Abstract
Polycystic ovary syndrome is a common endocrine disorder and a major cause of anovulatory sterility in women at reproductive age. Most patients with polycystic ovary syndrome have hyperandrogenism, caused by excess androgen synthesis. Bone morphogenetic protein 4 (BMP4) is an essential regulator of embryonic development and organ formation, and recent studies have also shown that BMP4 may be involved in female steroidogenesis process. However, the effect of BMP4 on hyperandrogenism remains unknown. Here, using a female mouse model of hyperandrogenism, we found that ovarian BMP4 levels were significantly decreased in hyperandrogenism. Elevated androgens inhibited BMP4 expression via activation of androgen receptors. Moreover, BMP4 treatment suppressed androgen synthesis in theca cells and promoted estrogen production in granulosa cells by regulating the expression of steroidogenic enzymes, including CYP11A, HSD3B2, CYP17A1, and CYP19A1. Consistently, knockdown of BMP4 augmented androgen levels and inhibited estrogen levels. Mechanistically, Smad signaling rather than the p38 MAPK pathway regulated androgen and estrogen formation, thereby mediating the effect of BMP4. Of note, BMP4-transgenic mice were protected against hyperandrogenism. Our observations clarify a vital role of BMP4 in controlling sex hormone levels and offer new insights into intervention for managing hyperandrogenism by targeting the BMP4-Smad signaling pathway.
Keywords: androgen, bone morphogenetic protein (BMP), cytochrome P450, endocrinology, SMAD transcription factor, hyperandrogenism, polycystic ovary syndrome
Introduction
Polycystic ovary syndrome (PCOS)4 is a highly heterogeneous and multifactorial endocrine disorder that is the leading cause of non-ovulation infertility in women of reproductive age (1, 2). Generally, PCOS is mainly characterized by two of the following three criteria: oligo-ovulation or non-ovulation, clinical/biochemical hyperandrogenism, and polycystic ovary appearance on ultrasonography. Its global prevalence is estimated to be between 6 and 10% and even up to 21%, depending on the diagnostic criteria and geographic location (3).
Hyperandrogenism is a key feature of PCOS and contributes to the clinical phenotype of PCOS patients, including menstrual and ovulatory dysfunction, hirsutism, and acne (4, 5). This condition is characterized by elevated serum level of androgens such as testosterone, dihydrotestosterone, and dehydroepiandrosterone (DHEA), primarily due to excess androgens production. In females, androgens are synthesized de novo from cholesterol in the ovarian theca cells (TCs) and the adrenal cortex. The ovarian TCs are stimulated by luteinizing hormone (LH) or insulin, which in turn activates a series of steroidogenic enzymes, including cytochrome P450scc (encoded by CYP11A), Δ5-isomerase-3β-hydroxysteroid dehydrogenase type 2 (3βHSD2, encoded by HSD3B2), and P450c17 (encoded by CYP17A1), thereby catalyzing the synthesis of androgens including testosterone and dihydrotestosterone (6, 7). The newly synthesized androgens then in part diffuse from TCs to granulosa cells (GCs), where they are aromatized by cytochrome P450arom (encoded by CYP19A1) to produce estrogens such as estrone and estradiol (6–8). These steroidogenic enzymes are essential to the level of sex hormones. Therefore, genetic mutation or aberrant expression of these genes may be highly related to pathogenesis of hyperandrogenism and PCOS (9). It is reported that the rate-limiting enzyme P450c17 is highly expressed and activated in ovarian TCs of PCOS patients, giving rise to increased androgens formation (10, 11). Thus, to investigate the steroidogenesis process and the regulation of steroidogenic enzymes it is necessary to understand the molecular basis underlying hyperandrogenism and PCOS development.
Bone morphogenetic protein-4 (BMP4) is a secreted protein and belongs to the bone morphogenetic protein family (12). It is well established that BMP4 plays a vital role in embryonic development and organ formation (13). BMP4, like other BMP family members, elicits its effects through two distinct serine–threonine kinase transmembrane receptors, type I and type II receptors (12, 14). Once binding with BMP4, type I receptors are activated and in turn transduce intracellular signals via mothers against dpp (Smad) and p38 mitogen-activated protein kinase (MAPK) pathway. In the former case, activated BMP type I receptors phosphorylate receptor-regulated Smads (R-Smads) at their carboxyl-terminal SSXS motifs, including Smad1, Smad5, and Smad8, which are BMP-specific R-Smads. The phosphorylated and activated R-Smad proteins form complexes with common partner Smad (coSmad); that is, Smad4, the BMP-specific coSmad. Smad complexes containing one Smad4 and two R-Smads move into the nucleus and associate with various transcriptional co-activators or co-repressors, then bind to regulatory elements of target genes to regulate their transcription (12, 14). In addition to Smad, p38 MAPK mediates BMP4 function in such physiological processes as cell differentiation and organ development. Upon phosphorylation by type I receptors, TGF-β-activated kinase 1 (TAK1) recruits TAK1-binding protein 1 and initiates the phosphorylation cascade with MAPK kinase kinase (MKK) and p38 MAPK. Phosphorylated/activated p38 MAPK then activates the expression of BMP target genes (12, 14).
We previously reported that BMP4 was required for commitment from pluripotent stem cells to the adipocyte lineage (15–16) and that BMP4 could improve systemic insulin sensitivity and energy homeostasis (17). The effect of BMP4 on metabolic improvement indicated its potential role in regulating PCOS and hyperandrogenism, as increasing evidence showed that hyperandrogenism was highly related with metabolic disorders (18). Additionally, it has been reported that BMPs promoted primordial follicle development (19) and might be involved in ovarian steroidogenesis process in vitro (20, 21). However, the effect of BMP4 on androgen production is still controversial, and the underlying molecular mechanism needs further clarification. In the current study we investigated the effect of BMP4 on coordination between androgen and estrogen synthesis and shed light on the mechanism underlying the regulation of steroidogenic enzymes by BMP4. Our results emphasized the essential role of the BMP4-Smad signaling pathway in the regulation of hyperandrogenism development.
Results
BMP4 expression in ovary was negatively related with the occurrence of hyperandrogenism
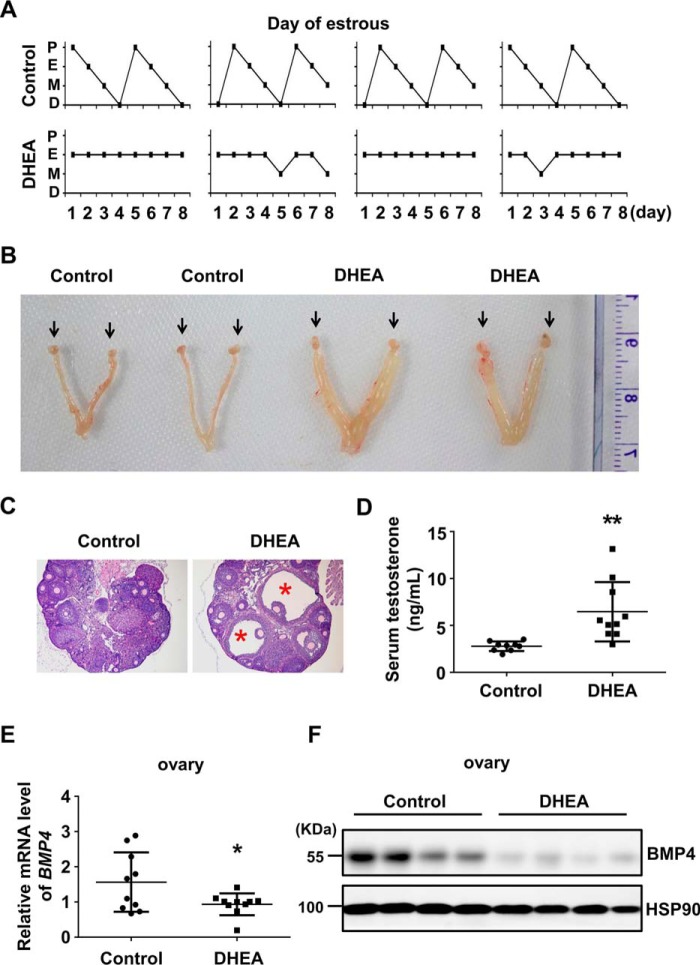
To clarify the expression of BMP4 on hyperandrogenism pathogenesis, we established PCOS mice model by consecutively injecting mice with DHEA for 20 days. Abnormal estrous cycles (Fig. 1A), dropsical ovary and uterus (Fig. 1B), increased cystic follicles (Fig. 1C), and elevated serum testosterone (Fig. 1D) were found in DHEA-treated mice, indicating that PCOS model was successfully developed. We, therefore, detected BMP4 expression in ovaries, finding out that both mRNA and protein levels of BMP4 were significantly decreased in hyperandrogenism ovaries compared with control (Fig. 1, E–F).
Figure 1.
BMP4 was suppressed in the ovary of hyperandrogenism. PCOS hyperandrogenism model was established by injecting DHEA in female 4-week-old mice (n = 9–10/group). A, estrous cycle was assessed by vaginal cytology for eight consecutive days. Control mice underwent 1–2 estrous cycles, whereas DHEA-treated mice lost regular estrous cycle. P, proestrus; E, estrus; M, metestrus; D, diestrus. B, morphology observation of ovary (arrows) and uterus edema. C, H&E staining showed cystic follicles (asterisk) in ovary from DHEA-induced mice. D, serum total testosterone of control and DHEA-treated mice was measured by chemiluminescence immunoassay. E, qPCR analysis of BMP4 mRNA levels in the ovaries from control and DHEA-induced mice. F, Western blot assay of BMP4 protein levels in the ovaries of control and DHEA-induced mice, with HSP90 as the loading control. *, p < 0.05; **, p < 0.01 compared with control group.
In addition to BMP4, we found that part of the BMP family members was down-regulated in hyperandrogenism ovaries, including BMP5, BMP6, BMP8b, BMP11, BMP12, and BMP15 (supplemental Fig. 1A), suggesting that BMP signaling might be impaired in hyperandrogenism. We also detected the expression of BMP antagonists and found that mRNA levels of Noggin, Chordin, Follistatin, and Gremlin were not affected in the hyperandrogenism condition (supplemental Fig. 1B).
Androgens inhibited BMP4 expression via androgen receptor (AR)
To identify the cell types that contributed to the down-regulated BMP4 expression, we isolated ovarian TCs and GCs. Consistent with previous report (22), BMP4 was mainly expressed in TCs (Fig. 2A). We then treated TCs and GCs with testosterone and DHEA. Quantitative PCR (qPCR) assays showed that both testosterone and DHEA induction could repress BMP4 expression in TCs and GCs (Fig. 2, B and C). As several androgen response elements were found in BMP4 promoter (data not shown), we then sought to confirm whether androgens regulated BMP4 promoter activity. To this end, we conducted reporter assays by inserting the BMP4 promoter to the upstream of the luciferase gene. We observed that BMP4 promoter activity was significantly inhibited by testosterone and DHEA treatment in a dose-dependent manner (Fig. 2D). Based on the notion that androgens function through AR activation (23), we therefore determined whether AR regulated BMP4 expression. We found that a deficiency of AR significantly reversed the down-regulated BMP4 expression caused by testosterone (Fig. 2E) and that AR could significantly attenuate BMP4 promoter activity (Fig. 2F). We then performed chromatin immunoprecipitation (ChIP) assays in TCs and GCs. ChIP-qPCR analysis showed that AR could bind to BMP4 promoter upon testosterone treatment (Fig. 2G). These results collectively illustrated that BMP4 was down-regulated by androgens through AR.
Figure 2.
Androgens inhibited BMP4 expression through AR. TCs and GCs were isolated from the ovaries of mice as described. A, qPCR analysis of BMP4 mRNA levels in TCs and GCs. B and C, TCs and GCs were treated with testosterone (B) or DHEA (C) at the concentration of 0, 1, 10, 25, 50, and 100 μm for 48 h. qPCR analysis was used to detect BMP4 expression. D, the 293T cells were transfected with BMP4 promoter and treated with testosterone and DHEA, with DMSO as a control. At 36 h after transfection, luciferase activity was measured. The luciferase data were normalized to DMSO-treated cells. E, TCs and GCs were transfected with siAR to disrupt AR expression, with siNC as the control, and then treated with testosterone (25 μm). qPCR analysis of AR and BMP4 expressions. *, p < 0.05 compared with siNC group; #, p < 0.05 compared with testosterone-treated siNC group (siNC+T). F, the 293T cells were transfected with BMP4 promoter and AR (0, 0.1, 0.2, 0.3, 0.4 μg) and then subjected to luciferase measurement 36 h after transfection. G, ChIP-qPCR was conducted in TCs and GCs with or without testosterone treatment for 24 h. Data were normalized to IgG in each group. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with control group.
BMP4 inhibited androgen synthesis and promoted estrogen production
It is well established that androgens were mainly synthesized in ovarian TCs, and estrogens were produced in GCs (9). We, therefore, isolated TCs and GCs and treated them with BMP4 and found that total testosterone from TC supernatant was significantly decreased by BMP4 in a dose-dependent manner, whereas estrogen level from GC supernatant was elevated (Fig. 3A). Then we detected expression of several steroidogenic enzymes, including CYP11A, HSD3B2, and CYP17A1, which were responsible for androgens synthesis in TCs, as well as CYP19A1, which was critical for estrogen formation in GCs (Fig. 3B). We found that mRNA levels of enzymes for androgen production in TCs, especially CYP17A1, was significantly suppressed by BMP4 treatment and that CYP19A1 in GCs was dramatically promoted by BMP4 (Fig. 3C). However, expression of SF1, a known transcription factor of CYP17A1 and CYP19A1 (24), was not affected by BMP4 (Fig. 3C). Consistently, CYP17A1 protein levels in TC were decreased, and CYP19A1 protein levels in GCs were increased by BMP4 (Fig. 3D). We then investigated the effect of Noggin on steroidogenic enzymes expression, which is a well known antagonist for BMP4 (25). We found that Noggin significantly reversed the altered steroidogenic genes expression caused by BMP4 in both TCs and GCs (Fig. 3E). In brief, Noggin augmented expression of CYP11A, HSD3B2, and CYP17A1 in TCs and repressed expression of CYP19A1 in GCs.
Figure 3.
BMP4 controlled synthesis of androgens and estrogens. TCs and GCs were isolated from mice ovaries and treated with BMP4 for 48 h at the concentration of 0, 10, 40, and 80 ng/ml. A, testosterone in TC supernatant and estrogen in GC supernatant were measured using chemiluminescence immunoassay. B, schematic diagram for androgens and estrogens production from cholesterol in ovaries. C, qPCR analysis of CYP11A, HSD3B2, CYP17A1, and SF1 mRNA levels in TCs (left) and CYP19A1 and SF1 mRNA levels in GCs (right) upon BMP4 treatment. D, protein level of CYP17A1 in TCs and CYP19A1 in GCs upon BMP4 treatment were detected by Western blot analysis. E, TCs and GCs were treated with BMP4 (40 ng/ml) and noggin (100 ng/ml) for 48 h as indicated. qPCR analysis of CYP11A, HSD3B2, CYP17A1, and SF1 mRNA levels in TCs (left) and CYP19A1 and SF1 mRNA levels in GCs (right) is shown. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with control group. #, p < 0.05; ##, p < 0.01 compared with BMP4-treated group.
We then disrupted BMP4 expression by adenovirus and measured androgen and estrogen production. We found that knockdown of BMP4 promoted androgen synthesis in TCs and inhibited estrogen in GCs (Fig. 4A). Consistently, knockdown of BMP4 augmented CYP11A, HSD3B2, and CYP17A1 expression in TCs but repressed CYP19A1 expression in GCs (Fig. 4B). Taken together, these data suggested that BMP4 inhibited androgens synthesis in ovarian TCs and promoted estrogens production in GCs.
Figure 4.
Knockdown of BMP4 modulated androgen and estrogen levels. TCs and GCs were isolated from mice ovaries and treated with adenovirus to disrupt BMP4 expression. A, testosterone in TCs supernatant and estrogen in GCs supernatant were measured using chemiluminescence immunoassay. B, qPCR analysis of CYP11A, HSD3B2, CYP17A1, and BMP4 mRNA levels in TCs (left) and CYP19A1 and BMP4 mRNA levels in GCs (right) upon BMP4 knockdown. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with control group.
BMP4 activated both Smad and p38 MAPK signaling pathways in TCs and GCs
BMP4 binds to two distinct type I and type II serine/threonine kinase receptors, which activates Smad and p38 MAPK signaling pathway, thereby regulating cellular processes (12, 14). We found that ovarian TCs and GCs mainly expressed type I receptor Bmpr1a and the type II receptors Bmpr2 and Acvr2a, indicating that these cells might be the direct target of BMP4 (Fig. 5A). Additionally, Western blot analyses showed that BMP4 activated both the Smad and p38 MAPK pathways in TCs and GCs, as indicated by increased phosphorylated (p)-Smad1/5/8 and p-p38 MAPK (Fig. 5B).
Figure 5.
BMP4 activated both the Smad and p38 MAPK signaling pathway in TCs and GCs. A, RT-PCR was used to detect BMP receptors including Bmpr1a, Bmpr1b, Bmpr2, Acvr2a, and Acvr2b in mice ovary and isolated TCs and GCs. B, TCs and GCs were treated with BMP4 at the indicated concentrations for 3 h and then subjected to Western blot assay for p-Smad1/5/8, Smad1, p-p38 MAPK, p38 MAPK, and HSP90.
Smad signaling was required for the effect of BMP4 on androgen and estrogen production
The data above implied that both Smad and p38 MAPK signaling pathways were activated by BMP4 in TCs and GCs. To explore which pathway mediated BMP4's effect, we respectively disrupted Smad4 and p38 MAPK expression by using two sets of siRNA. Smad4 is BMP4-specific coSmad, which is required for Smad complex formation to regulate target genes (12, 14). Hence, Smad signaling could be disrupted upon Smad4 deficiency. We found that knockdown of Smad4 by siSmad4-1 and siSmad4-2 increased total testosterone levels secreted by TCs and decreased estrogen levels from GCs even under the condition of BMP4 treatment (Fig. 6A). In contrast, knockdown of p38 MAPK had little effect on androgen and estrogen levels and even slightly increased estrogen levels in GCs (Fig. 6A). We then detected the expression of steroidogenic enzymes and found that Smad4 deficiency could reverse BMP4-induced expression changes of these enzymes. Briefly, a deficiency of Smad4 promoted CYP11A, HSD3B2, and CYP17A1 expression in TCs and inhibited CYP19A1 in GCs, whereas disruption of p38 MAPK could hardly affect these (Fig. 6, B and C). Collectively, these results suggested that the Smad pathway, instead of p38 MAPK, mediated BMP4 function in regulating androgens and estrogens synthesis.
Figure 6.
Smad signaling mediated the effect of BMP4 on androgen and estrogen production. Smad4 and p38 MAPK expression in TCs and GCs were, respectively, disrupted by transfecting two sets of siRNAs with siNC as the control. These cells were then treated with or without BMP4 (40 ng/ml) for 48 h. A, testosterone in TCs supernatant and estrogen in GCs supernatant were measured using chemiluminescence immunoassay. B, Western blot analysis of CYP17A1, CYP19A1, Smad4, p38 MAPK, and HSP90 in the indicated TCs and GCs. C, qPCR analysis of CYP11A, HSD3B2, and CYP17A1 expression in TCs and CYP19A1 in GCs. *, p < 0.05; **, p < 0.01 compared with control group. #, p < 0.05; ##, p < 0.01; ###, p < 0.001compared with BMP4-treated control group. D, the 293T cells were transfected with CYP17A1 promoter (0.25 μg), FLAG-Smad1 (left), or FLAG-Smad4 (right) at 0, 0.1, 0.2, 0.3, and 0.4 μg. 36 h after transfection, luciferase activity was measured, and data were normalized to the cells without Smad transfection. Western blot analysis with FLAG antibody was used to detect expression of FLAG-Smad1 and FLAG-Smad4. E, TCs were transfected with CYP17A1 promoter (0.3 μg), FLAG-Smad1 (0.3 μg), or FLAG-Smad4 (0.3 μg). 36 h after transfection, luciferase activity was conducted, and data were normalized to the cells without Smad transfection. *, p < 0.05.
The results above indicated that BMP4 regulated CYP17A1 expression through the Smad signaling pathway, which motivated the hypothesis that Smad protein directly modulated promoter activity of CYP17A1. To test the possibility, we transfected CYP17A1 promoter into 293T cells with Smad1 or Smad4, and a reporter assay was then performed finding that both Smad1 and Smad4 repressed CYP17A1 promoter activity in a dose-dependent manner (Fig. 6D). We further carried out reporter assay in TCs, with the same results (Fig. 6E). These results together indicated that Smads could regulate CYP17A1 expression.
BMP4-transgenic mice were protected against hyperandrogenism development
We then sought to demonstrate the effect of BMP4 in vivo. Because the adipose tissue is the main source of BMP4, we generated adipocyte-specific BMP4-transgenic (BMP4-TG) mice. As DHEA treatment might conceal the possible changes of androgen levels caused by BMP4, we established the PCOS model on BMP4-TG mice by injecting insulin and hCG for 3 weeks as described (Fig. 7A), which was a well acknowledged method to generate endogenous hyperandrogenism (26). No significant weight changes were observed between BMP4-TG and WT mice (Fig. 7B). Intriguingly, WT mice developed abnormal estrous cycles upon induction, whereas BMP4-TG mice underwent normal estrous cycles (Fig. 7C). Of note, the serum testosterone of BMP4-TG mice was significantly lower than WT mice (Fig. 7D), whereas no significant difference was found in estrogen levels (Fig. 7D). Consistently, decreased CYP17A1 levels and increased CYP19A1 levels were observed in the ovaries of BMP4-TG mice compared with WT mice (Fig. 7E). These results together suggested that BMP4 inhibited hyperandrogenism development, which validated the results in vitro.
Figure 7.
BMP4 inhibited hyperandrogenism occurrence in vivo. A, to establish a hyperandrogenism model, the 6-week-old WT and BMP4-TG mice were injected daily (subcutaneously) with insulin and hCG at the indicated dose for 3 weeks. In brief, the mice were injected with 0.07 IU of insulin on day 1, and the dosage was gradually increased to 0.84 IU on day 11. The dosage of insulin was then maintained at 0.84 IU from the day 12 until the day 22. The insulin was administered along with twice-daily injections of 0.21 IU of hCG. The mice were subjected to further investigation after administration. B–D, body weight (B), estrous cycle (C), serum estrogen (left in D), and androgen (right in D) were measured in WT and BMP4-TG mice (n = 3/group). E, Western blot analysis of CYP17A1 and CYP19A1 levels in ovaries of WT and BMP4-TG mice. *, p < 0.05.
Discussion
In the current study, we found that BMP4 was down-regulated by high levels of androgens. BMP4 treatment inhibited androgen synthesis in ovarian TCs and promoted estrogen production in GCs. The molecular mechanism is that BMP4 activated the Smad-signaling pathway, which in turn inhibited expression of CYP17A1. Based on these findings, we proposed a model in which BMP4 plays a role in controlling hyperandrogenism development by regulating androgens synthesis (Fig. 8). Normally, BMP4 coordinated the androgen and estrogen synthesis in the ovary through Smad pathway. Under androgen excess, ovarian BMP4 expression was suppressed by activated AR. The negative regulation of androgen synthesis was then relieved as a consequence of down-regulated BMP4; therefore, androgen production was further increased, and the hyperandrogenism eventually developed (Fig. 8). The negative feedback loop between BMP4 and androgen synthesis was critical for hyperandrogenism occurrence.
Figure 8.
Model of the role of BMP4 in regulating hyperandrogenism development. Normal BMP4 inhibited the androgen and promoted estrogen synthesis via Smad signaling in ovary. Under androgen excess, ovarian BMP4 expression was suppressed by activated AR. The negative regulation of androgen synthesis was then relieved as a result of down-regulated BMP4, leading to a further increase of androgen level. Accordingly, hyperandrogenism eventually developed.
In addition to endocrine dysfunction, hyperandrogenism and PCOS are closely related to metabolic syndrome, including obesity, insulin resistance, type 2 diabetes, and hyperlipidemia (18). It is estimated that among PCOS patients, 50–75% develop overweight or diabetes, and ∼70% manifest insulin resistance (27). As a consequence of insulin resistance, insulin secretion is compensatorily augmented and contributes to hyperinsulinemia. High levels of insulin associates synergistically with LH and activates P450c17, in turn promoting production and release of androgens (27). The role of insulin resistance and hyperinsulinemia in the occurrence of PCOS is supported by observations that improving insulin sensitivity through weight loss or drug therapy such as metformin treatment ameliorates the metabolic, hyperandrogenic, and reproductive features of PCOS (28). We previously found that BMP4 improved systemic insulin sensitivity and energy homeostasis (17). In combination with our current finding that BMP4 prevented the body from hyperandrogenism, it emphasizes the conception that hyperandrogenism is highly related with energy metabolism. Therefore, to identify the essential regulator for the coordination between metabolic conditions and hyperandrogenism may not only clarify the mechanisms underlying PCOS pathogenesis but also provide new strategies for PCOS treatment.
Regulation of BMP4 expression has seldom been studied so far. We found for the first time that testosterone and DHEA inhibited BMP4 expression through suppressing its promoter activity. It is well acknowledged that androgens exert their genomic effects via interaction with the AR (23). AR is a ligand-dependent nuclear transcription factor and belongs to the steroid hormone nuclear receptor family. In the absence of androgens, AR associates with chaperone proteins and keeps cytoplasmic (29). Upon binding with the ligands, AR dissociates itself from chaperone proteins, exposes the nuclear localization signal domain, and translocates to the nucleus, where it binds to androgen response elements and regulates target gene transcription (29). Here we found that AR bound to BMP4 promoter and inhibited BMP4 promoter activity, thereby repressing its expression. However, the underlying mechanism remains unknown. It is reported that the transcriptional activity of AR is modulated by specific co-activator or co-repressor (30). The best-studied AR corepressors so far are nuclear receptor corepressor (NCoR) and silencing mediator of retinoid and thyroid hormone receptors (SMRT) (31). AR could directly bind with NCoR and SMRT (32). Repression transcription of the NCoR–SMRT complex is mediated through recruitment of histone deacetylases (31). Therefore, it is of interest to investigate whether the NCoR–SMRT complex or other corepressors are involved in AR-mediated regulation of BMP4 expression.
BMP4 acts through two types of signaling pathway, the Smad and p38 MAPK pathways (12, 14). Smad and p38 MAPK seem to mediate distinct functions of BMP4. It is well known that Smad4 associates with R-Smad complexes and co-translocates into the nuclei, where they recruit co-factors to regulate target gene expression. BMP4-specific Smad complexes could not be assembled upon Smad4 ablation; therefore, Smad signaling transduction was reduced (12, 14). In the current study, disruption of Smad signaling, but not p38 MAPK, reversed BMP4's effect on androgen and estrogen synthesis. On the contrary, knockdown of p38 MAPK slightly augmented estrogen levels, probably due to the mild increase of CYP19A1 expression (Fig. 6, B and C). We then found that Smad inhibited CYP17A1 promoter activity and thereby repressed CYP17A1 expression. We also found that CYP19A1 expression was regulated by BMP4-Smad signaling, the underlying mechanism of which needs further investigation. Our observation indicated that the BMP4-Smad pathway is a negative regulator of androgen synthesis, which is an essential process in PCOS development. Interestingly, a recent report echoes our findings (33). It has been reported that disruption of Smad4 signaling in the ovary causes premature GC luteinization and impaired ovulation and cumulus expansion (33). Hence, the BMP4-Smad signaling pathway might be a potential target for PCOS intervention.
Besides BMP4, other BMPs have been showed to regulate hormone synthesis, including BMP2, BMP6, BMP7, and BMP15. For instance, BMP6 and BMP7 enhanced both basal and stimulated secretion of estradiol but repressed progesterone in GCs (34). It is reported that BMP6 and BMP7 inhibited basal and LH-induced androgen production by bovine theca interna cells (20), although the underlying mechanisms have not yet been clarified. It seems that BMP family members exert similar functions in modulating ovarian steroidogenesis. Therefore, the ovarian BMP signaling defect may be a leading cause for hyperandrogenic dysfunction, suggesting the BMP-Smad pathway as a potential target for hyperandrogenism treatment.
Experimental procedures
Animals and establishment of the PCOS mice model
Female C57BL/6J mice were purchased from the Model Animal Research Center of Nanjing University. To establish the PCOS model, 4-week-old mice were subcutaneously injected daily with DHEA (6 mg/100 g body weight) dissolved in camellia oil for 20 consecutive days, with camellia oil injection as the control. Mice estrous cycle was assessed by vaginal cytology for eight consecutive days. Adipose tissue-specific BMP4-transgenic mice were generated as previously described (17). To establish the PCOS model on BMP4-TG mice, 6-week-old BMP4-TG mice were subcutaneously injected daily with insulin and hCG for 3 weeks. Briefly, insulin was injected to mice along with twice-daily injections of 0.21 IU of hCG. The dosage of insulin was gradually increased from 0.07 IU on day 1 to 0.84 IU on day 11 and maintained at 0.84 IU from the 12th day until the 22nd day. The mice were subjected to further investigation after administration. All the animal studies were approved by the Animal Care and Use Committee of the Fudan University Shanghai Medical College and followed the National Institute of Health guidelines on the care and use of animals.
Estrous cycle analysis
For eight consecutive days, vaginal cells of the indicated mice were collected via normal saline lavage daily and visualized under light microscopy after Giemsa staining. Briefly, samples with primarily nucleated cells indicated the proestrus stage, primarily cornified epithelial cells indicated the estrus stage, both cornified cells and leukocytes indicated the metestrus stage, and primarily leukocytes indicated the diestrus stage.
Isolation and culture of ovarian TCs and GCs
The TCs and GCs were isolated from the ovaries of 6-week-old mice. The ovaries were freed from the lower back incision and then isolated from their connective tissues under a stereomicroscope, cleaned two times, and then collected in Lebovitz's L-15 medium (Gibco) with 10% FBS (Gibco), 100 units/ml penicillin, 0.1 mg/ml streptomycin. The GCs were released by puncturing the follicles with a sterile hypodermic needle, then collected by centrifugation (300 × g) for 3 min and cultured in McCoy's 5a medium (Gibco) with 10% FBS, 100 units/ml penicillin, 0.1 mg/ml streptomycin.
To isolate the TCs, the remaining ovary tissues, after releasing GCs, were washed twice and cut up into fragments using scissors in a McCoy's 5a medium containing 4 mg/ml collagenase (Sigma) and were then pipetted to facilitate cell dispersion. The suspension of ovarian fragments was incubated at 37 °C for 60 min and pipetted every 5 min. After digestion, the cell suspension was filtered through a 40-μm cell strainer (BD Biosciences) to remove the undigested ovarian fragments. The filtered TC suspension was centrifuged at 300 × g for 5 min and then washed twice and cultured in McCoy's 5a medium. TCs and GCs, both, were seeded on 24-well plates (5 × 104/well) and treated with BMP4 (R&D Systems, Minneapolis, MN) and testosterone or DHEA (Sigma) as indicated.
Plasmid construct
The mice Smad1 coding sequence was amplified via PCR using the primers ATAAAGCTTAATGTGACCAGCTTGTTTTCATTCACAAG (forward) and ATAGGATCCTTAAGACACGGATGAAATAGGATTGTGGG (reverse) and then cloned into Prk7-N-FLAG vector using HindIII (5′ end) and BamHI (3′ end) restriction sites. The mice Smad4 coding sequence was amplified via PCR using the primers ATAAAGCTTGACAATATGTCTATAACAAATACACC (forward) and ATAGAATTCTCAGTCTAAAGGCTGTGGGTCCGCAA (reverse) and then cloned into Prk7-N-FLAG vector using HindIII (5′ end) and EcoRI (3′ end) restriction sites. The FLAG tag was added to the amino-terminus of Smad1 and Smad4. The promoter regions of mouse BMP4 was amplified via PCR using the primers CGGAGCTCGGCCAAAGGTCACTTTATTGTC (forward) and CGCTCGAGTGCCGAACTCACCTAGCTTC (reverse) and then cloned into pGL4.20 luciferase vector (Promega Corp., Madison, WI) using SacI (5′ end) and XhoI (3′ end) restriction sites. The promoter regions of mouse CYP17A1 was amplified via PCR using the primers ATAACGCGTGGCCATAGTGTTTTATAGCCCAGGGTGA (forward) and ATAAGATCTGCAGAGAAGGAGAACTTTTAAAAGGC (reverse) and then cloned into pGL3-basic luciferase vector (Promega) using MluI (5′ end) and BglII (3′ end) restriction sites. All the plasmid constructs were verified by DNA sequencing.
RNA isolation, qPCR, and RT-PCR
Total RNA of the cells and tissues was extracted using TRIzol reagent (Invitrogen). The purified RNA was then subjected to reverse transcription using the PrimeScript reverse transcriptase kit (TaKaRa Bio, Otsu, Japan) followed by qPCR assays. qPCR was carried out using Power SYBR green PCR master mix (Applied Biosystems, Foster City, CA) and a Prism 7500 instrument (Applied Biosystems), with 18S rRNA as an endogenous control. Analysis was done in triplicate and repeated at least three times. Results were presented as the means ± S.D. from several independent samples. Forward and reverse primers (5′ to 3′) of qPCR are as follows: BMP4, TTCCTGGTAACCGAATGCTGA and CCTGAATCTCGGCGACTTTTT; AR, CTGGGAAGGGTCTACCCAC and GGTGCTATGTTAGCGGCCTC; CYP11A, AGGTCCTTCAATGAGATCCCTT and TCCCTGTAAATGGGGCCATAC; HSD3B2, GGTTTTTGGGGCAGAGGATCA and GGTACTGGGTGTCAAGAATGTCT; CYP17A1, GCCCAAGTCAAAGACACCTAAT and GTACCCAGGCGAAGAGAATAGA; CYP19A1, ATGTTCTTGGAAATGCTGAACCC and AGGACCTGGTATTGAAGACGAG; SF1, AGGTGTCGGGCTACCACTAC and CCACCCCGCATTCGATCAG; 18S rRNA, CGGCTACCACATCCAAGGAA and GCTGGAATTACCGCGGCT. Primers used to detect BMP family members and BMP antagonists are shown in supplemental Table 1. Additionally, RT-PCR was used to detect BMP receptors using specific primers. Forward and reverse primers (5′ to 3′) of RT-PCR are as follows: Bmpr1a, GCGAACTATTGCCAAACAG and GAGGTGGCACAGACCACAAG; Bmpr1b, GACACTCCCATTCCTCATC and GCTATTGTCCTTTGGACCAG; Bmpr2, AATCAAGAACGGCTGTGTGCA and CATGCTGTGAAGACCCTGTTT; Acvr2a, CGAAGCCACCCTATTACAAC and ATTAGCCACAGGTCCACATC; Acvr2b, ACCCCCAGGTGTACTTCTG and CATGGCCGTAGGGAGGTTTC.
Western blot analysis and antibodies
The cells and tissues were lysed with lysis buffer containing 2% sodium dodecyl sulfate (SDS), 50 mm Tris-HCl (pH 6.8), 10 mm dithiothreitol, 10% glycerol, 0.002% bromphenol blue, phosphatase inhibitors (10 mm Na3VO4, 10 mm NaF), and protease inhibitor mixture (Roche Applied Science). After quantification, equal amounts of protein were separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Millipore Corp., Bedford, MA). After blocking in 5% bovine serum albumin or nonfat milk, the membrane was immunoblotted with primary antibodies and visualized with horseradish peroxidase-coupled secondary antibodies (Jackson ImmunoResearch). The antibodies used in current study were: antibody against BMP4 (MAB1049) was from Millipore; CYP17A1 (ab125022) was from Abcam; FLAG (F1804) was from Sigma; CYP19A1 (sc-30086) and HSP90 (sc-7947) were from Santa Cruz; p-Smad1/5/8 (#9511), p-p38 MAPK (#9216S), Smad1 (#6944), Smad4 (#9515), and p38 MAPK (#9212S) were from Cell Signaling Technology (Danvers, MA).
Generation of recombinant adenovirus
Recombinant adenovirus for BMP4 knockdown was produced through BLOCK-iT Adenoviral RNAi Expression System (Invitrogen) as previously described (35). The adenoviral expression vector pAd/BLOCK-iT encoding short hairpin RNA (shRNA) of BMP4 was constructed, with shRNA for LacZ as the control. The sequences (5′ to 3′) for shRNAs were shBMP4 (CACCGGATTACATGAGGGATCTTTACGAATAAAGATCCCTCATGTAATCC) and shLacZ (CACCGCTACACAAATCAGCGATTTCGAAAAATCGCTGATTTGTGTAG). Recombinant adenovirus was produced and amplified in 293A cells. Then the adenovirus was used to infect TCs and GCs to disrupt BMP4 expression.
RNA interference
Synthetic stealth siRNA oligonucleotides specific for AR, Smad4, and p38 MAPK mRNA were designed and synthesized by Invitrogen. Two sets of siRNA were used to disrupt expression of Smad4 and p38 MAPK. Stealth siRNA negative control (siNC) duplexes with a similar GC content were used as controls. TCs or GCs were transfected with the siRNA oligonucleotide by using Lipofectamine RNAiMAX (Invitrogen) at 50% confluence as previously described (35, 36). The sequences for siRNAs were as follows: siAR, GCAAGUGCCCAAGAUCCUUTT; siSmad4-1, CAUACACACCUAAUUUGCCUCACCA; siSmad4-2, CACCUGGAAUUGAUCUCUCAGGAUU; sip38 MAPK-1, CCUUUGAAAGCAGGGACCUUCUCAU; sip38 MAPK-2, CCAGCAACCUAGCUGUGAACGAAGA.
ChIP
TCs and GCs were treated with or without testosterone for 24 h and then subjected to ChIP assay. ChIP was conducted as previously described (35) using anti-AR antibody (Abcam; ab74272), with rabbit IgG as a negative control. Immunoprecipitated DNA was purified and quantified by qPCR, with the DNA level in the input sample as an endogenous control. The data were normalized to IgG controls in each group. The primer (5′ to 3′) for ChIP-qPCR was BMP4 promoter (AAACTCAGGGAAGCCCAGAC and GACCGATGCCTCCAGCTC).
Measurement of total testosterone and estradiol
The levels of total testosterone and estradiol in mice serum and cell culture supernatant were measured by chemiluminescence immunoassay using Beckman coulter UniCel Dxi800 immunology analyzer.
Luciferase reporter assays
The promoter of CYP17A1 and BMP4 region were cloned into the luciferase vector (Promega) as described above. In luciferase reporter assays, 293T cells were transfected in triplicate with luciferase vector and the indicated transcription factors using the transfection kit Lipofectamine 2000 (Invitrogen) following the manufacturer's instructions. At the 36 h after transfection luciferase activity was measured using the dual-luciferase reporter assay (Promega), normalizing the firefly luciferase activity against Renilla luciferase activity.
Statistics
All experiments were independently repeated at least three times, with the data presented as the means ± S.D. p values were determined by unpaired two-tailed Student's t test. Differences were considered as significant when p < 0.05.
Author contributions
Y. L. and S.-Y. D. designed and conducted the experiments, analyzed the data, and wrote the manuscript. M. D., X. D., F.-F. Z., and Z.-Y. W. participated in carrying out the experiments in vivo. S.-W. Q. participated in generation of recombinant adenovirus. M. D., Z.-Y. W., S.-W. Q., and W. Z. reviewed the manuscript and offered critical advice. Q.-Q. T. and C.-J. X. directed the project and reviewed the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Supplementary Material
Acknowledgments
We thank Ya-xin Zhao (Fudan University) for assisting with generation of BMP4-Luc reporter plasmid and Rui Sun (Fudan University) for providing AR expression vector.
This work was supported partially by National Key Basic Research Project Grants 2011CB910201 and 2013CB530601 and The State Key Program of National Natural Science Foundation 31030048C120114 and 81390350 (to Q.-Q. T.); National Natural Science Foundation of China Grant 81601251 and Project funded by China Postdoctoral Science Foundation (to Y. L.); National Key Basic Research Project Grant 2016YFC1303100 (to C.-J. X.). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains supplemental Fig. 1 and Table 1.
- PCOS
- polycystic ovary syndrome
- BMP4
- bone morphogenetic protein 4
- DHEA
- dehydroepiandrosterone
- TC
- theca cell
- LH
- luteinizing hormone
- GC
- granulosa cell
- hGC
- human GC
- Smad
- mothers against dpp
- R-Smad
- receptor-regulated Smad
- coSmad
- common partner Smad
- AR
- androgen receptor(s)
- NCoR
- nuclear receptor corepressor
- SMRT
- silencing mediator of retinoid and thyroid hormone receptor
- qPCR
- quantitative PCR
- siNC
- siRNA negative control.
References
- 1. Azziz R., Carmina E., Chen Z., Dunaif A., Laven J. S., Legro R. S., Lizneva D., Natterson-Horowtiz B., Teede H. J., and Yildiz B. O. (2016) Polycystic ovary syndrome. Nat. Rev. Dis. Primers 2, 16057. [DOI] [PubMed] [Google Scholar]
- 2. Rosenfield R. L., and Ehrmann D. A. (2016) The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of pcos as functional ovarian hyperandrogenism revisited. Endocr. Rev. 37, 467–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Palomba S., and La Sala G. B. (2016) Pregnancy complications in women with polycystic ovary syndrome: importance of diagnostic criteria or of phenotypic features? Hum. Reprod. 31, 223–224 [DOI] [PubMed] [Google Scholar]
- 4. Deplewski D., and Rosenfield R. L. (2000) Role of hormones in pilosebaceous unit development. Endocr. Rev. 21, 363–392 [DOI] [PubMed] [Google Scholar]
- 5. Lizneva D., Gavrilova-Jordan L., Walker W., and Azziz R. (2016) Androgen excess: investigations and management. Best. Pract. Res. Clin. Obstet. Gynaecol. 37, 98–118 [DOI] [PubMed] [Google Scholar]
- 6. Luque-Ramírez M., and Escobar-Morreale H. F. (2015) Targets to treat androgen excess in polycystic ovary syndrome. Expert Opin. Ther. Targets 19, 1545–1560 [DOI] [PubMed] [Google Scholar]
- 7. Miller W. L., and Auchus R. J. (2011) The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 32, 81–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Steinkampf M. P., Mendelson C. R., and Simpson E. R. (1987) Regulation by follicle-stimulating hormone of the synthesis of aromatase cytochrome P-450 in human granulosa cells. Mol. Endocrinol. 1, 465–471 [DOI] [PubMed] [Google Scholar]
- 9. Shohat-Tal A., Sen A., Barad D. H., Kushnir V., and Gleicher N. (2015) Genetics of androgen metabolism in women with infertility and hypoandrogenism. Nat. Rev. Endocrinol. 11, 429–441 [DOI] [PubMed] [Google Scholar]
- 10. Escobar-Morreale H., Pazos F., Potau N., García-Robles R., Sancho J. M., and Varela C. (1994) Ovarian suppression with triptorelin and adrenal stimulation with adrenocorticotropin in functional hyperadrogenism: role of adrenal and ovarian cytochrome P450c17 α. Fertil. Steril. 62, 521–530 [DOI] [PubMed] [Google Scholar]
- 11. Wickenheisser J. K., Nelson-DeGrave V. L., Quinn P. G., and McAllister J. M. (2004) Increased cytochrome P450 17α-hydroxylase promoter function in theca cells isolated from patients with polycystic ovary syndrome involves nuclear factor-1. Mol. Endocrinol. 18, 588–605 [DOI] [PubMed] [Google Scholar]
- 12. Katagiri T., and Watabe T. (2016) Bone morphogenetic proteins. Cold Spring Harb. Perspect. Biol. 8, a021899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu M., Chen G., and Li Y. P. (2016) TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis, and disease. Bone Res. 4, 16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bragdon B., Moseychuk O., Saldanha S., King D., Julian J., and Nohe A. (2011) Bone morphogenetic proteins: a critical review. Cell. Signal. 23, 609–620 [DOI] [PubMed] [Google Scholar]
- 15. Huang H., Song T. J., Li X., Hu L., He Q., Liu M., Lane M. D., and Tang Q. Q. (2009) BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc. Natl. Acad. Sci. U.S.A. 106, 12670–12675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tang Q. Q., and Lane M. D. (2012) Adipogenesis: from stem cell to adipocyte. Annu. Rev. Biochem. 81, 715–736 [DOI] [PubMed] [Google Scholar]
- 17. Qian S. W., Tang Y., Li X., Liu Y., Zhang Y. Y., Huang H. Y., Xue R. D., Yu H. Y., Guo L., Gao H. D., Liu Y., Sun X., Li Y. M., Jia W. P., and Tang Q. Q. (2013) BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proc. Natl. Acad. Sci. U.S.A. 110, E798–E807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moran L. J., Norman R. J., and Teede H. J. (2015) Metabolic risk in PCOS: phenotype and adiposity impact. Trends Endocrinol. Metab. 26, 136–143 [DOI] [PubMed] [Google Scholar]
- 19. Nilsson E. E., and Skinner M. K. (2003) Bone morphogenetic protein-4 acts as an ovarian follicle survival factor and promotes primordial follicle development. Biol. Reprod 69, 1265–1272 [DOI] [PubMed] [Google Scholar]
- 20. Glister C., Richards S. L., and Knight P. G. (2005) Bone morphogenetic proteins BMP-4, -6, and -7 potently suppress basal and luteinizing hormone-induced androgen production by bovine theca interna cells in primary culture: could ovarian hyperandrogenic dysfunction be caused by a defect in thecal BMP signaling? Endocrinology 146, 1883–1892 [DOI] [PubMed] [Google Scholar]
- 21. Dooley C. A., Attia G. R., Rainey W. E., Moore D. R., and Carr B. R. (2000) Bone morphogenetic protein inhibits ovarian androgen production. J. Clin. Endocrinol. Metab. 85, 3331–3337 [DOI] [PubMed] [Google Scholar]
- 22. Erickson G. F., and Shimasaki S. (2003) The spatiotemporal expression pattern of the bone morphogenetic protein family in rat ovary cell types during the estrous cycle. Reprod. Biol. Endocrinol. 1, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Davey R. A., and Grossmann M. (2016) Androgen receptor structure, function, and biology: from bench to bedside. Clin. Biochem. Rev. 37, 3–15 [PMC free article] [PubMed] [Google Scholar]
- 24. Li D., Urs A. N., Allegood J., Leon A., Merrill A. H. Jr, and Sewer M. B. (2007) Cyclic AMP-stimulated interaction between steroidogenic factor 1 and diacylglycerol kinase θ facilitates induction of CYP17. Mol. Cell. Biol. 27, 6669–6685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Groppe J., Greenwald J., Wiater E., Rodriguez-Leon J., Economides A. N., Kwiatkowski W., Affolter M., Vale W. W., Izpisua Belmonte J. C., and Choe S. (2002) Structural basis of BMP signalling inhibition by the cystine knot protein Noggin. Nature 420, 636–642 [DOI] [PubMed] [Google Scholar]
- 26. Li H., Chen Y., Yan L. Y., and Qiao J. (2013) Increased expression of P450scc and CYP17 in development of endogenous hyperandrogenism in a rat model of PCOS. Endocrine 43, 184–190 [DOI] [PubMed] [Google Scholar]
- 27. Sirmans S. M., and Pate K. A. (2013) Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin. Epidemiol. 6, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Diaz R. (2011) Reproductive endocrinology: benefits of metformin in PCOS. Nat. Rev. Endocrinol. 7, 437. [DOI] [PubMed] [Google Scholar]
- 29. Matsumoto T., Sakari M., Okada M., Yokoyama A., Takahashi S., Kouzmenko A., and Kato S. (2013) The androgen receptor in health and disease. Annu. Rev. Physiol. 75, 201–224 [DOI] [PubMed] [Google Scholar]
- 30. van de Wijngaart D. J., Dubbink H. J., van Royen M. E., Trapman J., and Jenster G. (2012) Androgen receptor coregulators: recruitment via the coactivator binding groove. Mol. Cell. Endocrinol. 352, 57–69 [DOI] [PubMed] [Google Scholar]
- 31. Perissi V., Jepsen K., Glass C. K., and Rosenfeld M. G. (2010) Deconstructing repression: evolving models of co-repressor action. Nat. Rev. Genet. 11, 109–123 [DOI] [PubMed] [Google Scholar]
- 32. Hodgson M. C., Astapova I., Cheng S., Lee L. J., Verhoeven M. C., Choi E., Balk S. P., and Hollenberg A. N. (2005) The androgen receptor recruits nuclear receptor CoRepressor (N-CoR) in the presence of mifepristone via its N and C termini revealing a novel molecular mechanism for androgen receptor antagonists. J. Biol. Chem. 280, 6511–6519 [DOI] [PubMed] [Google Scholar]
- 33. Yu C., Zhang Y. L., and Fan H. Y. (2013) Selective Smad4 knockout in ovarian preovulatory follicles results in multiple defects in ovulation. Mol. Endocrinol. 27, 966–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Glister C., Kemp C. F., and Knight P. G. (2004) Bone morphogenetic protein (BMP) ligands and receptors in bovine ovarian follicle cells: actions of BMP-4, -6, and -7 on granulosa cells and differential modulation of Smad-1 phosphorylation by follistatin. Reproduction 127, 239–254 [DOI] [PubMed] [Google Scholar]
- 35. Liu Y., Ge X., Dou X., Guo L., Liu Y., Zhou S. R., Wei X. B., Qian S. W., Huang H. Y., Xu C. J., Jia W. P., Dang Y. J., Li X., and Tang Q. Q. (2015) Protein inhibitor of activated STAT 1 (PIAS1) protects against obesity-induced insulin resistance by inhibiting inflammation cascade in adipose tissue. Diabetes 64, 4061–4074 [DOI] [PubMed] [Google Scholar]
- 36. Liu Y., Zhang Y. D., Guo L., Huang H. Y., Zhu H., Huang J. X., Liu Y., Zhou S. R., Dang Y. J., Li X., and Tang Q. Q. (2013) Protein inhibitor of activated STAT 1 (PIAS1) is identified as the SUMO E3 ligase of CCAAT/enhancer-binding protein β (C/EBPβ) during adipogenesis. Mol. Cell Biol. 33, 4606–4617 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.