Abstract
Background and Objectives
The matricellular protein osteopontin is involved in the pathogenesis of both kidney and cardiovascular disease. However, whether circulating and urinary osteopontin levels are associated with the risk of these diseases is less studied.
Design, Setting, Participants, and Measurements
A community-based cohort of elderly men (Uppsala Longitudinal Study of Adult Men [ULSAM]; n = 741; mean age: 77 years) was used to study the associations between plasma and urinary osteopontin, incident chronic kidney disease, and the risk of cardiovascular death during a median of 8 years of follow-up.
Results
There was no significant cross-sectional correlation between plasma and urinary osteopontin (Spearman ρ = 0.07, p = 0.13). Higher urinary osteopontin, but not plasma osteopontin, was associated with incident chronic kidney disease in multivariable models adjusted for age, cardiovascular risk factors, baseline glomerular filtration rate, urinary albumin/creatinine ratio, and the inflammatory markers interleukin 6 and high-sensitivity C-reactive protein (odds ratio for 1 standard deviation [SD] of urinary osteopontin, 1.42, 95% CI 1.00-2.02, p = 0.048). Conversely, plasma osteopontin, but not urinary osteopontin, was independently associated with cardiovascular death (multivariable hazard ratio per SD increase, 1.35, 95% CI 1.14-1.58, p < 0.001, and 1.00, 95% CI 0.79-1.26, p = 0.99, respectively). The addition of plasma osteopontin to a model with established cardiovascular risk factors significantly increased the C-statistics for the prediction of cardiovascular death (p < 0.002).
Conclusions
Higher urinary osteopontin specifically predicts incident chronic kidney disease, while plasma osteopontin specifically predicts cardiovascular death. Our data put forward osteopontin as an important factor in the detrimental interplay between the kidney and the cardiovascular system. The clinical implications, and why plasma and urinary osteopontin mirror different pathologies, remain to be established.
Keywords: Protein, Atherosclerosis, Cardiovascular risk marker, Renal disease, Epidemiology
Introduction
Osteopontin, an acidic glycoprotein [1], has been found both as an intra- and extracellular tissue protein as well as a soluble cytokine in urine and plasma [2]. Expressed in several different cell types, such as epithelial and endothelial cells, macrophages, and smooth muscle cells [3], osteopontin has been ascribed to various physiological and pathophysiological processes, including tissue and bone remodeling, inflammation, cell survival [2], atherosclerosis [4], and kidney damage [5]. Yet, the role and possible importance of circulating and urinary osteopontin as a candidate biomarker is less studied. Previous observational studies are mainly based on clinical samples of patients with cardiovascular or kidney disease [6, 7], and community-based data are scarce.
Given previous experimental data supporting an important role for osteopontin in the interplay between the kidney and the cardiovascular system, we hypothesized that circulating and urinary osteopontin may reflect prognostic information regarding these two diseases. Therefore, our study aimed at discovering the longitudinal associations between circulating and urinary osteopontin and a decline of the glomerular filtration rate (GFR), the incidence of chronic kidney disease, and the risk of cardiovascular death in a community-based cohort of elderly individuals.
Research Design and Methods
Study Sample
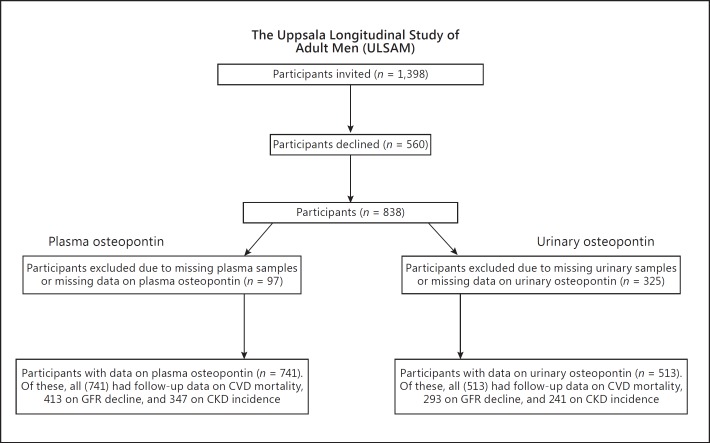
The Uppsala Longitudinal Study of Adult Men (ULSAM) study cohort, described in detail on http://www.pubcare.uu.se/ulsam, is an ongoing study since 1970, focusing on cardiovascular risk factors [8]. Inclusion criteria were male sex, age 50 years (birth year 1920-1924), and resident in Uppsala County, Sweden. The present study uses data from the 4th examination cycle of ULSAM when participants were approximately 77 years old. At this examination cycle, 1,398 participants were invited and 838 agreed to participate. We excluded 97 participants due to missing plasma samples or missing data on plasma osteopontin, leaving 741 participants as the present study sample. Urinary osteopontin was available in 513 participants. During follow-up, we also used the 5th examination cycle (2003-2005) when participants were 82 years old in order to identify those who had progressed to chronic kidney disease. After exclusion of individuals with missing samples or missing data on plasma or urinary osteopontin at baseline and individuals without data on GFR at both examinations, 706 had follow-up data for the analyses with GFR decline and 588 for incidence of chronic kidney disease (Fig. 1).
Fig. 1.
Flow chart describing the number of participants included in the cohort. CVD, cardiovascular disease; GFR, glomerular filtration rate; CKD, chronic kidney disease.
Covariates
The collection of clinical and biochemical characteristics has been described in detail elsewhere [8]. In brief, calculation of body mass index (kg/m2) and assessment of blood pressure were performed using standardized methods. Participants were asked to fill in questionnaires regarding socioeconomic status, medical history, smoking habits, medication, and physical activity [8]. Diabetes mellitus was diagnosed based on fasting plasma glucose (≥7.0 mmol/L or ≥126 mg/dL) or use of antidiabetic medication. Venous blood samples for biochemical analyses were drawn in the morning after an overnight fast and kept in −70°C pending analyses. Measurements and sampling on glucose, cholesterols, and creatinine were assessed by standard analytical methods in direct connection with the participants' visit at the clinic. Human osteopontin was analyzed by a commercial sandwich enzyme-linked immunosorbent assay (ELISA) kit (DY1433, R&D Systems, Minneapolis, MN, USA), in which a monoclonal antibody specific for the peptide was coated onto microtiter plates. After blocking the wells with bovine serum albumin, standards and samples were pipetted into the wells, and the peptide was bound to the immobilized antibodies. After washing, a biotinylated anti-peptide antibody was added to the wells. After another incubation and washing cycle, a streptavidin-horseradish peroxidase conjugate was added. Finally, after incubation and washing, a substrate solution was added. The development was stopped and the absorbance was measured in a SpectraMax 250 (Molecular Devices, Sunnyvale, CA, USA). The peptide concentrations in the samples were determined by comparing the optical density of the sample with the standard curve. The assays were calibrated against highly purified recombinant human osteopontin. The total coefficient of the assay was approximately 6%. The assay was performed blinded without knowledge of the clinical diagnosis. Urinary osteopontin levels were adjusted for urinary creatinine levels by creating a urinary osteopontin/creatinine ratio. The prerequisite for this adjustment was to compensate for variations in urine concentrations, assuming a steady osteopontin-creatinine excretion rate, thus reducing the variation of the biomarker.
High-sensitivity C-reactive protein (CRP) measurements were performed by latex-enhanced reagent (Dade Behring, Deerfield, IL, USA) using a Behring BN ProSpec analyzer (Dade Behring), and high-sensitivity interleukin 6 (IL-6) was analyzed by an ELISA kit (high-sensitivity IL-6 HS, R&D Systems) as previously described [9]. GFR was estimated from serum cystatin C performed by latex-enhanced reagent (N Latex Cystatin C, Dade Behring) using a Behring BN ProSpec analyzer (Dade Behring) with the formula: eGFR = 77.24 × cystatin C − 1.2623, which has been shown to be closely correlated with iohexol clearance [10].
Study Outcome
The Swedish Cause-of-Death register was used to obtain the outcomes of total and cardiovascular mortality. Cardiovascular mortality was defined as International Classification of Diseases (10th Revision) codes I00-I99. GFR was estimated at baseline and after 5 years. Incident chronic kidney disease was defined as having a GFR <60 mL/min/1.73 m2 after 5 years in individuals with a GFR ≥60 mL/min/1.73 m2 at baseline.
Statistical Analyses
Statistical software STATA 12 (StataCorp, College Station, TX, USA) was used in all analyses. The threshold for statistical significance (2-sided p value) was set at <0.05. Logarithmic transformation was used to promote a normal distribution of plasma osteopontin.
We investigated the association between plasma and urinary osteopontin, incident chronic kidney disease (logistic regression), GFR decline/year (linear regression), and cardiovascular disease (Cox regression) using the following multivariable models:
-
–
A. Age-adjusted model.
-
–
B. Kidney function model (model A + cystatin C-estimated GFR and urinary albumin/creatinine ratio) to determine if osteopontin provides information beyond established kidney disease markers.
-
–
C. Inflammation model (model A + high-sensitivity CRP and high-sensitivity IL-6) to determine if osteopontin provides information beyond the most widely used inflammatory markers.
-
–
D. Cardiovascular risk factor model (model A + lipid-lowering treatment, cardiovascular diagnosis, body mass index, diabetes, antihypertensive treatments, systolic blood pressure, total and high-density lipoprotein [HDL] cholesterol, and smoking).
-
–
E. Total model (all covariates from models A-D included).
In all analyses, urinary and plasma osteopontin were expressed per standard deviation increase. A Kaplan-Meier graph was used to graphically evaluate the cumulative incidence between plasma osteopontin and cardiovascular disease. We also performed the above analyses stratified by prevalent cardiovascular disease or chronic kidney disease to determine whether the effect is seen as a continuum in specific clinically relevant groups.
Multiple imputation methods were used to account for the potential influence of missing data with reference to the covariates (age, inflammatory markers, cardiovascular risk factors, and kidney function). In the cohort, 623 individuals had no missing data, 105 individuals had 1 variable missing, 12 individuals had 2 variables missing, and 1 individual had 5 variables missing.
Differences in C-statistics after the addition of plasma osteopontin to the model with established cardiovascular risk factors were estimated in order to evaluate improvement in model discrimination (Harrell's C). In addition, we examined correlations of both plasma and urinary osteopontin with baseline characteristics using Spearman correlation (for continuous variables) and ANOVA (for categorical variables).
Results
Baseline Characteristics and Clinical Correlates of Circulating and Urinary Osteopontin
A summary of general characteristics of the ULSAM study cohort is shown in Table 1. The mean level of plasma and urinary osteopontin was 55 ± 26 and 113 ± 64 ng/mL, respectively.
Table 1.
Baseline characteristics of the ULSAM cohort
| Subjects, n | 741 |
| Age, years | 77.5 ± 0.8 |
| Body mass index | 26.3 ± 3.5 |
| Systolic blood pressure, mm Hg | 151 ± 21 |
| Diastolic blood pressure, mm Hg | 81 ± 10 |
| Urinary albumin/creatinine ratio | 0.8 (0.3 – 2.1) |
| Glomerular filtration ratea | 73 ± 17 |
| Total cholesterol, mmol/L | 5.4 ± 1.0 |
| HDL cholesterol, mmol/L | 1.3 ± 0.3 |
| Fasting plasma glucose, mmol/L | 5.8 ± 1.2 |
| C-reactive protein, mg/L | 1.8 (0.96 – 4.2) |
| Interleukin 6, ng/L | 2.9 (2.1 – 4.9) |
| Plasma osteopontin, ng/mL | 55 ± 26 |
| Urinary osteopontin, ng/mLb | 113 ± 64 |
| Lipid-lowering treatment | 125 (17) |
| Antihypertensive treatment | 352 (48) |
| Acetylsalicylic acid | 213 (29) |
| Chronic kidney disease | 155 (21) |
| Cardiovascular disease | 204 (28) |
| Ischemic heart disease | 140 (19) |
| Cerebrovascular disease | 73 (10) |
| Congestive heart failure | 35 (5) |
| Diabetes | 86 (12) |
| Smoking | 59 (8) |
Normally distributed continuous variables are presented as means ± standard deviations, skewed continuous variables as medians (interquartile ranges; IQR25–IQR75), and categorical variables as n (%). HDL, high-density lipoprotein.
Glomerular filtration rate was estimated from serum cystatin C (mL/min/1.73 m2).
Creatinine-adjusted osteopontin.
Cross-Sectional Associations at Baseline
There was no significant association between plasma and urinary osteopontin (Spearman ρ = 0.07, p = 0.13). In univariate analysis, urinary albumin/creatinine ratio, fasting plasma glucose and inflammatory markers, and high-sensitivity IL-6 and high-sensitivity CRP were positively associated with plasma osteopontin, whereas body mass index, systolic and diastolic blood pressure, GFR, and total and HDL cholesterol were negatively associated with plasma osteopontin (online suppl. Table 1; for all online suppl. material, see www.karger.com/doi/10.1159/000476001). Moreover, higher plasma osteopontin was seen in participants with lipid-lowering, antihypertensive, and acetylsalicylic acid treatment, cardiovascular disease, chronic kidney disease, and diabetes and among smokers (p < 0.001; online suppl. Table 2). Urinary osteopontin exhibited a positive association with systolic and diastolic blood pressure, GFR, and fasting plasma glucose and a negative association with age at baseline, urinary albumin/creatinine ratio, HDL cholesterol, and high-sensitivity IL-6 (online suppl. Table 1). Higher urinary osteopontin was seen in those on lipid-lowering, antihypertensive, and acetylsalicylic acid treatment, those with chronic kidney disease, in smokers, and in those with diabetes, and lower urinary osteopontin was seen in those with cardiovascular disease (online suppl. Table 2).
Osteopontin, Incident Chronic Kidney Disease, and GFR Decline/Year
Multivariable logistic regression models of the association between osteopontin and the incidence of chronic kidney disease (GFR <60 mL/min/1.73 m2) in individuals with a GFR ≥60 mL/min/1.73 m2 are shown in Table 2. One standard deviation higher urinary osteopontin was significantly associated with incident chronic kidney disease in the kidney function model (model B, p = 0.020) and in the total model (model E, p = 0.048; Table 2). Moreover, urinary osteopontin was associated with GFR decline in models A (p = 0.035), C (p = 0.037), and D (p = 0.046) but not in models B and E (Table 2). However, in subgroup analyses in those with GFR ≥60 mL/min/1.73 m2 at baseline, urinary osteopontin was associated with GFR decline in all multivariable models (online suppl. Table 3). The association between plasma osteopontin and GFR decline was nonsignificant in all models (Table 2).
Table 2.
Standard deviation increments in creatinine-adjusted urinary osteopontin and plasma osteopontin and the associated 5-year progression of chronic kidney disease (logistic and linear regression models)
| Incidence |
GFR decline |
|||
|---|---|---|---|---|
| urinary osteopontin | plasma osteopontin | urinary osteopontin | plasma osteopontin | |
| Model A | 1.16 (0.88 to 1.53) | 1.24 (0.91 to 1.68) | −0.03 (−0.06 to −0.002)* | 0.008 (−0.01 to 0.03) |
| Model B | 1.48 (1.06 to 2.07)* | 1.15 (0.82 to 1.61) | −0.02 (−0.05 to 0.005) | −0.009 (−0.03 to 0.01) |
| Model C | 1.16 (0.87 to 1.53) | 1.25 (0.90 to 1.74) | −0.03 (−0.06 to −0.002)* | 0.005 (−0.02 to 0.03) |
| Model D | 1.12 (0.83 to 1.51) | 1.25 (0.92 to 1.70) | −0.03 (−0.06 to −0.0005)* | 0.005 (−0.02 to 0.03) |
| Model E | 1.42 (1.00 to 2.02)* | 1.22 (0.84 to 1.77) | −0.02 (−0.05 to 0.008) | −0.01 (-0.03 to 0.01) |
Values are odds ratios (95% confidence intervals) for incidence and regression coefficients (95% confidence intervals) for GFR decline. For the adjustments made in the models, see Statistical Analyses section. Incidence: plasma osteopontin, n = 347; urinary osteopontin, n = 241. GFR decline: plasma osteopontin, n = 413; urinary osteopontin, n = 293. GFR, glomerular filtration rate; model A, age-adjusted model; model B, kidney function model (model A + cystatin C-estimated GFR and albuminuria); model C, inflammation model (model A + C-reactive protein and interleukin 6); model D, cardiovascular risk factor model (model A + lipid-lowering treatment, cardiovascular diagnosis, body mass index, diabetes, antihypertensive treatments, systolic blood pressure, high-density lipoprotein cholesterol, total cholesterol, and smoking); model E, total model (combination of all variables included in model A-D).
Significance level: p < 0.05.
Osteopontin and Cardiovascular Disease Mortality
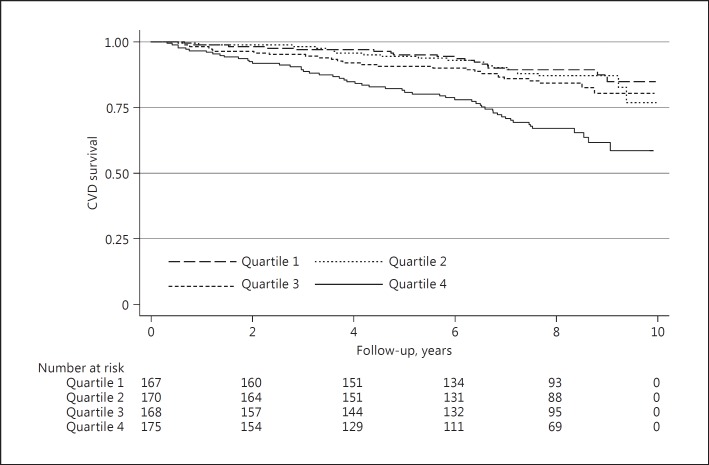
In multivariable Cox regression analyses, the association between plasma osteopontin and the risk of cardiovascular mortality was significant in all multivariable models (p < 0.001; model A-E). Kaplan-Meier curves showed an association between plasma osteopontin and cardiovascular mortality (Fig. 2). In contrast, the association between urinary osteopontin and cardiovascular mortality was not statistically significant in any of the models (Table 3). In secondary analysis, we performed stratified analyses in those with and without prevalent cardiovascular disease. Plasma osteopontin appeared to be associated with the risk of cardiovascular death in both strata in all multivariable models (with the exception of model E in those without prevalent cardiovascular disease at baseline, p = 0.084; online suppl. Table 4).
Fig. 2.
Kaplan-Meier curves and individuals at risk of cardiovascular mortality according to quartiles of plasma osteopontin distribution. CVD, cardiovascular disease.
Table 3.
The association between creatinine-adjusted urinary osteopontin and plasma osteopontin and cardiovascular mortality (Cox regression)
| Cardiovascular mortality |
||||
|---|---|---|---|---|
| urinary osteopontin, ng/mL |
plasma osteopontin, ng/mL |
|||
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Model A | 0.98 (0.78 – 1.21) | 0.82 | 1.55 (1.35 – 1.76) | <0.001 |
| Model B | 1.03 (0.83 – 1.29) | 0.77 | 1.41 (1.22 – 1.63) | <0.001 |
| Model C | 1.00 (0.80 – 1.24) | 0.97 | 1.44 (1.25 – 1.67) | <0.001 |
| Model D | 0.90 (0.72 – 1.12) | 0.34 | 1.51 (1.31 – 1.74) | <0.001 |
| Model E | 1.00 (0.79 – 1.26) | 0.99 | 1.35 (1.14 – 1.58) | <0.001 |
Data are HR per standard deviation increment. Plasma osteopontin, n = 739; urinary osteopontin, n = 513. HR, hazard ratio; CI, confidence interval; model A, age-adjusted model; model B, kidney function model (model A + cystatin C-estimated glomerular filtration rate and albuminuria); model C, inflammation model (model A + C-reactive protein and interleukin 6); model D, cardiovascular risk factor model (model A + lipid-lowering treatment, cardiovascular diagnosis, body mass index, diabetes, antihypertensive treatments, systolic blood pressure, high-density lipoprotein cholesterol, total cholesterol, and smoking); model E, total model (combination of all variables included in model A–D).
In the participants without cardiovascular disease at baseline, the C-statistic increased significantly for the prediction of cardiovascular mortality when plasma osteopontin was incorporated into a model with established cardiovascular risk factors (C-statistics for base model [model C], 0.610, and for base model + plasma osteopontin, 0.665, p = 0.002).
Discussion
Principal Findings
In the present study, data from a community-based cohort of elderly men demonstrated that higher urinary osteopontin, but not plasma osteopontin, was associated with incident chronic kidney disease and GFR decline, while higher plasma osteopontin, but not urinary osteopontin, was independently associated with an increased risk for cardiovascular death. These associations were independent of age, established cardiovascular risk factors, inflammatory markers, GFR, and albuminuria. Our data confirm and extend our understanding of the importance of osteopontin in the interplay between the kidney and the cardiovascular system and suggest that osteopontin may have specific roles in different tissues.
Comparison with the Literature
Previous observational studies on plasma osteopontin have been performed in select patient groups, such as patients with stable ischemic heart disease [11], calcified coronary plaques [12], and symptomatic carotid atherosclerosis [6] and in arteries of diabetic patients [13]. In these studies, higher plasma osteopontin has generally been shown to be associated with more severe atherosclerosis and cardiovascular disease. Furthermore, in a cohort of critically ill patients with acute kidney injury (AKI) requiring renal replacement therapy, plasma osteopontin was found to be significantly elevated compared to critically ill controls without AKI [7]. We are aware of no previous community-based studies reporting the longitudinal association between plasma osteopontin and cardiovascular death.
Urinary osteopontin appears to be less evaluated as a biomarker. One clinical study in newborns put forward urinary osteopontin as a promising marker for AKI [14]; otherwise, these studies are scares. To our knowledge, the association of urinary osteopontin with chronic kidney disease incidence and GFR decline has not been reported before.
Potential Mechanisms
It is not possible to substantiate causal relationships between osteopontin and cardiorenal disease in the present study due to our observational study design. There is, however, some previous experimental evidence that may provide an explanation for our findings.
Our study aimed to investigate separate associations of plasma and urinary osteopontin with kidney and cardiovascular outcome. Indeed, osteopontin has been shown to be expressed both in the vascular wall, the myocardium, and locally in both fetal and mature human kidneys [15, 16, 17], with a particularly high subcellular expression in the tubuli (http://www.proteinatlas.org/ENSG00000118785-SPP1/tissue/kidney#imid_7707072). Yet, it is not known whether the plasma and urinary levels of osteopontin originate from these tissues.
Experimental studies performed in various animal models and humans suggest that osteopontin plays a pivotal role in the development of atherosclerosis, vascular remodeling, and restenosis [18, 19, 20]. Osteopontin has been shown to be an important contributor to the migration of vascular smooth muscle cells through degradation of extracellular matrix in animal studies using angiotensin II-induced hypertension models [21]. Interestingly, osteopontin has been shown to be significantly reduced by atherosclerosis-modifying treatment with both statin and angiotensin II blockade compared to controls [22], which provides additional support for the view of a possible causal role of osteopontin in the atherosclerotic process.
Furthermore, angiotensin II is one of the main players in the pathogenesis of hypertensive nephropathy and subsequent chronic and end-stage renal damage [23] and has been used as an experimental model in rodents, which exhibit upregulation of osteopontin in inflammation, oxidation, and fibrosis in the course of angiotensin II-induced renal injury [24]. Moreover, osteopontin has been shown to be constitutively expressed in human kidney and locally upregulated during inflammation and fibrosis, strengthening the inflammatory function and expression of osteopontin in the renal compartments [17]. Whether urinary levels of osteopontin reflect these pathways remains to be established.
In a rat model, urinary osteopontin concentrations were significantly increased following induction of AKI [25], and urinary osteopontin has in 1 clinical study been suggested as a promising biomarker for AKI [14]. Yet, in the present study, the prevalence of AKI was low (or absent), which indicates that urinary osteopontin likely also reflects chronic tubular damage.
Both plasma and urinary osteopontin were associated with several established cardiovascular risk factors in cross-sectional analyses. However, the longitudinal association between osteopontin and outcome was generally independent of these factors, which would argue against confounding of these factors as a major explanation of our findings.
Clinical Implications
As osteopontin plays an important role in both cardiovascular and kidney disease, it may be a suitable target for pharmaceutical intervention. As described above, treatment with statins and antihypertensive agents has previously been shown to influence osteopontin activity [22]. Remarkably, studies done in mice showed that inhibition of renal osteopontin by liver X receptor agonists may have a therapeutic value for diabetic nephropathy [26], and several other preclinical animal studies using loss-of-function approaches have demonstrated osteopontin as a therapeutic target [27]. Whether the measurement of plasma or urinary osteopontin will be useful in guiding the initiation and monitoring of osteopontin-modulating treatments remains to be established.
In clinical practice, there is a need for an improved prediction of cardiovascular and kidney disease, and the measurement of novel biomarkers may be a means to achieve this. Our findings suggest that plasma osteopontin, as evident by the substantial increase in the C-statistic, could improve the risk stratification beyond the established cardiovascular risk factors. Yet, this result should be interpreted with caution given the narrow age range of the cohort and the inclusion of elderly men only. External validation is warranted.
Strengths and Limitations
The strengths of our investigation include our longitudinal study design, the use of perhaps the largest community-based cohort in which both circulating and urinary levels of osteopontin were analyzed, and the detailed description of the study participants with respect to cardiovascular and kidney phenotypes. Limitations comprise the inability to replicate the results in an independent cohort and the inability to generalize the results to women and other age and ethnic groups. Additional large-scale studies in these groups are needed to accurately validate our findings in order to identify individuals at an increased risk.
Conclusions
Higher urinary osteopontin specifically predicts incident chronic kidney disease, while plasma osteopontin specifically predicts cardiovascular death. Our data put forward osteopontin as an important factor in the detrimental interplay between the kidney and the cardiovascular system. The clinical implications, and why plasma and urinary osteopontin mirror different pathologies, remain to be established.
Statement of Ethics
All study participants gave written informed consent in adherence to the Declaration of Helsinki, and the Ethics Committee of Uppsala University approved the study protocols.
Disclosure Statement
The authors declare no conflicts of interest.
Funding Sources
This study was supported by The Swedish Research Council, Swedish Heart-Lung Foundation, the Marianne and Marcus Wallenberg Foundation, Dalarna University, and Uppsala University. The funding sources did not play any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Dr. Ärnlöv is the guarantor of this work, had full access to all the data, and takes full responsibility for the integrity of data and the accuracy of data analysis.
Supplementary Material
Supplementary data
References
- 1.Fisher LW, et al. Small integrin binding ligand N-linked glycoprotein gene family expression in different cancers. Clin Cancer Res. 2004;10:8501–8511. doi: 10.1158/1078-0432.CCR-04-1072. [DOI] [PubMed] [Google Scholar]
- 2.Denhardt DT, et al. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 2001;107:1055–1061. doi: 10.1172/JCI12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Brien ER, et al. Osteopontin is synthesized by macrophage, smooth muscle, and endothelial cells in primary and restenotic human coronary atherosclerotic plaques. Arterioscler Thromb. 1994;14:1648–1656. doi: 10.1161/01.atv.14.10.1648. [DOI] [PubMed] [Google Scholar]
- 4.Bruemmer D, et al. Angiotensin II-accelerated atherosclerosis and aneurysm formation is attenuated in osteopontin-deficient mice. J Clin Invest. 2003;112:1318–1331. doi: 10.1172/JCI18141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorenzen J, et al. Circulating levels of osteopontin are closely related to glomerular filtration rate and cardiovascular risk markers in patients with chronic kidney disease. Eur J Clin Invest. 2010;40:294–300. doi: 10.1111/j.1365-2362.2010.02271.x. [DOI] [PubMed] [Google Scholar]
- 6.Golledge J, et al. Osteoprotegerin and osteopontin are expressed at high concentrations within symptomatic carotid atherosclerosis. Stroke. 2004;35:1636–1641. doi: 10.1161/01.STR.0000129790.00318.a3. [DOI] [PubMed] [Google Scholar]
- 7.Lorenzen JM, et al. Osteopontin predicts survival in critically ill patients with acute kidney injury. Nephrol Dial Transplant. 2011;26:531–537. doi: 10.1093/ndt/gfq498. [DOI] [PubMed] [Google Scholar]
- 8.Byberg L, et al. Plasminogen activator inhibitor-1 activity is independently related to both insulin sensitivity and serum triglycerides in 70-year-old men. Arterioscler Thromb Vasc Biol. 1998;18:258–264. doi: 10.1161/01.atv.18.2.258. [DOI] [PubMed] [Google Scholar]
- 9.Florvall G, et al. Microalbuminuria measured by three different methods, blood pressure and cardiovascular risk factors in elderly Swedish males. Anal Chem Insights. 2008;3:69–74. [PMC free article] [PubMed] [Google Scholar]
- 10.Larsson A, et al. Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest. 2004;64:25–30. doi: 10.1080/00365510410003723. [DOI] [PubMed] [Google Scholar]
- 11.Georgiadou P, et al. Osteopontin as a novel prognostic marker in stable ischaemic heart disease: a 3-year follow-up study. Eur J Clin Invest. 2010;40:288–293. doi: 10.1111/j.1365-2362.2010.02257.x. [DOI] [PubMed] [Google Scholar]
- 12.Fitzpatrick LA, et al. Diffuse calcification in human coronary arteries. Association of osteopontin with atherosclerosis. J Clin Invest. 1994;94:1597–1604. doi: 10.1172/JCI117501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen NX, Moe SM. Arterial calcification in diabetes. Curr Diab Rep. 2003;3:28–32. doi: 10.1007/s11892-003-0049-2. [DOI] [PubMed] [Google Scholar]
- 14.Askenazi DJ, et al. Urine biomarkers predict acute kidney injury in newborns. J Pediatr. 2012;161:270–275. doi: 10.1016/j.jpeds.2012.02.007. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Momiyama Y, et al. Associations between plasma osteopontin levels and the severities of coronary and aortic atherosclerosis. Atherosclerosis. 2010;210:668–670. doi: 10.1016/j.atherosclerosis.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kadoglou NP, et al. The relationship between serum levels of vascular calcification inhibitors and carotid plaque vulnerability. J Vasc Surg. 2008;47:55–62. doi: 10.1016/j.jvs.2007.09.058. [DOI] [PubMed] [Google Scholar]
- 17.Hudkins KL, et al. Osteopontin expression in fetal and mature human kidney. J Am Soc Nephrol. 1999;10:444–457. doi: 10.1681/ASN.V103444. [DOI] [PubMed] [Google Scholar]
- 18.Giachelli CM, et al. Osteopontin is elevated during neointima formation in rat arteries and is a novel component of human atherosclerotic plaques. J Clin Invest. 1993;92:1686–1696. doi: 10.1172/JCI116755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isoda K, et al. Osteopontin plays an important role in the development of medial thickening and neointimal formation. Circ Res. 2002;91:77–82. doi: 10.1161/01.res.0000025268.10302.0c. [DOI] [PubMed] [Google Scholar]
- 20.Seo KW, et al. Mechanical stretch enhances the expression and activity of osteopontin and MMP-2 via the Akt1/AP-1 pathways in VSMC. J Mol Cell Cardiol. 2015;85:13–24. doi: 10.1016/j.yjmcc.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38((3 Pt 2)):581–587. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- 22.Lorenzen JM, et al. Angiotensin II receptor blocker and statins lower elevated levels of osteopontin in essential hypertension – results from the EUTOPIA trial. Atherosclerosis. 2010;209:184–188. doi: 10.1016/j.atherosclerosis.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Black C, van der Veer SN. Unlocking the value of variation in CKD prevalence. J Am Soc Nephrol. 2015;29:2015111280. doi: 10.1681/ASN.2015111280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolak T, et al. Osteopontin modulates angiotensin II-induced inflammation, oxidative stress, and fibrosis of the kidney. Kidney Int. 2009;76:32–43. doi: 10.1038/ki.2009.90. [DOI] [PubMed] [Google Scholar]
- 25.Won AJ, et al. Discovery of urinary metabolomic biomarkers for early detection of acute kidney injury. Mol Biosyst. 2016;12:133–144. doi: 10.1039/c5mb00492f. [DOI] [PubMed] [Google Scholar]
- 26.Tachibana H, et al. Activation of liver X receptor inhibits osteopontin and ameliorates diabetic nephropathy. J Am Soc Nephrol. 2012;23:1835–1846. doi: 10.1681/ASN.2012010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiefer FW, et al. Neutralization of osteopontin inhibits obesity-induced inflammation and insulin resistance. Diabetes. 2010;59:935–946. doi: 10.2337/db09-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data