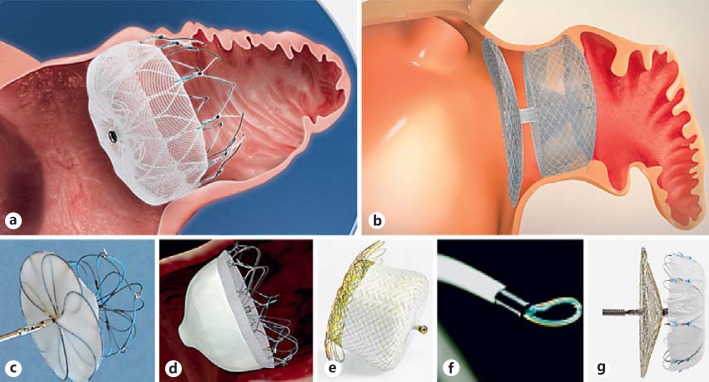
The Watchman (Boston Scientific, MapleGrove, MN, USA; Fig. 1a), a self-expanding nitinol cage with fixation barbs, covered with porous polyethylene membrane on the proximal face, received the CE mark (Conformité Européenne) in 2005 and is the only FDA-approved device for transcatheter LAA occlusion. The PROTECT-AF [50, 51, 52] is a multicenter, prospective randomized clinical trial comparing Watchman device with long-term warfarin therapy. It demonstrated the noninferiority of the Watchman device to traditional medical therapy using stroke, cardiovascular or unexplained death and systemic embolism as the primary end point at 1 year follow-up. The PROTEC-AF trial achieved statistical superiority for the composite primary efficacy end point at 4 years of follow-up. In this trial, the rate of procedure-related serious cardiovascular complications was 8.7%, mainly due to periprocedural pericardial effusion. The number of procedural adverse events was higher in the early period of device implantation. In the PREVAIL [53] study, implant success rate increased to 95% (from 90% of PROTECT) and procedural adverse events at 7 days decreased to 4.4%. A meta-analysis by Holmes et al. [54] included 2,406 patients with 5,931 patient-years of follow-up from the PROTECT-AF and the PREVAIL trials (and their respective continued access registries). They concluded that in patients with nonvalvular AF at increased risk for stroke or bleeding who are candidates for chronic anticoagulation, LAA occlusion with watchman device resulted in decreased rates of hemorrhagic stroke, cardiovascular/unexplained death, and nonprocedural bleeding compared to warfarin.
Fig. 1.
a The Watchman device (Boston Scientific, Marlborough, MA, USA). b The Amulet device (St Jude Medical, St Paul, MN, USA). c The Ultrasept LAA Occluder (Cardia Inc., Eagan, MN, USA). d The Wavecrest device (Coherex Medical, Salt Lake City, UT, USA). e The Occlutech LAA Occluder (Occlutech International AB, Helsingborg, Sweden). f The Lariat device (SentreHEART, Palo Alto, CA, USA). g The LAmbre LAA closure system (Lifetech Scientific Corp., China).
The Amulet device (AGA; St Jude Medical, Minneapolis, MN, USA, Fig. 1b), a newer model of the Amplatzer Cardiac Plug (ACP) device, is a self-expanding nitinol occluder of the LAA consisting of a lobe with fixation barbs (that anchors the prosthesis inside the appendage), a disk that seals the ostium of the appendage and a waist (that connects the lobe to the disk). Most of the clinical evidence for ACP and Amulet devices is from pooled multicenter registry data outside the US. A pooled analysis of 1,047 consecutive patients from 22 centers, reports a procedural success of 97.3% with 5% of periprocedural major adverse events [55]. Santoro et al. [56] reported an ischemic stroke rate of 0.8/100 person-years, an embolic event rate of 2.5/100 person-years and all-cause mortality of 2.5% over the follow-up period in a population of 134 patients implanted with an ACP device. A number of other devices are currently under development or clinical investigation (Cardia, WaveCrest, Occlutech, Lariat, LAmbre; Fig. 1c-g).
Patient Selection and Preprocedural Screening
The most commonly accepted indication for LAA occlusion is the prevention of stroke in patients with AF at high risk for thromboembolic events and contraindications to oral anticoagulants [57]. Interestingly, most of the data on safety and efficacy were obtained in patients without contraindications to anticoagulants. The indication of LAA occlusion as alternative to oral anticoagulation when medical therapy is possible remains a point of discussion. Dual antiplatelet therapy is generally indicated after the procedure for at least 1–6 months followed by the lifelong use of a single antiplatelet agent. Antiplatelet agents have a comparable hemorrhagic risk to that of warfarin, which is why the duration of dual antiplatelet therapy is kept to a minimum after the LAA is excluded. Often, the antiplatelet therapy is tailored according to the patient: balancing the risk of stroke and hemorrhagic events. Patients with advanced renal failure represent a particular population in which the best strategy for stroke prevention in the case of AF remains uncertain. NOACs are in fact not recommended in the presence of severe renal impairment. The use of warfarin may increase tissue calcification and atherosclerosis and at the same time exposes patients to an increased risk of bleeding. Moreover, patients with severe renal failure have been usually excluded from most important clinical trials. A recent registry study assessed the safety and efficacy of LAAO in 1,014 patients with available renal function: 375 with and 639 without CKD. Procedural and occlusion success were similarly high in all stages of CKD. No differences between patients with and without CKD were observed in periprocedural major adverse events. The annual rate of thromboembolic cerebral events and the observed bleeding rate were similar among patients with and without CKD. LAA occlusion seems to have a similar safety profile in patients with CKD compared to patients with normal renal function and offers an important reduction of stroke and bleeding rates in all stages of CKD, as compared to expected annual risk [58]. The indication for LAA closure for stroke prevention in patients with AF and renal disease should be discussed with a multidisciplinary team as well as with the patient, determining on a case by case basis the risks and benefits of anticoagulation versus LAA closure. Transesophageal echocardiogram (TEE) is the best imaging modality to assess LAA anatomy and rule out the presence of thrombus. Presence of thrombus is a contraindication to proceed with percutaneous LAA occlusion due to the risk of thrombus dislodgement. CT angiography can be useful in order to evaluate the shape and size of the appendage before the procedure.
Procedure Overview
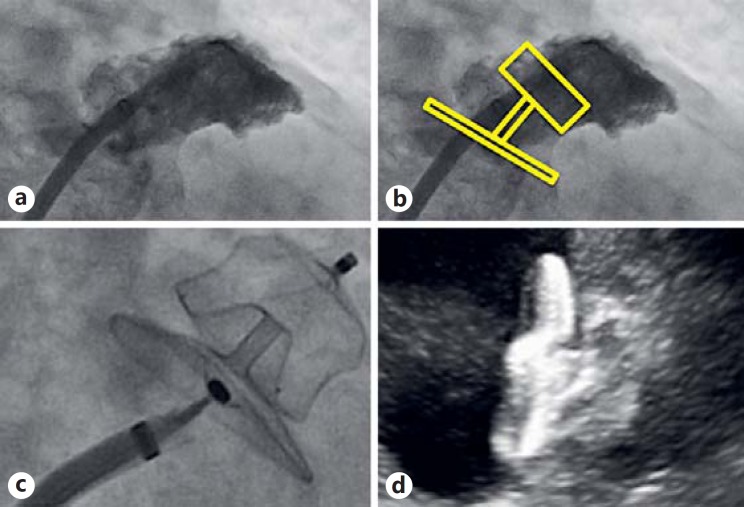
Usually, the procedure is performed under general anesthesia with TEE and angiographic guidance. Some operators have developed an expertise with intracardiac echocardiography (ICE) [59, 60]. The advantage of this technique is that the procedure can be accomplished without sedation. The theoretical disadvantage is the need for an additional venous access and images that may be limited compared to TEE. Once the femoral venous access is obtained with the Seldinger technique, a transseptal kit consisting of a sheath and a needle is advanced into the right atrium at the level of the septum. The transseptal puncture is performed under TEE or ICE guidance to facilitate left atrial catheterization and avoid complications such as cardiac tamponade. A bolus of unfractionated heparin is administered usually once the catheter is advanced into the left atrium. Angiographic images of the LAA (Fig. 2a) can typically be obtained in the right caudal or right cranial views. Angiographic measurements of the LAA can be compared with ones obtained by ICE or TEE to decide the correct device size. A delivery catheter is then advanced into the LAA, and the device is positioned under echocardiographic and fluoroscopic guidance with the aim to obtain a complete sealing and exclusion of the appendage (Fig. 2b-d). After safety, stability, and efficacy assessments, the device is released and the delivery catheter is withdrawn. Hemostasis of the venous access site is generally obtained by mechanical compression.
Fig. 2.
a Angiographic view of the LAA. b Simulation of LAA occlusion with the Amulet device. Angiographic (c) and ICE (d) view of the Amulet device deployed in the LAA.
Conclusions
Patients with AF and severe renal failure are at high risk for stroke and thromboembolism, but at the same time they are also at increased risk for serious bleeding events. This population has not been adequately studied as they have been excluded from most important clinical trials. This complicates the assessment of the risk/benefit balance of thromboprophylaxis in patients with renal disease. Warfarin has been for many years the only option for oral anticoagulation therapy, but data on patients with advanced renal failure show a high rate of bleeding events, with uncertain protection against thromboembolic events. Among patients with normal renal function, NOACs demonstrate a better safety profile and similar efficacy compared to warfarin. The use of NOACs in patients with mild and moderate CKD has been shown to have a good safety profile. Lau et al. [61] proposed an algorithm for oral anticoagulation choices in patients with AF and CKD with the possibility to prescribe apixaban 5 mg b.i.d. even in patients with CrCl <15 mL/min, or ESRD on renal replacement therapy. NOACs, however, are usually not recommended in patients with ESRD because of the risk of drug accumulation since they are dependent, to some extent, on renal elimination.
A promising therapeutic option is the anatomical exclusion of the site of thrombus formation with the percutaneous occlusion of the LAA, and preliminary data indicate that it seems to have a similar efficacy and safety profile in patients with CKD compared to patients with normal renal function. Figure 3 shows a proposed algorithm for the treatment of patients with AF and CKD. Further investigations are indicated to evaluate the best strategy for thromboprophylaxis in patients with AF and severe renal disease.
Fig. 3.
Proposed algorithm of strategies for stroke prevention in patients with atrial fibrillation and chronic kidney disease. eGFR, estimated glomerular filtration rate; TTR, time in therapeutic range; LAA, left atrial appendage; OAC, oral anticoagulation.
Statement of Ethics
For this review paper, no approval by an appropriate ethics committee was required.
Disclosure Statement
Dr. P. Mazzone is proctor for St Jude Medical and Boston Scientific.
Funding Sources
The authors declare no financial support for this paper.
References
- 1.Go A, Hylek E, Phillips K, et al. Prevalence of diagnosed atrial fibrillation in adults. National implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Miyasaka Y, Barnes M, Gersh B, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980-2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 3.Heeringa J, Van Der Kulp D, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949–953. doi: 10.1093/eurheartj/ehi825. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd-Jones D, Wang T, Leip E, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 5.Soliman EZ, Prineas RJ, Go AS, et al. Chronic kidney disease and prevalent atrial fibrillation: the Chronic Renal Insufficiency Cohort (CRIC) Am Heart J. 2010;159:1102–1107. doi: 10.1016/j.ahj.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baber U, Howard VJ, Halperin JL, et al. Association of chronic kidney disease with atrial fibrillation among adults in the United States: REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Circ Arrhythm Electrophysiol. 2011;4:26–32. doi: 10.1161/CIRCEP.110.957100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genovesi S, Vincenti A, Rossi E, et al. Atrial fibrillation and morbidity and mortality in a cohort of long-term hemodialysis patients. Am J Kidney Dis. 2008;2:255–262. doi: 10.1053/j.ajkd.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 8.Go AS, Fang MC, Udaltsova N, et al. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation. The Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. Circulation. 2009;199:1363–1369. doi: 10.1161/CIRCULATIONAHA.108.816082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmerman D, Sood MM, Rigatto C, et al. Systematic review and meta-analysis of incidence, prevalence and outcomes of atrial fibrillation in patients on dialysis. Nephrol Dial Transplant. 2012;27:3816–3822. doi: 10.1093/ndt/gfs416. [DOI] [PubMed] [Google Scholar]
- 10.Dahal K, Kunwar S, Rijal J, et al. Stroke, major bleeding, and mortality outcomes in warfarin users with atrial fibrillation and chronic kidney disease: a meta-analysis of observational studies. Chest. 2016;149:951–959. doi: 10.1378/chest.15-1719. [DOI] [PubMed] [Google Scholar]
- 11.Genovesi S, Rossi E, Gallieni M, et al. Warfarin use, mortality, bleeding and stroke in haemodialysis patients with atrial fibrillation. Nephrol Dial Transplant. 2015;30:491–498. doi: 10.1093/ndt/gfu334. [DOI] [PubMed] [Google Scholar]
- 12.Dahal K, Kunwar S, Rijal J, et al. Stroke, major bleeding and mortality outcomes in warfarin users with atrial fibrillation and chronic kidney disease: a meta-analysis of observational studies. Chest. 2016;149:951–959. doi: 10.1378/chest.15-1719. [DOI] [PubMed] [Google Scholar]
- 13.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 14.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 15.Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 16.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 17.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:2246–2280. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 18.Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist anticoagulants in patients with non valvular atrial fibrillation. Europace. 2015;17:1467–1507. doi: 10.1093/europace/euv309. [DOI] [PubMed] [Google Scholar]
- 19.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothorac Surg. 2016;50:e1–e88. doi: 10.1093/ejcts/ezw313. [DOI] [PubMed] [Google Scholar]
- 20.Chan KE, Giugliano RP, Patel MR, et al. Nonvitamin K anticoagulant agents in patients with advanced chronic kidney disease or on dialysis with atrial fibrillation. J Am Coll Cardiol. 2016;67:2888–2899. doi: 10.1016/j.jacc.2016.02.082. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 24.Hijazi Z, Hohnloser SH, Oldgren J, et al. Efficacy and safety of dabigatran compared with warfarin in relation to baseline renal function in patients with atrial fibrillation: a RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) trial analysis. Circulation. 2014;129:961–970. doi: 10.1161/CIRCULATIONAHA.113.003628. [DOI] [PubMed] [Google Scholar]
- 25.Böhm M, Ezekowitz MD, Connolly SJ, Eikelboom JW, et al. Changes in renal function in patients with atrial fibrillation: an analysis from the RE-LY trial. J Am Coll Cardiol. 2015;65:2481–2493. doi: 10.1016/j.jacc.2015.03.577. [DOI] [PubMed] [Google Scholar]
- 26.Fox KAA, Piccini JP, Wojdyla D, et al. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J. 2011;32:2387–2394. doi: 10.1093/eurheartj/ehr342. [DOI] [PubMed] [Google Scholar]
- 27.Hohnloser SH, Hijazi Z, Thomas L, et al. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J. 2012;33:2821–2830. doi: 10.1093/eurheartj/ehs274. [DOI] [PubMed] [Google Scholar]
- 28.Hijazi Z, Hohnloser SH, Andersson U, et al. Efficacy and safety of apixaban compared with warfarin in patients with atrial fibrillation in relation to renal function over time insights from the ARISTOTLE randomized clinical trial. JAMA Cardiol. 2016;1:451–460. doi: 10.1001/jamacardio.2016.1170. [DOI] [PubMed] [Google Scholar]
- 29.Bohula EA, Giugliano RP, Ruff CT, et al. Impact of renal function on outcomes with edoxaban in the ENGAGE AF-TIMI 48 trial. Circulation. 2016;134:24–36. doi: 10.1161/CIRCULATIONAHA.116.022361. [DOI] [PubMed] [Google Scholar]
- 30.Chan KE, Edelman ER, Wenger JB, Thadhani RI, Maddux FW. Dabigatran and rivaroxaban use in atrial fibrillation patients on hemodialysis. Circulation. 2015;131:972–979. doi: 10.1161/CIRCULATIONAHA.114.014113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Vriese AS, Caluwé R, Bailleul E, et al. Dose finding study of rivaroxaban in hemodialysis patients. Am J Kidney Dis. 2015;66:91–98. doi: 10.1053/j.ajkd.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 32.Stangier J, Rathgen K, Stähle H, et al. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatranetexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet. 2010;49:259–268. doi: 10.2165/11318170-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Tirucherai G, Marbury TC, et al. Pharmacokinetics, pharmacodynamics, and safety of apixaban in subjects with end-stage renal disease on hemodialysis. J Clin Pharmacol. 2016;56:628–636. doi: 10.1002/jcph.628. [DOI] [PubMed] [Google Scholar]
- 34.Chang M, Yu Z, Shenker A, et al. Effect of renal impairment on the pharmacokinetics, pharmacodynamics, and safety of apixaban. J Clin Pharmacol. 2016;56:637–645. doi: 10.1002/jcph.633. [DOI] [PubMed] [Google Scholar]
- 35.Deal EN, Pope H, Ross W. Apixaban use among patients with severe renal impairment. Ann Pharmacother. 2014;48:1667. doi: 10.1177/1060028014554446. [DOI] [PubMed] [Google Scholar]
- 36.Holmes DR, Doshi SK, Kar S, et al. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2015;65:2614–2623. doi: 10.1016/j.jacc.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 37.Piccini JP, Sievert H, Patel MR. Left atrial appendage occlusion: rationale, evidence, devices, and patient selection. Eur Heart J. 2016;pii:ehw330. doi: 10.1093/eurheartj/ehw330. [DOI] [PubMed] [Google Scholar]
- 38.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guidelines for the management of patients with atrial fibrillation. J Am Coll Cardiol. 2014;64:e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 39.O'Brien EC, Homes DJN, Ansell JE, et al. Physician practices regarding contraindications to oral anticoagulation in atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Am Heart J. 2014;167:601–609. doi: 10.1016/j.ahj.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 40.Go AS, Hylek EM, Borowsky LH, Phillips KA, Selby JV, Singer DE. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann Intern Med. 1999;131:927–934. doi: 10.7326/0003-4819-131-12-199912210-00004. [DOI] [PubMed] [Google Scholar]
- 41.Stafford RS, Singer DE. Recent national patterns of warfarin use in atrial fibrillation. Circulation. 1998;97:1231–1233. doi: 10.1161/01.cir.97.13.1231. [DOI] [PubMed] [Google Scholar]
- 42.Al-Saady NM, Obel OA, Camm AJ. Left atrial appendage: structure, function, and role in thromboembolism. Heart. 1999;82:547–554. doi: 10.1136/hrt.82.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Virchow R. Gesammelte Abhandlungen zur wissenschaftlichen Medicin. Frankfurt am Main: Von Meidinger and Sohn; 1856. Thrombose und Embolie. Gefässentzündung und septische Infektion. pp. 219–732. [Google Scholar]
- 44.Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow's triad revisited. Lancet. 2009;373:155–166. doi: 10.1016/S0140-6736(09)60040-4. [DOI] [PubMed] [Google Scholar]
- 45.Madden JL. Resection of the left auricular appendix. JAMA. 1948;140:769–772. doi: 10.1001/jama.1949.02900440011003. [DOI] [PubMed] [Google Scholar]
- 46.Tsaia YC, Phan K, Munkholm-Larsenc S, et al. Surgical left atrial appendage occlusion during cardiac surgery for patients with atrial fibrillation: a meta-analysis. Eur J Cardiothorac Surg. 2015;47:847–854. doi: 10.1093/ejcts/ezu291. [DOI] [PubMed] [Google Scholar]
- 47.Crystal E, Lamy A, Connolly SJ, et al. Left Atrial Appendage Occlusion Study (LAAOS): a randomized clinical trial of left atrial appendage occlusion during routine coronary artery bypass graft surgery for long-term stroke prevention. Am Heart J. 2003;145:174–178. doi: 10.1067/mhj.2003.44. [DOI] [PubMed] [Google Scholar]
- 48.Whitlock R, Healey J, Vincent J, et al. Rationale and design of the Left Atrial Appendage Occlusion Study (LAAOS) III. Ann Cardiothorac Surg. 2014;3:45–54. doi: 10.3978/j.issn.2225-319X.2013.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bayard Y, Omran H, Kramer P, Matthews R, Reisman M, Block P, Ostermayer S, Leetz M, Fischer E, Sievert H. Worldwide experience of percutaneous left atrial appendage transcatheter occlusion (PLAATO) J Neurol Sci. 2005;238:S70–S70. [Google Scholar]
- 50.Holmes DR, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomized non-inferiority trial. Lancet. 2009;374:534–542. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]
- 51.Reddy VY, Doshi SK, Holmes DR, et al. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3 year follow-up of the PROTECT-AF Trial. Circulation. 2013;127:720–729. doi: 10.1161/CIRCULATIONAHA.112.114389. [DOI] [PubMed] [Google Scholar]
- 52.Reddy VY, Sievert H, Halperin J, et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312:1988–1998. doi: 10.1001/jama.2014.15192. [DOI] [PubMed] [Google Scholar]
- 53.Holmes DR, Saibal K, Reddy VY, et al. Prospective randomized evaluation of the watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy. J Am Coll Cardiol. 2014;64:1–12. doi: 10.1016/j.jacc.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 54.Holmes DR, Doshi SK, Kar S, Price MJ, Sanchez JM, Sievert H, Valderrabano M, Reddy VY. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2015;65:2614–2623. doi: 10.1016/j.jacc.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 55.Tzikas A, Shakir S, Gafoor SA, Omran H, et al. Left atrial appendage occlusion for stroke prevention in atrial fibrillation: multicentre experience with the AMPLATZER Cardiac Plug. Eurointervention. 2016;11:1170–1179. doi: 10.4244/EIJY15M01_06. [DOI] [PubMed] [Google Scholar]
- 56.Santoro G, Meucci F, Stolcova M, Rezzaghi M, Mori F, Palmieri C, Paradossi U, Pastormerlo LE, Rosso G, Berti S. Percutaneous left atrial appendage occlusion in patients with non-valvular atrial fibrillation: implantation and up to four years follow-up of the AMPLATZER Cardiac Plug. Eurointervention. 2016;11:1188–1194. doi: 10.4244/EIJY14M10_13. [DOI] [PubMed] [Google Scholar]
- 57.Pison L, Potpara TS, Chen J, et al. Left atrial appendage closure – indications, techniques, and outcomes: results of the European Heart Rhythm Association Survey. Europace. 2015;17:642–646. doi: 10.1093/europace/euv069. [DOI] [PubMed] [Google Scholar]
- 58.Kefer J, Tzikas A, Freixa X, et al. Impact of chronic kidney disease on left atrial appendage occlusion for stroke prevention in patients with atrial fibrillation. Int J Cardiol. 2016;207:335–340. doi: 10.1016/j.ijcard.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 59.Berti S, Paradossi U, Meucci F, et al. Periprocedural intracardiac echocardiography for left atrial appendage closure a dual-center experience. J Am Coll Cardiol Intv. 2014;7:1036–1044. doi: 10.1016/j.jcin.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 60.Ronco F, Pascotto A, Barbierato M, et al. Percutaneous left atrial appendage closure in awake non intubated patient: multimodality intraprocedural imaging. Cardiovasc Revasc Med. 2012;13:360–361. doi: 10.1016/j.carrev.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 61.Lau YC, Proietti M, Guiducci E, et al. Atrial fibrillation and thromboembolism in patients with chronic kidney disease. J Am Coll Cardiol. 2016;68:1452–1464. doi: 10.1016/j.jacc.2016.06.057. [DOI] [PubMed] [Google Scholar]