Abstract
Purpose
To measure in vivo brain gamma-aminobutyric acid (GABA) concentrations, and assess regional and hemispheric differences, using MR spectroscopy (1H-MRS).
Materials and Methods
GABA concentrations were measured bilaterally in the frontal cortex (FC), parietal cortex (PC), and occipital cortex (OC) of 21 healthy young subjects (age range 20–29 years) using 3 Tesla Philips scanner. A univariate general linear model analysis was carried out to assess the effect of region and hemisphere as well as their interaction on GABA concentrations while controlling for sex and gray matter differences.
Results
Results indicated a significant regional dependence of GABA levels [F(2,89) =11.725, P < 0.001, ] with lower concentrations in the FC compared with both PC (P < 0.001) and OC (P < 0.001) regions. There was no significant hemispheric differences in GABA levels [F(1,89) =.172; P =0.679; ].
Conclusion
This study reports the concentrations of GABA in the FC, PC, and OC brain regions of healthy young adults. GABA distribution exhibits hemispheric symmetry, but varies across regions; GABA levels in the FC are lower than those in the PC and OC.
Gamma-aminobutyric acid (GABA) is the primary inhibitory neurotransmitter in the mammalian brain (for extensive review, see Petroff).1 GABAergic inhibition counteracts glutamatergic excitation to regulate neuronal activity in the healthy brain.2 Regional alterations in GABA have been associated with a myriad of neurological and psychiatric disorders.3 For instance, disrupted GABA levels have been reported in the parietal region in patients with Alzheimer’s disease,4 and in the occipital region in patients with autism.5 Studies are ongoing to assess the potential of brain GABA levels as a neurochemical biomarker for various neurological and psychiatric disorders.6,7 Consequently, reliable and accurate estimation of GABA levels in key brain regions of healthy individuals has clinical value.
Despite the evident need and relevance of characterizing the normal distribution of GABA in the brain, there is a paucity of such studies in the literature. A few studies have indicated inhomogeneous distribution of GABA in the human brain.8,9 A recent study that assessed the ratio of GABA to N-acetyl aspartate (NAA) signal in various brain regions of eight subjects showed significant regional differences in GABA/NAA ratios between the frontal lobe and basal ganglia, lateral temporal lobe, and right hippocampus, providing preliminary indication of inhomogeneous distribution of GABA in the brain.10
Furthermore, there is very little information on the left–right hemispheric distribution of GABA in the brain. Thus far, one study has looked into hemispheric asymmetry of in vivo GABA levels; this study showed laterality of GABA in the primary motor cortex to be dependent on handedness11
Until recently, robust spectroscopic quantitation of GABA with proton (1H) MR spectroscopy (1H-MRS) has been challenging due to its low concentrations, which are near the detection limit for in vivo MRS at ~1 mM. Additionally, the GABA resonance is obscured by high signal-to-noise ratio signal from other metabolites, especially from the creatine (Cr) resonance at 3 ppm.12 Consequently, robust quantitation of GABA requires usage of spectral editing methods such as Mescher-Garwood point resolved spectroscopy (MEGA-PRESS)13 and double quantum filter,14 pulse sequences, which are tailored specifically to isolate GABA signals from in vivo spectra.
In this study, we aim to estimate GABA concentrations bilaterally in three cerebral regions, i.e. frontal cortex (FC), parietal cortex (PC), and occipital cortex (OC), of healthy young adults and investigate regional and hemispheric differences in GABA concentrations.
Materials and Methods
Subjects
Twenty-one healthy young adults (10 males and 11 females; age 23.8 ± 2.5 years; age range 20–29 years; education 17 ± 2.16 years) were recruited. All subjects were right-handed and reported normal or corrected-to-normal vision. Exclusion criteria included MR incompatibility, claustrophobia, and history or presence of any neurological or psychiatric disorders. All subjects were explained the purpose of the study and written informed consent was obtained before participation in the study. The study protocol was approved by the human ethics committee at National Brain Research Centre.
MR Data Acquisition
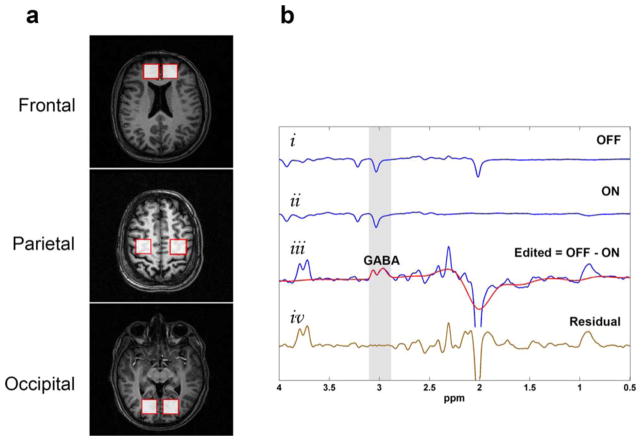
All 1H-MRS data were acquired with a 3 Tesla MR scanner (Achieva, Philips, The Netherlands) equipped with eight-channel dual tuned transmit-receive volume coil. The spectroscopy data were acquired from six single voxel volumes corresponding to the bilateral FC, PC, and OC regions of each subject (Fig. 1a). Care was taken to ensure the standard placement of voxels in all subjects, and MRS data acquisition was performed by one of us (P.K.M.) for consistency.
FIGURE 1.
Detection of GABA resonance at 3 ppm through MEGA-PRESS editing technique. (a) MRS voxel placement for regions of interest in the bilateral frontal, parietal, and occipital regions. (b) Representative stacked plot showing (i) the sub-spectrum acquired with the J-editing pulses OFF; (ii) the sub-spectrum acquired with the J-editing pulses ON; (iii) the edited spectrum with the GABA resonance at 3 ppm (blue line) together with the fitted spectrum (red line); (iv) the residual difference between the experimental and fitted GABA spectrum (brown line).
At each of the six brain regions, GABA measurements were performed using MEGA-PRESS pulse sequence from individual voxel volumes of 15.6 cc (voxel dimension = 2.5 × 2.5 × 2.5 cm3) with the following experimental parameters: repetition time (TR) = 2000 ms; echo time (TE) = 68 ms; spectral width = 2000 Hz; number of samples = 2048; number of signal averages = 320. The GABA resonance at 3 ppm was detected by application of refocusing pulse at 1.9 ppm during ON spectra and at 7.5 ppm during OFF spectra (Fig. 1b). Suppression of water signal was achieved by chemical shift selective (CHESS) imaging technique.15 First- and second-order shimming was performed using pencil beam-volume, resulting in water linewidths of < 16 Hz for FC, < 12 Hz for PC, and < 13 Hz for OC. The total scan time for all six brain regions was approximately one hour.
Following 1H-MRS acquisition, T1-weighted anatomical image was acquired with following acquisitions parameters: TR = 8.4 ms; TE =3.48 ms; field of view = 210 × 210 × 160 mm3; flip angle = 8°; total number of slices = 145; voxel size = 1 × 1 × 1.1 mm3.
Postprocessing and Quantitation
The postprocessing and spectral fitting of 1H-MRS data were performed using an in-house MATLAB-based toolbox, KALPANA. All sub-spectra were initially phase corrected with respect to first data point, apodized with 4 Hz Gaussian window function, and zero-filled to 4096 time points, followed by Fourier transformation. Each sub-spectrum was then corrected for phase and frequency shift errors with respect to the Cr peak. Before summation, individual sub-spectra were rejected in pairs if any of the parameter estimates for the Cr peak, i.e. amplitude, frequency, phase, or linewidth, deviated more than 3 SD (as estimated from all sub-spectra).16 Subsequently, the summed ON spectrum was subtracted from the summed OFF spectrum to obtain the difference edited spectrum. The edited spectrum was inverse Fourier transformed to time-domain and residual water was suppressed using the Hankel singular value decomposition method.17
The GABA signal at 3 ppm was fitted as two Gaussian peaks (Fig. 1b) using an iterative baseline estimation and peak fitting approach.18 This approach has been previously shown to yield better quantitation results as compared to single pass optimization method that models baseline and metabolite signal together.19 We used singular spectrum analysis20 for baseline estimation, and time-domain nonlinear least square cost function optimization for spectral fitting. The quality of spectral fitting was assessed using Cramer-Rao lower bound (CRLB)21 values for the time-domain GABA amplitude estimates and fitted spectra with CRLB >20% were rejected.
The anatomical images were segmented using the fuzzy c-means method22 and tissue composition within each voxel volume was calculated. The percentage GM in each voxel volume after excluding cerebrospinal fluid was used to account for the difference in GM fraction between different voxels.
Absolute Quantitation
GABA concentrations in mM (institutional units), i.e. [GABA], were estimated using Cr as internal reference according to the equation:
where IGABA and ICr are the signal intensities of GABA and Cr, respectively; [Cr] is the concentration of Cr (assumed to be 7.1 mM); 3/2 is the ratio of number of protons in Cr and GABA; MMcor is the scaling factor to account for contribution of macromolecules (MM) to the GABA peak (.45)23; eff is the editing efficiency (.5).23 Both IGABA and ICr were individually corrected for T1 and T2 relaxation effects by using literature reported values of T1 and T2 relaxation times.24–26
Statistical Analysis
Data were analyzed using the Statistical Package for Social Sciences (SPSS, version 22.0, Chicago, IL). Normality and homoscedasticity of GABA levels were assessed using Shapiro-Wilk test and Levene’s test, respectively. As the criterion for normality was not met in some of the cases, GABA concentrations were log-transformed. The effect of REGION (FC, PC, OC) and HEMISPHERE (left and right) factors on Log GABA was assessed with univariate general linear model (GLM) analyses. To control for the possible confounding effects of sex and voxel GM content on GABA levels, sex of subjects and percentage GM in voxels were included as covariates. The main effects of REGION and HEMISPHERE along with the interaction factor REGION × HEMISPHERE were assessed. Post hoc analysis were carried out using the Bonferroni method for multiple comparison correction. All results are reported as mean ± SD. Significance level was set at P < 0.05 for all analysis.
Results
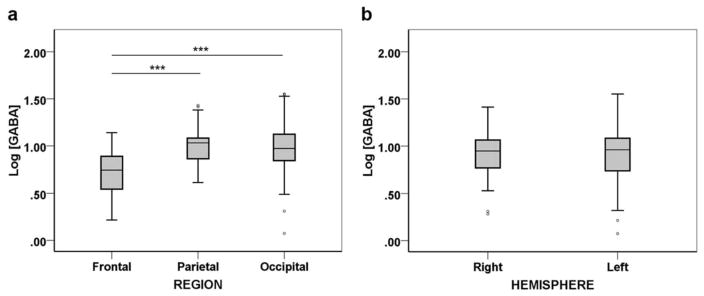
The mean and SD values of GABA for each region and hemisphere are tabulated in Table 1. The GLM analysis indicated a significant main effect of REGION on Log GABA after controlling for sex and voxel GM fraction [F(2,89) =11.725; P < 0.001; ] (Fig. 2a). Post hoc analysis between different regions indicated that the FC had significantly lower Log GABA compared with both PC (P < 0.001) and OC (P < 0.001) (Fig. 2a). No significant difference was found for Log GABA between the PC and OC (P =0.523).
TABLE 1.
GABA Concentrations in mM (institutional units) for Different Regions and Hemispheres
| Mean | SD | 95% Confidence interval
|
||
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| Regions: | ||||
| Frontal | 2.09 | .50 | 1.89 | 2.29 |
| Parietal | 2.78 | .56 | 2.59 | 2.96 |
| Occipital | 2.74 | .84 | 2.45 | 3.04 |
| Hemispheres: | ||||
| Right | 2.54 | .59 | 2.37 | 2.71 |
| Left | 2.62 | .82 | 2.38 | 2.86 |
FIGURE 2.
Regional and hemispheric distribution of GABA. a: Log GABA concentrations in the frontal, parietal, and occipital regions. b: Log GABA concentrations in the right and left hemispheres. Boxes represent median and interquartile range of the Log GABA distribution. Whiskers extend to cases that are 1.5 times the interquartile range. ***P < 0.001.
Assessment of hemispheric GABA distribution revealed no significant hemispheric asymmetry in Log GABA [F(1,89) =.172; P =0.679; ] (Fig. 2b). Moreover, there was no significant effect of REGION × HEMISPHERE interaction on Log GABA [F(2,89) =.313; P =0.732; ].
Discussion
In this study, we investigated regional and hemispheric variations of in vivo GABA levels using single voxel MEGA-PRESS after controlling for both sex and GM fraction. GABA concentrations found in our study are in line with previously published concentrations of GABA.11,27 The key finding of our study is that GABA levels are symmetrically distributed between left and right hemispheres but vary across regions.
Previous studies have indicated inhomogeneous distribution of GABA in the human brain.8–10 Our finding of lower GABA levels in the FC compared with the OC are in line with a recent study that showed a similar, albeit insignificant, trend in GABA/NAA ratio between the FC and OC.10 These regional variations in GABA concentrations likely relate to variation in the number and synaptic density of GABAergic neurons in these regions, although MRS does not allow for distinction of intracellular versus extracellular GABA.3 The observed regional GABA distribution in this study highlights the importance of considering regional differences while investigating between-population differences in GABA levels, particularly in context of neurological and psychiatric disorders.
There exists extensive evidence for brain lateralization from anatomical, functional, and biochemical studies. However, there is very little in vivo evidence of neurochemical laterality. Function-related regional asymmetry of GABA distribution has previously been reported in one MRS study that indicated higher in vivo GABA levels in the left primary motor cortex than in the right motor cortex of right-handed subjects.11 A postmortem biochemical analysis study has also shown asymmetrical distribution of GABA.28 Our data, however, do not support the idea of GABA laterality in the FC, OC, and PC regions of the brain.
It is important to discuss the findings of this study in light of its limitations. Previous studies indicate significant effect of menstrual cycle on GABA concentrations.9,29 However, we did not control for the phase of menstrual cycle during GABA signal acquisitions, which may have confounded the obtained GABA concentrations. Furthermore, the generalizability of the study findings is limited to healthy young adults (20–29 years) and to selective brain regions (frontal, parietal and occipital cortices). Future large-scale studies that target other brain regions and different age groups are required to gain further insights into regional GABA distribution in the brain.
It should also be noted that, although the relative contribution of MM to the GABA peak has been accounted for in our study, we did not control for variability in GABA to MM ratio. However, the relative contribution of MM to the GABA peak has been shown to remain relatively stable across anatomical regions of the brain,30 suggesting that the observed regional variations reflect changes in absolute GABA concentrations.
In conclusion, this study reports bilateral GABA concentrations in three key brain regions and demonstrates significant regional variations in brain GABA levels. It evidences hemispheric symmetry in GABA distribution. The findings in this study underscore the importance of factoring regional effects while assessing GABA levels in various clinical studies are under active consideration.
Acknowledgments
Contract grant sponsor: Department of Biotechnology, Ministry of Science and Technology; contract grant number: BT/PR7361/MED/30/953/2013; NIH; contract grant number: R01 EB016089; P41 EB015909
Dr. Pravat K. Mandal thanks the Department of Biotechnology, Ministry of Science and Technology, Government of India, for funding. Financial support to Dr. Mandal from Tata Innovation Fellowship, Department of Biotechnology, Government of India, is also acknowledged. Dr. Richard A.E. Edden thanks NIH for support. Dr. Mandal thanks Sarah A. Khan, Shammi More, and Shipra Jain for helping with subject recruitment. Dr. Sandeep Kalra (Director Radiology, Medanta Africare, Nairobi, Kenya) is also acknowledged for his help with MRI report assessments.
References
- 1.Petroff OA. GABA and glutamate in the human brain. Neuroscientist. 2002;8:562–573. doi: 10.1177/1073858402238515. [DOI] [PubMed] [Google Scholar]
- 2.Neto FL, Ferreira-Gomes J, Castro-Lopes JM. Distribution of GABA receptors in the thalamus and their involvement in nociception. Adv Pharmacol. 2006;54:29–51. doi: 10.1016/s1054-3589(06)54002-5. [DOI] [PubMed] [Google Scholar]
- 3.Levy LM, Degnan AJ. GABA-based evaluation of neurologic conditions: MR spectroscopy. Am Soc Neuroradiology. 2013;34:259–265. doi: 10.3174/ajnr.A2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai X, Edden RA, Gao F, et al. Decreased gamma-aminobutyric acid levels in the parietal region of patients with Alzheimer’s disease. J Magn Reson Imaging. 2015;41:1326–1331. doi: 10.1002/jmri.24665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robertson Caroline E, Ratai E-M, Kanwisher N. Reduced GABAergic action in the autistic brain. Curr Biol. 2016;26:80–85. doi: 10.1016/j.cub.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 6.El-Ansary A, Al-Ayadhi L. GABAergic/glutamatergic imbalance relative to excessive neuroinflammation in autism spectrum disorders. J Neuroinflammation. 2014;11:189. doi: 10.1186/s12974-014-0189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cawley N, Solanky BS, Muhlert N, et al. Reduced gamma-aminobutyric acid concentration is associated with physical disability in progressive multiple sclerosis. Brain. 2015;138:2584–2595. doi: 10.1093/brain/awv209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans CJ, McGonigle DJ, Edden RAE. Diurnal stability of γ-aminobutyric acid concentration in visual and sensorimotor cortex. J Magn Reson Imaging. 2010;31:204–209. doi: 10.1002/jmri.21996. [DOI] [PubMed] [Google Scholar]
- 9.Harada M, Kubo H, Nose A, Nishitani H, Matsuda T. Measurement of variation in the human cerebral GABA level by in vivo MEGA-editing proton MR spectroscopy using a clinical 3 T instrument and its dependence on brain region and the female menstrual cycle. Hum Brain Mapp. 2011;32:828–833. doi: 10.1002/hbm.21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durst CR, Michael N, Tustison NJ, et al. Noninvasive evaluation of the regional variations of GABA using magnetic resonance spectroscopy at 3 Tesla. Magn Reson Imaging. 2015;33:611–617. doi: 10.1016/j.mri.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Oeltzschner G, Hoogenboom N, Baumgarten T, Wittsack H, Jr, Schnitzler A. Absolute GABA spectroscopy with MEGA-PRESS and watermapping in sensorimotor and visual cortex and correlation to handedness. Eur J Med Res. 2014;19(Suppl 1):S28. [Google Scholar]
- 12.Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 13.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11:266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 14.Keltner JR, Wald LL, Frederick BDB, Renshaw PF. In vivo detection of GABA in human brain using a localized double-quantum filter technique. Magn Reson Med. 1997;37:366–371. doi: 10.1002/mrm.1910370312. [DOI] [PubMed] [Google Scholar]
- 15.Haase A, Frahm J, Hanicke W, Matthaei D. 1H NMR chemical shift selective (CHESS) imaging. Phys Med Biol. 1985;30:341–344. doi: 10.1088/0031-9155/30/4/008. [DOI] [PubMed] [Google Scholar]
- 16.Edden RA, Puts NA, Harris AD, Barker PB, Evans CJ. Gannet: a batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J Magn Reson Imaging. 2014;40:1445–1452. doi: 10.1002/jmri.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barkhuijsen H, de Beer R, van Ormondt D. Improved algorithm for noniterative time-domain model fitting to exponentially damped magnetic resonance signals. J Magn Reson. 1987;73:553–557. [Google Scholar]
- 18.Young K, Soher BJ, Maudsley AA. Automated spectral analysis II: application of wavelet shrinkage for characterization of non-parameterized signals. Magn Reson Med. 1998;40:816–821. doi: 10.1002/mrm.1910400606. [DOI] [PubMed] [Google Scholar]
- 19.Soher BJ, Young K, Maudsley AA. Representation of strong baseline contributions in 1H MR spectra. Magn Reson Med. 2001;45:966–972. doi: 10.1002/mrm.1129. [DOI] [PubMed] [Google Scholar]
- 20.Golyandina N, Nekrutkin V, Zhigljavsky AA. Analysis of time series structure: SSA and related techniques. Boca Raton, FL: CRC Press; 2001. p. 320. [Google Scholar]
- 21.Cavassila S, Deval S, Huegen C, van Ormondt D, Graveron-Demilly D. Cramer-Rao bounds: an evaluation tool for quantitation. NMR Biomed. 2001;14:278–283. doi: 10.1002/nbm.701. [DOI] [PubMed] [Google Scholar]
- 22.Bezdek JC. Pattern recognition with fuzzy objective function algorithms. New York: Plenum Press; 1981. p. 256. [Google Scholar]
- 23.Mullins PG, McGonigle DJ, O’Gorman RL, et al. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage. 2014;86:43–52. doi: 10.1016/j.neuroimage.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puts NA, Barker PB, Edden RA. Measuring the longitudinal relaxation time of GABA in vivo at 3 Tesla. J Magn Reson Imaging. 2013;37:999–1003. doi: 10.1002/jmri.23817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edden RA, Intrapiromkul J, Zhu H, Cheng Y, Barker PB. Measuring T2 in vivo with J-difference editing: application to GABA at 3 Tesla. J Magn Reson Imaging. 2012;35:229–234. doi: 10.1002/jmri.22865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mlynarik V, Gruber S, Moser E. Proton T1 and T2 relaxation times of human brain metabolites at 3 Tesla. NMR Biomed. 2001;14:325–331. doi: 10.1002/nbm.713. [DOI] [PubMed] [Google Scholar]
- 27.Harris AD, Puts NAJ, Anderson BA, et al. Multi-regional investigation of the relationship between functional MRI blood oxygenation level dependent (BOLD) activation and GABA concentration. PLoS One. 2015;10:e0117531. doi: 10.1371/journal.pone.0117531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glick SD, Ross DA, Hough LB. Lateral asymmetry of neurotransmitters in human brain. Brain Res. 1982;234:53–63. doi: 10.1016/0006-8993(82)90472-3. [DOI] [PubMed] [Google Scholar]
- 29.De Bondt T, De Belder F, Vanhevel F, Jacquemyn Y, Parizel PM. Pre-frontal GABA concentration changes in women-influence of menstrual cycle phase, hormonal contraceptive use, and correlation with pre-menstrual symptoms. Brain Res. 2015;1597:129–138. doi: 10.1016/j.brainres.2014.11.051. [DOI] [PubMed] [Google Scholar]
- 30.Kegeles LS, Mao X, Gonsalez R, Shungu DC. Evaluation of anatomic variation in macromolecule contribution to the GABA signal using metabolite nulling and the J-editing technique at 3.0 T. Proceedings of the 15th Annual Meeting of ISMRM; Berlin, Germany. 2007. (abstract 1391) [Google Scholar]