Abstract
FREP1 in mosquito midguts facilitates Plasmodium falciparum parasite transmission. The fibrinogen-like (FBG) domain of FREP1 is highly conserved (>90% identical) among Anopheles species from different continents, suggesting that anti-FBG antibodies may block malaria transmission to all anopheline mosquitoes. Using standard membrane-feeding assays, anti-FREP1 polyclonal antibodies significantly blocked transmission of Plasmodium berghei and Plasmodium vivax to Anopheles gambiae and Anopheles dirus, respectively. Furthermore, in vivo studies of mice immunized with FBG achieved >75% blocking efficacy of P. berghei to A. gambiae without triggering immunopathology. Anti-FBG serum also reduced >81% of P. falciparum infection to A. gambiae. Finally, we showed that FBG interacts with Plasmodium gametocytes and ookinetes, revealing the molecular mechanism of its antibody transmission-blocking activity. Collectively, our data support that FREP1-mediated Plasmodium transmission to mosquitoes is a conserved pathway and that targeting the FBG domain of FREP1 will limit the transmission of multiple Plasmodium species to multiple Anopheles species.
Keywords: infection, ligand-binding protein, malaria, parasite, vaccine, malaria transmission–blocking vaccine
Introduction
Malaria death rates have dropped by 47% between 2000 and 2013 globally and by 54% in Africa because of application of several anti-malaria strategies, including anti-malaria drugs, insecticide-treated nets, and indoor insecticide spraying. Despite these efforts, more than 587,000 people still died, and 90% of these deaths occurred in sub-Saharan Africa in 2014 (1). The rapid spread of drug-resistant malaria parasites and insecticide-resistant mosquitoes along with the absence of efficient vaccines against malaria present major challenges for malaria control. Therefore, new approaches are urgently needed. Transmission-blocking vaccines (TBVs)3 have been considered as promising measure to combat malaria. TBVs are designed to block parasite development in the mosquito midgut upon ingestion with the human antibodies against antigens from either parasites or mosquitoes.
Human malaria is caused by Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, Plasmodium malariae, and Plasmodium knowlesi, of which P. falciparum and P. vivax are responsible for ∼99% of malaria cases. Because only gametocytes can infect mosquitoes, antigens on the surface of gametocytes and/or ookinetes, such as Pfs25, Pfs48/45, and Pfs230, have been evaluated as TBV candidates in preclinical studies (2–4). Among them, Pfs25 and its ortholog Pvs25 from P. vivax are the only candidates to progress to clinical trials. Pfs25 is a 25-kDa sexual stage–specific protein expressed on the surface of the parasite during several sexual developmental stages, including gamete, zygote, and ookinete (5). Clinical trials of Pfs25 only showed moderate levels of transmission-blocking activity (6), underscoring the need to identify additional and novel antigens for TBV development.
About 30 anopheline mosquito species transmit malaria (7). The major malaria vectors in Africa are Anopheles gambiae, Anopheles arabiensis, and Anopheles funestus. In Asia, the most important species are Anopheles stephensi and Anopheles dirus. In South America, Anopheles minimus, Anopheles albimanus, and Anopheles darlingi are responsible for malaria transmission (2, 8, 9). To successfully transmit malaria, Plasmodium parasites must complete a complex developmental cycle in both human and mosquito hosts. Thus, mosquito midgut molecules that facilitate ookinete invasion are likely to serve as ideal targets for TBVs. Previous studies showed that polyclonal antibodies against mosquito alanyl aminopeptidase 1 or carboxypeptidases B (2, 10) inhibited 73% and 51% parasite development in mosquito midguts, respectively, using the P. berghei mouse infection system.
Because human malaria is caused by several Plasmodium species and transmitted by numerous Anopheles species, and many endemic areas have both P. falciparum and P. vivax malaria cases transmitted by several different Anopheles species, an ideal TBV antigen would effectively block malaria transmission of multiple parasite species to multiple mosquito species. We recently reported that FREP1 plays a pivotal role in ookinete invasion of the mosquito midgut (11). FREP1 is a tetramer that localizes within the peritrophic matrix and facilitates Plasmodium invasion through direct binding to gametocytes and ookinetes. In this study, we demonstrate that a highly conserved FBG domain within FREP1 is a broad-spectrum TBV antigen that blocks transmission of multiple Plasmodium species to multiple Anopheles species, which supports FREP1-mediated Plasmodium invasion to mosquitoes as a conserved pathway. In particular, an in vivo mouse model demonstrates FBG as a vaccine that blocks >75% transmission of P. berghei, better than reported mosquito TBV antigens (aminopeptidase 1 and carboxypeptidases B) (2, 10). It is worth noting that only three mosquito proteins have been identified to be suitable for malaria TBV antigens. Membrane feeding assays showed that anti-FBG serum blocked >81% transmission of P. falciparum, which meets the PATH guideline for malaria TBVs for clinical trials (>80%).4
Results
The FREP1 fibrinogen-like domain is highly conserved in anopheline mosquitoes
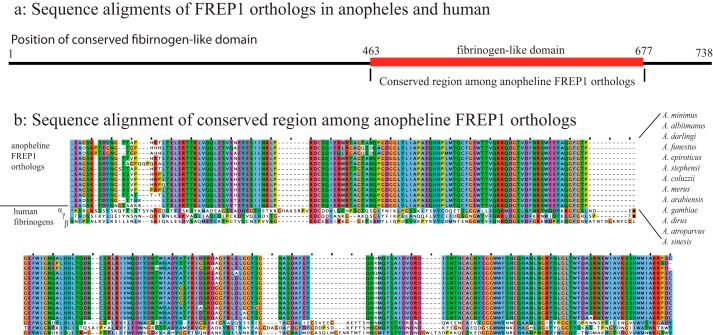
We examined the FREP1 orthologs among anopheline mosquitoes to find conserved regions. We obtained 13 orthologs from major malaria vectors in Africa (A. gambiae, A. funestus, A. arabiensis, Anopheles coluzzii, Anopheles merus, and A. albimanus), South America (A. darlingi), Asia (Anopheles sinesis, A. stephensi, A. minimus, and Anopheles epiroticus), and Europe (Anopheles atroparvus). The results from multisequence alignment revealed a highly conserved region between amino acids 463 and 677 of A. gambiae FREP1 (Fig. 1a). Detailed analyses of this conserved region found that more than 90% of the protein sequences are identical among all 13 anopheline species (Fig. 1b), suggesting that antibodies raised against this domain might be able to block the transmission of multiple Plasmodium species to multiple Anopheles mosquitoes. Because this conserved region is a FBG domain, we also compared FREP1 with human fibrinogens α, β, and γ chains. Multiple sequence alignment found less than 10% identical sequences between the mosquito conserved FBG domain and human fibrinogens, supporting that vaccination with recombinant mosquito FREP1 or the FBG domain protein would be unlikely to trigger autoimmune reactions.
Figure 1.
Multiple sequence alignment of FREP1 from A. gambiae and other major malaria vectors. a, overview of sequence alignments of FREP1 orthologs in Anopheles mosquitoes and human fibrinogens. b, detailed alignment of conserved FBG domains. Dots and dashes are insertions or deletions, respectively. The ClustalX color scheme is used to depict letters when the amino acid profile of the alignment at that position meets criteria and residue types to highlight the conservations.
Rabbit anti-FREP1 antibodies inhibit malaria transmission in P. berghei and P. vivax in A. gambiae and A. dirus, respectively
Previously, we reported that anti-FREP1 rabbit polyclonal antibodies effectively blocked P. falciparum in a major malaria vector, A. gambiae (11). Because there is a highly conserved FBG domain among anopheline orthologs, we determined whether anti-FREP1 antibody would also inhibit transmission of other Plasmodium species to additional Anopheles species. To address this question, A. gambiae mosquitoes were fed with P. berghei–infected blood mixed with rabbit anti-FREP1 serum (1:1 dilution, final titer units, 5 × 104), and we subsequently examined the number of developing oocysts in mosquito midguts. Rabbit preimmune serum was used as a negative control. The results showed that anti-FREP1 serum significantly reduced the number of P. berghei oocysts compared with the control group, in which we substituted anti-FREP1 serum with preimmune serum (Fig. 2a). The average number of P. berghei oocysts per midgut significantly decreased from 10 in the control group to three in the experimental group (p < 0.0001). The results were consistent in two biological replicates, which were conducted with different P. berghei–infected mouse blood.
Figure 2.
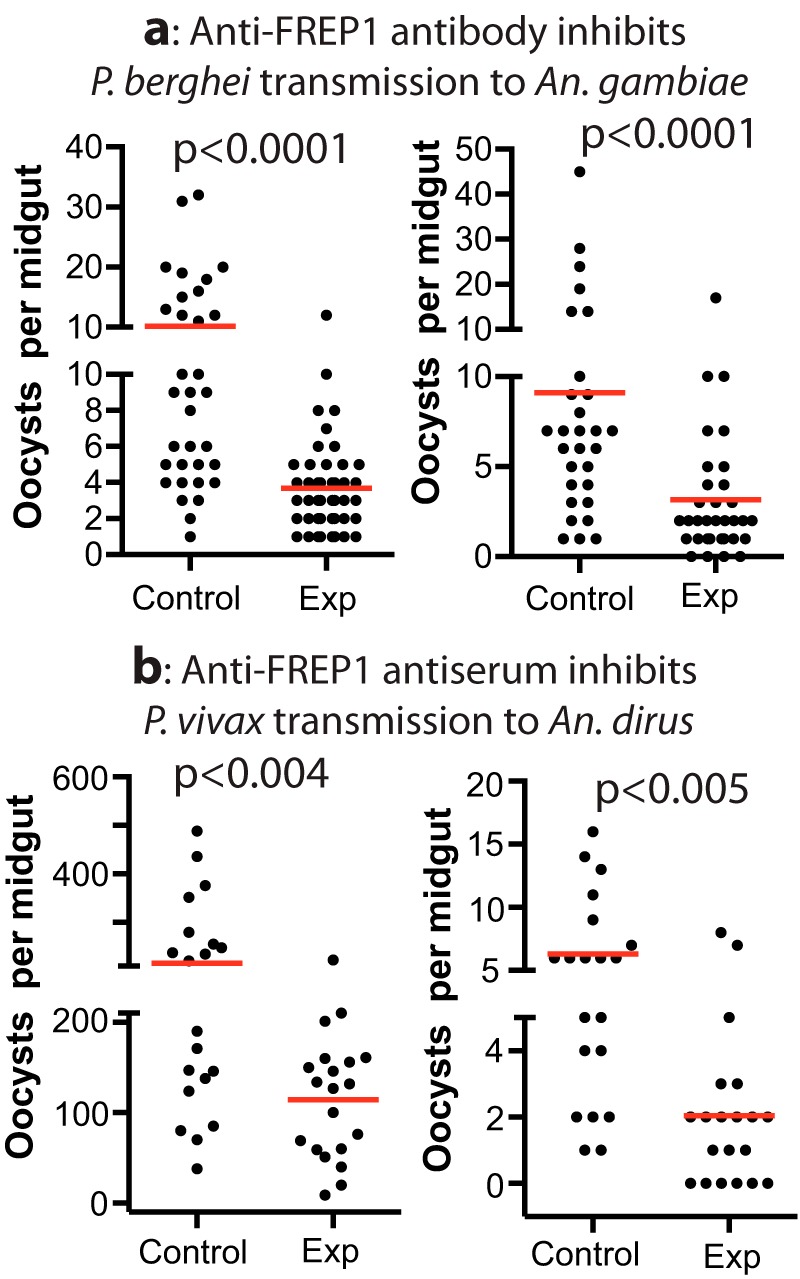
Rabbit anti-FREP1 antiserum inhibits P. berghei (a) and P. vivax (b) infection of A. gambiae and A. dirus mosquitoes, respectively. Control, preimmune rabbit serum was used; experiment (Exp), anti-FREP1 rabbit serum was used. The p values were calculated using Mann-Whitney-Wilcoxon test.
Next we tested whether the anti-FREP1 antibody could inhibit the transmission of another major human malaria pathogen, P. vivax, to another major malaria vector in Asia, A. dirus. P. vivax–infected blood (two field isolates) was mixed with rabbit anti-FREP1 serum (1:1 dilution, final titer units, 5 × 104) and fed to A. dirus. The results showed that the anti-FREP1 antibody significantly reduced the number of oocysts per midgut, more than 2-fold, compared with the control serum (Fig. 2b). Statistical analysis showed that the inhibitory effect of anti-FREP1 antibodies against P. vivax infection in A. dirus is significant (p < 0.005). The results were consistent in two biological replicates that were conducted with different P. vivax–infected human blood. Together, these data support that anti-FREP1 antibodies can block the transmission of multiple species of malaria parasites to multiple mosquito species.
Experimental immunization of mice with FREP1 does not trigger toxicity or elicit antibodies that cross-react with human fibrinogen
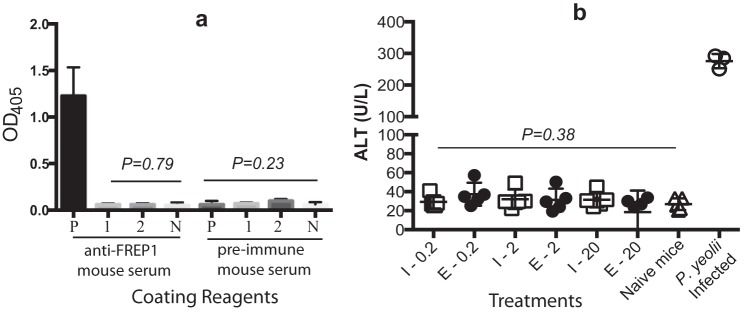
Our sequence alignment displayed a minor degree of homology between mosquito FREP1 and mammalian fibrinogens. Despite this, we also investigated whether immunization of mice with FREP1 causes any autoimmune response. We expressed the recombinant FREP1 protein in Escherichia coli and insect cells and purified the recombinant proteins using nickel-nitrilotriacetic acid affinity columns (11). For both E. coli– and insect cell–expressed recombinant FREP1, mice were immunized with a series of FREP1 doses (0.2, 2, or 20 μg) and boosted with the same dose twice at 3-week intervals. Preimmune human plasma–coated ELISA assays were performed to assess the cross-reactivity between anti-FREP1 polyclonal antibodies raised in mice and human blood plasma (human fibrinogens). Wells coated with bovine serum albumin or insect cell–expressed recombinant FREP1 were used as negative and positive controls, respectively. The results showed no cross-specific recognition between the anti-FREP1 antibodies generated by E. coli– or insect cell–expressed FREP1 to human plasma fibrinogen (Fig. 3a).
Figure 3.
FREP1 immunizations do not trigger autoimmune reactions against mammalian or human fibrinogens and are nontoxic to mice. a, mouse anti-FREP1 antiserum does not cross-react with human fibrinogens, and no autoimmunity was induced. P, coated with insect cell–expressed recombinant FREP1; 1, coated with human plasma; 2, coated with 10-fold diluted human plasma; N, wells coated with BSA. b, serum ALT activity does not change significantly between FREP1-immunized mice and control mice. I-, insect cell–expressed recombinant FREP1; E-, E. coli–expressed recombinant FREP1. The p values were calculated with one-way analysis of variance.
Complementarily, we examined whether immunization with recombinant FREP1 triggered toxicity or caused inflammatory immunopathology in mammals. Alanine aminotransferase (ALT) is found mainly in the liver, and the liver is very sensitive to inflammation and toxins. Therefore, the activities of ALT in immunized mice were examined. The positive control was the blood from P. yoelii–infected mice, which exhibited high levels of ALT, characteristic of inflammation and liver damage (Fig. 3b). Notably, the levels of ALT activity in mice injected with E. coli– or insect cell–expressed FREP1 protein were similar to the baseline activity observed in naïve mouse serum (p > 0.38), supporting that immunization of FREP1 does not cause inflammatory autoimmune or toxicity responses.
Immunizing mice with FBG inhibits the transmission of P. berghei to mosquitoes in vivo using direct feeding on mice
Although anti-FREP1 antibodies block the transmission of multiple Plasmodium species to multiple Anopheles species, only FBG domains are conserved among anopheline mosquitoes. Therefore, we next determined whether the highly conserved FREP1 FBG domain alone was an effective TBV antigen in vivo and in vitro. We cloned the FREP1 FBG domain (from 463 to 729 amino acids) and expressed it in E. coli. Mice were immunized with purified FBG protein mixed with Alhydrogel adjuvant using an optimal prime-boost regimen. High-titer antibody levels (3 × 106 on average) against FBG were achieved in antiserum. Mice in the control group were immunized with buffer mixed with Alhydrogel under the same regimen. Ten days after the second boost, mice were infected with P. berghei. Seven days after infection, the parasitemia was about 10% in mice. To induce maturation of gametocytes, phenylhydrazine hydrochloride in 0.9% NaCl was injected into mice intraperitoneally (60 μg/g body weight). Two days later, mice at a similar level of infection (parasitemia, 10–12%; gametocytemia, 1–1.5%) were used to infect A. gambiae directly. Results showed that A. gambiae mosquitoes fed on FBG-immunized mice had 4.2 and 3.5 oocysts per midgut on average in two experiments, which was significantly (p < 0.0004) fewer than mosquitoes fed on mock-immunized mice that had 16.7 and 19.8 oocysts on average in two experiments (Fig. 4a). Anti-FBG antibodies reduced the number of oocysts by 75% and 82% in these two independent replicates. We collected the mouse sera and measured titer units of anti-FBG antibodies by ELISA end point titer assays.
Figure 4.
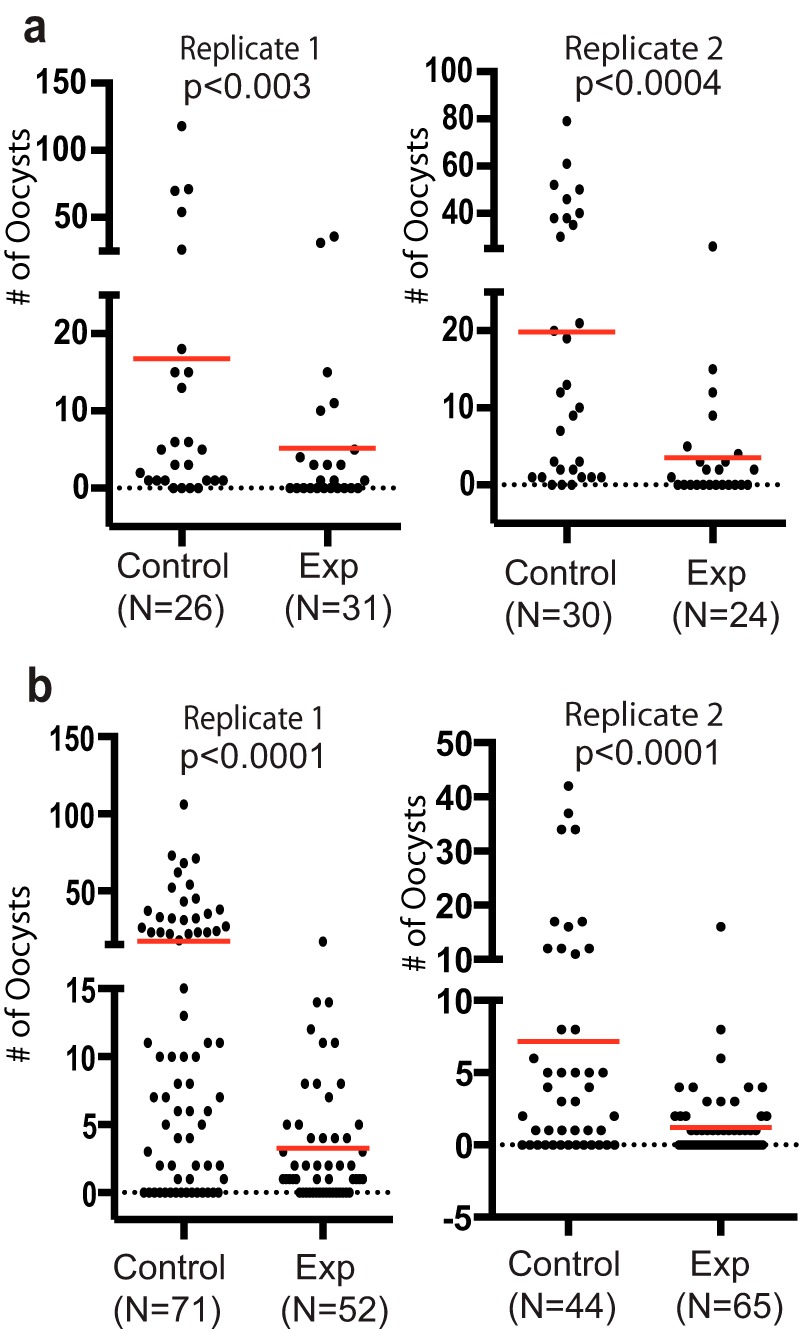
Immunization of mice with purified FBG formulated with Alhydrogel inhibits P. berghei transmission to A. gambiae in in vivo studies and P. falciparum transmission to A. gambiae in vitro with the SMFA. a, results of direct feeding assays using two FBG-immune mice and two control mice show the transmission-blocking activity of the in vivo generated anti-FBG antibody response against P. berghei to A. gambiae mosquitoes. Exp, experiment. b, serum collected from FBG-immune mice also blocks P. falciparum (NF54 strain) infection to A. gambiae mosquitoes. Control or anti-FREP1–immunized mouse sera were mixed with human serum (1:4 ratio) and mixed with P. falciparum–infected packed human blood prior to its use in the SMFA. The final anti-FBG antibody titer was 4 × 105. The p values were calculated using Mann-Whitney-Wilcoxon test.
Anti-FBG antiserum inhibits the transmission of P. falciparum to mosquitoes in membrane-feeding assays
To test whether mouse anti-FBG serum also exhibits transmission-blocking activity against P. falciparum, we added immune serum from immunized mice to P. falciparum-infected blood containing gametocytes and fed to mosquitoes using an SMFA. The average number of oocyst per midgut was reduced from 17.4 and 7.2 in control groups to 3.3 and 1.2 in experimental groups, respectively, in two replicates (Fig. 4b). The anti-FBG serum significantly (p < 0.0001) reduced the number of oocysts of P. falciparum by more than 81%. These in vivo and in vitro experiments demonstrate that the conserved FBG alone is a potent universal TBV antigen.
FBG binds sexual-stage ookinetes
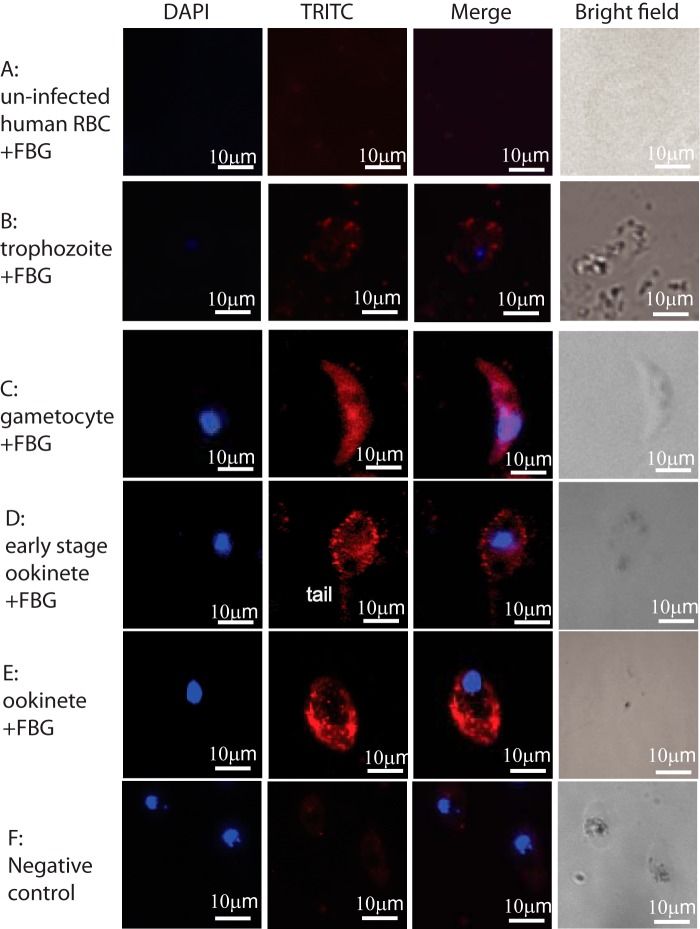
We determined the molecular relationship between FBG and parasites. We showed previously that FREP1 binds to P. falciparum (NF54) sexual-stage gametocytes and ookinetes (11). In this study, we examined the capacity for the purified FBG domain of FREP1 to bind P. falciparum parasites, gametocytes, and ookinetes in particular. Non-infected human RBCs, P. falciparum gametocytes, and ookinetes were fixed on coverslips. After incubation with insect cell-expressed FBG recombinant protein, we detected parasite-bound FBG protein by indirect immunofluorescence assays. The results showed that FREP1 FBG bound gametocytes (Fig. 5, row C), early-stage ookinetes (just merged with a sharp tail; Fig. 5, row D), and ookinetes (Fig. 5, row E). The binding signals were not observed when non-infected human RBCs were assayed (Fig. 5, row A), confirming that FBG does not bind healthy human RBCs. The red fluorescence intensity for asexual-stage parasites was lower (Fig. 5, row B) than that for gametocytes and ookinetes. Sexual-stage parasites and gametocytes are within red blood cells; however, the fluorescence signals depicted gametocytes well (Fig. 5, row C). This indicates that FBG binds to parasites directly. Because gametocytes stretch cells, parasites, particularly gametocytes inside cells, might change red blood cell permeability or make infected cells more fragile to 4% paraformaldehyde treatment than uninfected cells. Additional negative controls included incubation of P. falciparum gametocytes with an irrelevant protein (chloramphenicol acetyltransferase, CAT) expressed in the same system. As shown in Fig. 5, row E, there were no binding signals in control groups. Therefore, the FBG fragment is a functional domain of FREP1 that is responsible for the interaction between FREP1 protein and Plasmodium parasites.
Figure 5.
Insect cell–expressed recombinant FREP1 FBG protein interacts with P. falciparum detected by indirect IFAs. The first and second columns depict cell nuclei stained with DAPI and binding FBG proteins, respectively. Merging the first and second columns generated the third column, showing the co-localization of P. falciparum (nuclei) and FBG protein binding. The fourth column shows bright views of the cells. Row A, FBG does not bind to uninfected RBCs. Row B, the interaction between FBG and asexual P. falciparum is weak. Row C, FBG binds to P. falciparum gametocytes. Rows D and E, FBG binds to P. falciparum ookinetes. Row F, an irrelevant control protein (CAT) does not bind P. falciparum parasites. TRITC, tetramethylrhodamine isothiocyanate.
FBG binds the mosquito midgut peritrophic matrix (PM)
Previously, we showed that FREP1 bound the PM (11). Here we determined which domain (FBG or N-FREP1) is responsible for this interaction. Because PM forms only after a blood meal, blood-fed mosquito midguts and naïve mosquito midguts were incubated with BSA, recombinant FREP1, N-FREP1, and FBG separately. Ten midguts were used in each treatment. Three independent experimental replicates were performed. BSA and FREP1 were negative and positive controls, respectively. Because naïve mosquito midguts do not contain a PM, they were used as negative controls as well. After incubation, midguts and their binding proteins were homogenized, and extracts were used to coat an ELISA plate. The bound recombinant FREP1, N-FREP1, and FBG were probed with anti-His monoclonal antibody. After development with ELISA reagents, FREP1 and FBG incubated with blood-fed mosquito midguts exhibited a significantly (p < 0.005) higher signal than BSA-incubated midguts. There was no significant (p > 0.4) difference between N-FREP1–incubated midguts and negative controls (Fig. 6). These results support FBG binding to the PM.
Figure 6.
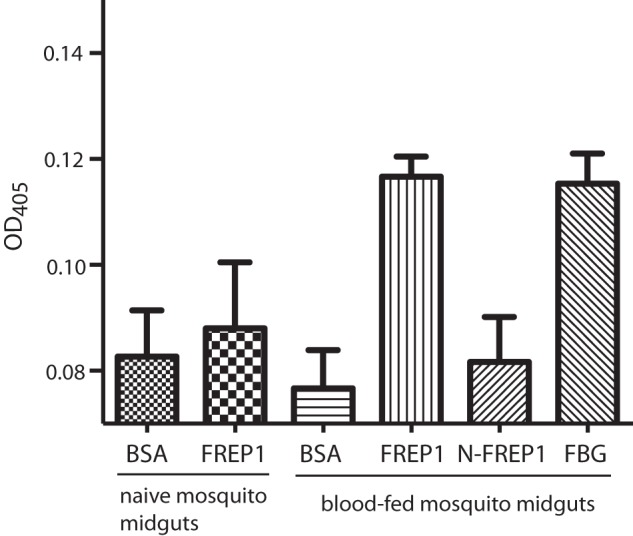
FBG binds to the mosquito PM. Mosquito midguts were obtained from naïve mosquitoes that do not have a PM (as a negative control) and from 18-h post-blood-fed mosquitoes that contain a PM. BSA (negative control) and recombinant proteins of FREP1 (positive control), N-FREP1, and FBG were incubated with midguts. After removing the supernatant, the pellets were extracted, and the remaining recombinant proteins were developed with anti-His monoclonal antibodies.
Anti-FBG antibodies prevent FREP1 from binding to Plasmodium parasites
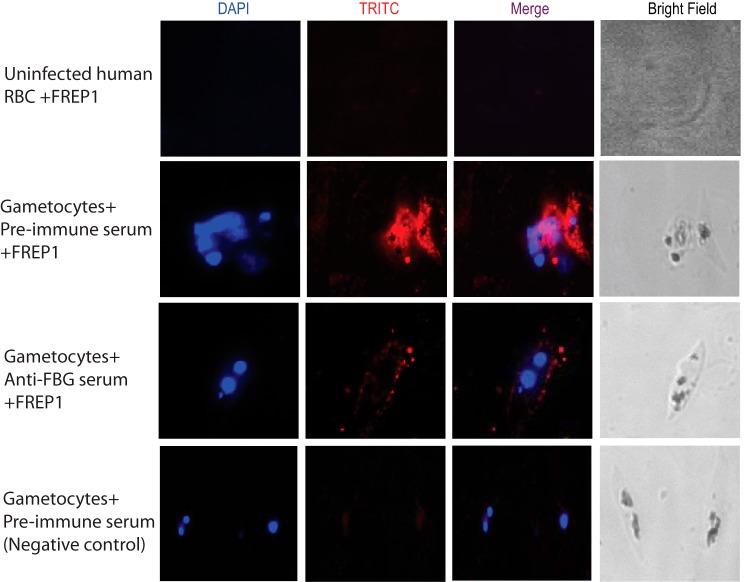
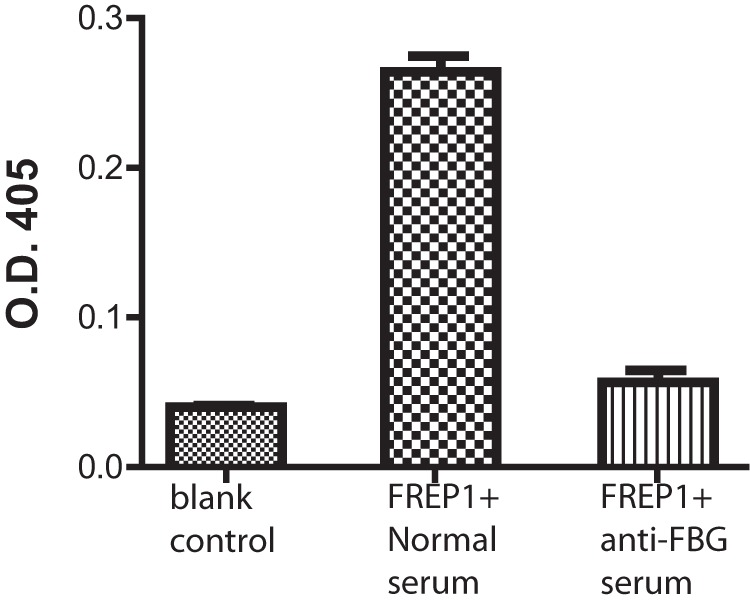
Because anti-FBG antiserum blocks Plasmodium transmission and the FBG domain of FREP1 binds Plasmodium parasites, anti-FBG antiserum should inhibit the interaction between FREP1 and parasites. We used an ELISA and IFA to test this hypothesis. For the ELISA, we coated a plate with the lysate of sexual-stage parasites. The purified FREP1 and anti-FBG serum were mixed and added to coated wells. The normal mouse serum replacing anti-FBG antiserum was used as a negative control (no blocking activity). Incubation of the coated plate without FREP1 was used as a positive control (completely blocked). The parasite-bound FREP1 was quantified by antibodies against N-FREP1 protein. The results indicated that anti-FBG antiserum significantly (p < 0.01 by t test) reduced binding signals between FREP1 and parasites (Fig. 7). To confirm that anti-FBG antiserum blocks the interaction between FREP1 and sexual-stage parasites, IFA assays were conducted. As shown in Fig. 8, P. falciparum gametocytes and ookinetes incubated with FREP1 mixed with anti-FBG serum had much weaker signals than those mixed with preimmune mouse serum.
Figure 7.
Anti-FBG antibodies block the interaction between the mosquito peritrophic membrane and recombinant FREP1 protein. 18 h after the blood meal, mosquito midguts were incubated separately with BSA (blank control), recombinant FREP1 premixed with preimmune mouse serum, and recombinant FREP1 premixed with anti-FBG mouse serum. After removing the supernatant, the midgut-bound recombinant FREP1 was quantified by antibodies against N-FREP1 protein using ELISA.
Figure 8.
Anti-FBG antibody prevents FREP1 from binding to parasites. Stage V gametocytes were deposited onto glass coverslips and incubated with FREP1 mixed with preimmune mouse serum (second row) or anti-FBG mouse serum (third row). The bound recombinant FREP1 protein was quantified by anti-His monoclonal antibody. The first row shows that uninfected human red blood cells do not bind FREP1. The same experiments as in the second row without FREP1 showed no red signals (fourth row). TRITC, tetramethylrhodamine isothiocyanate.
Discussion
Malaria TBVs are novel approaches for malaria control. However, there are few efficacious antigen targets that have been described. We identified a novel Plasmodium invasion pathway, e.g. FREP1-mediated Plasmodium invasion through direct binding to P. falciparum gametocytes and ookinetes (11). Because FREP1 localizes in the mosquito midgut peritrophic matrix, it is readily accessible to antibodies in co-ingested blood. Therefore, mosquito FREP1 is an ideal target for a TBV.
One of the scientific hurdles in developing TBVs is that malaria is caused by multiple species of parasites transmitted by multiple species of mosquito vectors. Identification of an antigen with the potential to limit the transmission of multiple major parasite species by multiple major vectors is critical for developing a successful TBV. Previously, we demonstrated that the anti-FREP1 antibody effectively inhibited the infection of A. gambiae, a major malaria vector in Africa, by P. falciparum, a major human malaria pathogen (11). Here we show that the anti-FREP1 antibody also significantly reduced the transmission of P. vivax, another important human malaria pathogen, by A. dirus, another major mosquito malaria vector in Asia. Moreover, our data show that anti-FREP1 antibodies also inhibit transmission of P. berghei to mosquitoes. P. berghei is a distantly related parasite causing rodent malaria. Together, we propose that FREP1 is an excellent candidate as a universal TBV antigen.
We examined the orthologs of FREP1 in anopheline mosquitoes. The length of FREP1 orthologs is various, from 311 amino acids in A. dirus to 738 amino acids in A. gambiae. However, they share a highly conserved FBG domain that is about 210 amino acids in length. Notably, antibodies against the FREP1 FBG domain effectively blocked the transmission of P. berghei and P. falciparum to mosquitoes. Because the E. coli–expressed FREP1 FBG domain used for immunization was purified under a denaturing condition, and the transmission-blocking activity of anti-FBG antibody was functional, a fully folded FBG is not required as a vaccine antigen. This is an advantage for this highly conserved mosquito midgut antigen (3, 13, 14). We discovered that the FBG domain in FREP1 binds Plasmodium gametocytes and ookinetes. Anti-FBG serum prevents parasites from binding to FREP1, which explains the molecular mechanisms of FREP1 FBG as a universal TBV antigen. Contrary to the FBG domain, the N-FREP1 did not bind to parasites. Because FREP1 localized in the mosquito midgut PM, our data support our prediction (11) that the FBG domain interacts with the PM as well.
We also investigated the immunogenicity, toxicity, and autoimmunity following FREP1 and FBG immunization of mice. Immunization with either E. coli– or insect cell–expressed FREP1 proteins elicited high titer units of antibodies, supporting that recombinant FREP1 and FBG is highly immunogenic. High-titer unit functional antibodies are required for TBVs. The principal mode of action of this approach depends on high levels of antibodies circulating in the blood of the human host at the time when the malaria mosquito vector takes a bite for the antibodies to prevent parasites from invading mosquito guts. Importantly, FBG immunization of mice in a clinically relevant adjuvant (Alhydrogel) did not induce autoimmune reactions or immunopathology or elicit cross-reactive antibodies against endogenous or human fibrinogens.
Our data collectively demonstrate that the FREP1-mediated Plasmodium invasion pathway is highly conserved in Plasmodium parasites and Anopheles mosquitoes. Indeed, a mixture of vaccine antigens that includes both FBG and the parasite-expressed FREP1 binding partners would be predicted to synergistically increase transmission-blocking efficacy and potentially enable the vaccines to completely inhibit malaria transmission.
Experimental procedures
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Mice were used according to approved protocols (R15-012) by the University of Oklahoma Institutional Animal Care and Use Committee (A3240-01). P. vivax–infected patient blood was used to examine the efficacy of antibodies. We followed the National Institutes of Health Human Subjects Policies and Guidance. The Ethical Review Committee of the Faculty of Tropical Medicine of Mahidol University approved the P. vivax infection protocols (MUTM 2011-040-05).
Mosquito and P. falciparum maintenance
The A. gambiae G3 strain and A. dirus were reared in an insectary room maintained at 27 °C, 80% humidity with a 12-h day/night cycle. Larvae were fed with ground koi fish food (0.1 mg/larva/day). Adult mosquitoes were maintained on 8% (w/v) sucrose and fed with mouse blood for egg production. P. falciparum parasites (NF54 strain from MR4, Manassas, VA) were maintained in RPMI 1640 medium (Life Technologies) supplemented with 10% heat-inactivated (56 °C for 45 min) human AB+ serum (Interstate Blood Bank, Memphis, TN), 12.5 μg/ml hypoxanthine, and 4% hematocrit (O+ human blood) in a candle jar at 37 °C as described previously (11).
In vitro transmission-blocking assay of P. berghei infection in A. gambiae with anti-FREP1 antibodies
Mouse blood infected with P. berghei (ANKA GFPcon strain) was precipitated by centrifugation (2000 × g for 3 min). The blood pellet was then mixed with an equal volume of antiserum. The SMFA (15) was conducted using 3-day-old female naïve mosquitoes. After feeding for 20 min, the engorged mosquitoes were maintained on 8% sugar (w/v) at 19 °C. Seven days after infection, mosquitoes were dissected, and the midguts were stained with 0.2% mercurochrome and examined using light microscopy to count the number of oocysts. Data were analyzed with nonparametric Wilcoxon test implemented in R-project software.
In vitro transmission-blocking assay of P. vivax infection in A. dirus mosquitoes with anti-FREP1 antibodies
Field isolates of P. vivax were collected from patients attending malaria clinics in Ubonratchanthani province, Thailand. Within 10 h after collecting blood, 350-μl aliquots of infected blood were prepared. The infected blood was centrifuged at 1500 × g for 5 min, and plasma was removed. Packed blood was washed once with RPMI 1640 incomplete medium. The antiserum was mixed with P. vivax–infected packed blood at 1:1 ratio (v/v). The suspension was incubated at room temperature for 15 min before being fed to 100 female A. dirus (age, 5–7 days) per treatment for 30 min using membrane feeding device. The packed infected blood mixed with naïve human AB serum at a 1:1 ratio (v/v) was used as a control. Engorged mosquitoes were kept on 10% sugar solution. The number of oocysts in mosquito midguts was determined under a microscope on day 7 after the blood meal.
Gene cloning, protein expression, and purification
The full-length coding sequence of A. gambiae FREP1 was amplified as described previously (11). The FREP1 FBG fragment was amplified with the gene-specific primers 5′-ACGCATGCCACGAGTACGAGTCGATC-3′, 5′-TGCAAGCTTGTACTCGTGCTCGACCGACTC-3′, and the PCR fragment was cloned into the pQE30 vector and expressed in E. coli strain M15[pREP4] (Qiagen, Valencia, CA) with 0.5 mm isopropyl 1-thio-β-d-galactopyranoside at room temperature. The recombinant protein was purified on a nickel-nitrilotriacetic acid column (Qiagen) and eluted with buffer D (20 mm sodium phosphate, 500 mm NaCl, and 8 m urea (pH 4.0)). The eluted fractions were analyzed on 12% SDS-PAGE gel, followed by Coomassie Blue staining to examine the purity. All fractions containing protein were then combined, and protein concentration was determined using the Bradford protein assay (16).
Expression of recombinant FREP1 protein in insect High Five cells
As described previously (11), the full-length FREP1 was cloned into plasmid pIB/V5-His (Life Technologies). After being amplified in E. coli DH5α, plasmids were purified using GenElute endotoxin-free plasmid preparation kits (Sigma-Aldrich, St. Louis, MO). The purified recombinant plasmids pIB-FREP1 and pIB-CAT (as a control) were transformed into 40–60%-confluent cabbage looper ovarian cell-derived High Five cells. Cellfectin® reagent (Invitrogen) was mixed with each individual plasmid (1 μl of Cellfectin/1 μg of plasmid) in 5–6 ml of Express Five® SFM medium (Invitrogen) to transfect into cells. After five generations of dilution, the cells that stably expressed the recombinant protein were cultured in 25-cm2 CELLSTAR® cell culture flasks (Greiner Bio-One, Monroe, NC) for 48 h at 27 °C. The secreted FREP1 protein in medium was purified using a nickel-nitrilotriacetic acid column and dissolved in PBS for use.
Immunization of mice with FREP1 and FBG
Five Hsd:ND4 female (20–25 g, 7 to 8 weeks old) mice were primed via subcutaneous (s.c.) injection and boosted twice at 3-week intervals via s.c. injection with 20 μg of FREP1 per mouse in 200 μl of PBS adsorbed (1:1) in Alhydrogel adjuvant. The control mice were primed or boosted with PBS only and adsorbed (1:1) with Alhydrogel. Similarly, five mice were injected s.c. with FBG protein under the same regime for optimal-prime boosting with 20 μg of the purified recombinant protein in 0.5× buffer D, with the control group comprised of five mice injected with 0.5× buffer D adsorbed in Alhydrogel instead. Serum was collected from each mouse prior to each priming and boosting immunization. Animals were sacrificed following in vivo feeding assays, and blood was collected via the orbital sinus (17). Blood samples were centrifuged at 2000 × g for 20 min at 4 °C for serum separation.
In vivo transmission-blocking assay with immunized mice
Mice were immunized with the same regime for optimal-prime boosting as described above. Ten days after the second boost, naïve and optimally prime-boosted Hsd:ND4 mice were infected via the intraperitoneal route with P. berghei–infected blood. When parasitemia reached <6%, mice were injected with phenylhydrazine hydrochloride (60 mg/kg) (Santa Cruz Biotechnology, Dallas, Texas) to stimulate gametocytemia. Mice harboring circulating gametocytes (confirmed by Giemsa staining) were anesthetized and used to feed 100 3-day-old A. gambiae female mosquitoes for 20 min. After feeding, unengorged mosquitoes were removed, and engorged mosquitoes were transferred to a cage and kept at 19 °C and 8% (w/v) sucrose. Seven days after infection, mosquitoes were dissected, and the midguts were stained with 0.2% mercurochrome and examined using light microscopy to count the number of oocysts. Two independent experiments were performed for each treatment.
Determining antibody titer units with ELISAs
Mouse sera were collected and assayed for the presence of FREP1-specific IgG antibodies using ELISA. In brief, recombinant affinity-purified FREP1 was diluted to 0.5 μg/ml in 0.1 m sodium phosphate (Na2HPO4, pH 9.0) binding buffer and used to coat 96-well plate MediSorpTM plates (Nunc, Denmark) overnight at 4 °C. Wells were washed three times with 0.2% Tween 20 in PBS (PBST) and blocked with 2.5% BSA and 1% normal goat serum in PBS for 2 h at room temperature. Serum samples were prepared by 1:5 serial dilutions starting at 1:100 to 1:8 × 106, and 50 μl was added into each well and incubated at room temperature for 1 h. Bound IgG antibodies were detected using alkaline phosphatase (AP)-conjugated goat anti-mouse IgG (Sigma-Aldrich) at 1:800 in blocking solution for 2 h at room temperature and visualized using p-nitrophenyl phosphate (Sigma-Aldrich) as the substrate. Absorbance at 450 nm was measured using an Epoch microplate spectrophotometer (Biotek, Winooski, VT). Antibody titer unit was expressed as end point titer unit, the highest dilution of serum that gives a reading above the cutoff (2× S.D. of the signal generated from preimmune serum).
Cross-reactivity of mouse FREP1 serum to human plasma fibrinogen
Human blood (AB+) was purchased from the Oklahoma Blood Institute. Whole blood collected from donors was transferred into Vacutainer® blood collection tubes (BD Biosciences) coated with ethylenediaminetetraacidic acid dipotassium salt dihydrate (K2EDTA) to prevent coagulation. Blood samples were centrifuged at 3000 × g for 5 min at 4 °C to separate the plasma. 50 μl of human plasma samples without dilution (one sample) or diluted 10-fold in PBS (two samples) were coated overnight at 4 °C in a 96-well plate (Brand, Wertheim, Germany). 50 μl of insect cell–expressed recombinant FREP1 (0.5 μg/ml) and BSA (10 mg/ml) were coated as positive and negative controls, respectively. Samples were diluted in 0.1 m Na2HPO4 (pH 9.0). Each well was then incubated with the following solutions at room temperature: 150 μl of blocking buffer (2.5% BSA and 1% normal goat serum in PBS) for 2 h; 50 μl of anti-FREP1 mouse serum (1:1000), control mouse serum (1:1000), and anti-FREP1 rabbit serum (1:2000) diluted in blocking buffer for 1 h; and 50 μl of alkaline phosphatase–conjugated goat anti-mouse IgG (1:800) and goat anti-rabbit IgG (1:20,000) for 1.5 h. Wells were washed for 5 min with PBST three times between incubations. At the end, the samples were developed with 50 μl of p-nitrophenyl phosphate solution (Sigma-Aldrich). There were three replicates for each sample.
Immunofluorescence assays (IFAs)
Immunofluorescence assays were performed as described previously (11). In brief, P. falciparum cultures containing cells and parasites were smeared on coverslips (Fisher Scientific, Waltham, MA) and fixed in 4% paraformaldehyde in PBS at room temperature for 30 min. After quenching with 0.1 m glycine in PBS, coverslips were blocked overnight at 4 °C in 2.5% BSA and 1% normal goat serum in PBS (blocking solution). Cells were incubated for 2 h at room temperature with High Five cell–expressed FBG (100 μg/ml) in blocking solution. Sequentially, cells were incubated for 1 h with mouse monoclonal anti-His antibody (1:1000) and goat anti-mouse (1:1000) secondary antibody (Alexa Fluor® 555, Life Technologies) for 45 min, both diluted in blocking solution. Cells were washed between each step three times for 5 min in PBST. Cells in the control group were incubated with High Five–expressed 100 μg/ml CAT. Coverslips were mounted on glass slides using 20 μl of Vectashield mounting medium (Vector Laboratories, Burlingame, CA) and visualized using a Nikon Eclipse Ti fluorescence microscope.
Alignment of FREP1 sequences from multiple species of anopheline mosquitoes
The orthologs of FREP1 in various anopheline species were obtained from the Vector-Base genome server (18). The multiple sequence alignment of A. gambiae FREP1 with its orthologs was built using ClustalO program version 1.2.1 (19) and visualized with Jalview (20).
Using the ELISA approach to demonstrate that anti-FBG serum inhibits the interaction between FREP1 and parasites
15-day cultured P. falciparum NF54 cells were lysed in PBST. About 50 μl of supernatant (2.0 mg/ml) per well was used to coat a 96-well plate. Purified recombinant full-length FREP1 was mixed with preimmune mouse serum (1:150) or anti-FBG serum (1:150; final titer units, 1 × 104). BSA (2.5 mg/ml) was used as a negative control. The bound FREP1 in wells was probed with anti-N-FREP1 mouse serum (1:1000 dilution), followed by incubation with alkaline phosphatase–conjugated goat anti-mouse IgG (1:20,000). After development with p-nitrophenyl phosphate (Sigma), A405 (OD405) was measured using an Epoch microplate reader. Three replicate experiments were conducted.
Binding assays between FBG and PM
3- to 5-day-old G3 A. gambiae mosquitoes were fed with mouse blood. The engorged mosquitoes were maintained in the insectary (27 °C, 80% humidity) for 18 h. Ice-anesthetized mosquitoes were then dissected to obtain midguts, and the blood bolus inside a midgut was removed by puncturing it. Blood-fed mosquito midguts and naïve mosquito midguts were incubated with 200 μl of 0.1 mg/ml BSA, full-length FREP1, N-FREP1, and FBG separately for 2 h at room temperature. Ten midguts were used in each treatment. Three independent experimental replicates were performed. After incubation, midguts were homogenized with pestles in 300 μl of 0.5% Tween 20 in PBS. Midgut lysate supernatants (50 μl) were used to coat 96-well plates overnight at 4 °C. Because recombinant proteins of FREP1, FBG, and N-FREP1 contain a His tag, the bound protein was probed with 50 μl of mouse anti-His monoclonal antibody and developed as we mentioned above.
Statistical analyses
As described previously (12), the p values of oocyst difference for in vivo infection assays and the SMFA were obtained with a nonparametric Mann-Whitney-Wilcoxon test using R package software. The p values for binding assays were obtained using two-tailed Student's t test. The p values of cross-reactions triggered by FREP1 were calculated with one-way analysis of variance in R package software.
Author contributions
G. N. and C. F. prepared proteins, vaccinated mice, and infected mice and mosquitoes. G. Z. was involved in infecting A. gambiae mosquitoes. W. R., W. N., and J. P. designed and conducted experiments related to P. vivax and A. dirus. X. W. participated in data analysis. N. S. B. was involved in experimental design and vaccination of mice. J. L. designed the project, conducted experiments, and analyzed the data. G. N., C. F., and J. L. wrote the manuscripts. All authors edited the manuscript.
This work was supported by National Institutes of Health Grant R21AI115178 and National Science Foundation Career Award 1742644. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or National Science Foundation.
PATH. (2010) PATH Malaria Vaccine Initiative: Workshop on Malaria Transmission-Blocking Vaccines: http://www.malariavaccine.org/sites/www.malariavaccine.org/files/content/resource/files/TBV-workshop-summary-20140331.pdf. (Please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party–hosted site.)
- TBV
- transmission-blocking vaccine
- FBG
- fibrinogen
- ALT
- alanine aminotransferase
- SMFA
- standard membrane-feeding assay
- CAT
- chloramphenicol acetyltransferase
- PM
- peritrophic matrix
- IFA
- immunofluorescence assay
- s.c.
- subcutaneous(ly).
References
- 1. Murray C. J., Rosenfeld L. C., Lim S. S., Andrews K. G., Foreman K. J., Haring D., Fullman N., Naghavi M., Lozano R., and Lopez A. D. (2012) Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 379, 413–431 [DOI] [PubMed] [Google Scholar]
- 2. Dinglasan R. R., and Jacobs-Lorena M. (2008) Flipping the paradigm on malaria transmission-blocking vaccines. Trends Parasitol. 24, 364–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kapulu M. C., Da D. F., Miura K., Li Y., Blagborough A. M., Churcher T. S., Nikolaeva D., Williams A. R., Goodman A. L., Sangare I., Turner A. V., Cottingham M. G., Nicosia A., Straschil U., Tsuboi T., et al. (2015) Comparative assessment of transmission-blocking vaccine candidates against Plasmodium falciparum. Sci. Rep. 5, 11193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mlambo G., Maciel J., and Kumar N. (2008) Murine model for assessment of Plasmodium falciparum transmission-blocking vaccine using transgenic Plasmodium berghei parasites expressing the target antigen Pfs25. Infect. Immun. 76, 2018–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaslow D. C., Quakyi I. A., Syin C., Raum M. G., Keister D. B., Coligan J. E., McCutchan T. F., and Miller L. H. (1988) A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature 333, 74–76 [DOI] [PubMed] [Google Scholar]
- 6. Wu Y., Ellis R. D., Shaffer D., Fontes E., Malkin E. M., Mahanty S., Fay M. P., Narum D., Rausch K., Miles A. P., Aebig J., Orcutt A., Muratova O., Song G., Lambert L., et al. (2008) Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS ONE 3, e2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kiszewski A., Mellinger A., Spielman A., Malaney P., Sachs S. E., and Sachs J. (2004) A global index representing the stability of malaria transmission. Am. J. Trop. Med. Hyg. 70, 486–498 [PubMed] [Google Scholar]
- 8. malERA Consultative Group on Vaccines (2011) A research agenda for malaria eradication: vaccines. PLoS Med. 8, e1000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Birkett A. J., Moorthy V. S., Loucq C., Chitnis C. E., and Kaslow D. C. (2013) Malaria vaccine R&D in the decade of vaccines: breakthroughs, challenges and opportunities. Vaccine 31, B233–B243 [DOI] [PubMed] [Google Scholar]
- 10. Dinglasan R. R., Kalume D. E., Kanzok S. M., Ghosh A. K., Muratova O., Pandey A., and Jacobs-Lorena M. (2007) Disruption of Plasmodium falciparum development by antibodies against a conserved mosquito midgut antigen. Proc. Natl. Acad. Sci. U.S.A. 104, 13461–13466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang G., Niu G., Franca C. M., Dong Y., Wang X., Butler N. S., Dimopoulos G., and Li J. (2015) Anopheles midgut FREP1 mediates Plasmodium invasion. J. Biol. Chem. 290, 16490–16501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li J., Wang X., Zhang G., Githure J. I., Yan G., and James A. A. (2013) Genome-block expression-assisted association studies discover malaria resistance genes in Anopheles gambiae. Proc. Natl. Acad. Sci. U.S.A. 110, 20675–20680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miura K., Takashima E., Deng B., Tullo G., Diouf A., Moretz S. E., Nikolaeva D., Diakite M., Fairhurst R. M., Fay M. P., Long C. A., and Tsuboi T. (2013) Functional comparison of Plasmodium falciparum transmission-blocking vaccine candidates by the standard membrane-feeding assay. Infect. Immun. 81, 4377–4382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Armistead J. S., Morlais I., Mathias D. K., Jardim J. G., Joy J., Fridman A., Finnefrock A. C., Bagchi A., Plebanski M., Scorpio D. G., Churcher T. S., Borg N. A., Sattabongkot J., and Dinglasan R. R. (2014) Antibodies to a single, conserved epitope in Anopheles APN1 inhibit universal transmission of Plasmodium falciparum and Plasmodium vivax malaria. Infect. Immun. 82, 818–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Niu G., Wang B., Zhang G., King J. B., Cichewicz R. H., and Li J. (2015) Targeting mosquito FREP1 with a fungal metabolite blocks malaria transmission. Sci. Rep. 5, 14694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bradford M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 17. Parasuraman S., Raveendran R., and Kesavan R. (2010) Blood sample collection in small laboratory animals. J. Pharmacol. Pharmacother. 1, 87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Giraldo-Calderón G. I., Emrich S. J., MacCallum R. M., Maslen G., Dialynas E., Topalis P., Ho N., Gesing S., VectorBase Consortium, Madey G., Collins F. H., and Lawson D. (2015) VectorBase: an updated bioinformatics resource for invertebrate vectors and other organisms related with human diseases. Nucleic Acids Res. 43, D707–D713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sievers F., Wilm A., Dineen D., Gibson T. J., Karplus K., Li W., Lopez R., McWilliam H., Remmert M., Söding J., Thompson J. D., and Higgins D. G. (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Waterhouse A. M., Procter J. B., Martin D. M., Clamp M., and Barton G. J. (2009) Jalview Version 2: a multiple sequence alignment editor and analysis workbench. Bioinformatics 25, 1189–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]